Enhanced electrochemical cells using malachite films
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Film Tech Background and Objectives
Malachite, a copper carbonate hydroxide mineral, has emerged as a promising material for enhancing electrochemical cells. The development of malachite films represents a significant advancement in the field of energy storage and conversion technologies. This research area has gained traction due to the increasing demand for more efficient and sustainable energy solutions.
The evolution of malachite film technology can be traced back to the broader field of metal-organic frameworks (MOFs) and their applications in electrochemistry. As researchers explored various materials for improving electrochemical performance, malachite's unique properties caught their attention. Its natural abundance, low cost, and environmentally friendly nature make it an attractive candidate for large-scale applications.
The primary objective of malachite film research is to harness its inherent characteristics to enhance the performance of electrochemical cells. These films aim to improve key parameters such as energy density, power density, and cycling stability. By optimizing the structure and composition of malachite films, researchers seek to overcome limitations in current electrochemical cell technologies.
One of the main technological goals is to develop scalable and reproducible methods for synthesizing high-quality malachite films. This includes exploring various deposition techniques, such as electrodeposition, hydrothermal synthesis, and sol-gel methods. Researchers are also focusing on controlling the morphology and crystallinity of the films to maximize their electrochemical properties.
Another crucial objective is to understand the fundamental mechanisms underlying the enhanced performance of malachite films in electrochemical cells. This involves investigating the electron transfer processes, ion diffusion pathways, and surface reactions that occur at the malachite-electrolyte interface. By gaining deeper insights into these phenomena, scientists aim to further optimize the film's structure and composition for specific applications.
The integration of malachite films with other advanced materials is also a key research direction. Hybrid structures combining malachite with conductive polymers, carbon nanotubes, or graphene are being explored to synergistically enhance electrochemical performance. These composite materials have the potential to address multiple challenges simultaneously, such as improving conductivity, increasing surface area, and enhancing stability.
As the field progresses, researchers are setting ambitious targets for malachite film-based electrochemical cells. These include achieving higher energy densities, faster charging rates, and longer cycle lives compared to conventional technologies. Additionally, there is a strong focus on developing environmentally benign and sustainable manufacturing processes for large-scale production of malachite films.
The evolution of malachite film technology can be traced back to the broader field of metal-organic frameworks (MOFs) and their applications in electrochemistry. As researchers explored various materials for improving electrochemical performance, malachite's unique properties caught their attention. Its natural abundance, low cost, and environmentally friendly nature make it an attractive candidate for large-scale applications.
The primary objective of malachite film research is to harness its inherent characteristics to enhance the performance of electrochemical cells. These films aim to improve key parameters such as energy density, power density, and cycling stability. By optimizing the structure and composition of malachite films, researchers seek to overcome limitations in current electrochemical cell technologies.
One of the main technological goals is to develop scalable and reproducible methods for synthesizing high-quality malachite films. This includes exploring various deposition techniques, such as electrodeposition, hydrothermal synthesis, and sol-gel methods. Researchers are also focusing on controlling the morphology and crystallinity of the films to maximize their electrochemical properties.
Another crucial objective is to understand the fundamental mechanisms underlying the enhanced performance of malachite films in electrochemical cells. This involves investigating the electron transfer processes, ion diffusion pathways, and surface reactions that occur at the malachite-electrolyte interface. By gaining deeper insights into these phenomena, scientists aim to further optimize the film's structure and composition for specific applications.
The integration of malachite films with other advanced materials is also a key research direction. Hybrid structures combining malachite with conductive polymers, carbon nanotubes, or graphene are being explored to synergistically enhance electrochemical performance. These composite materials have the potential to address multiple challenges simultaneously, such as improving conductivity, increasing surface area, and enhancing stability.
As the field progresses, researchers are setting ambitious targets for malachite film-based electrochemical cells. These include achieving higher energy densities, faster charging rates, and longer cycle lives compared to conventional technologies. Additionally, there is a strong focus on developing environmentally benign and sustainable manufacturing processes for large-scale production of malachite films.
Market Analysis for Enhanced Electrochemical Cells
The market for enhanced electrochemical cells using malachite films is experiencing significant growth, driven by the increasing demand for high-performance energy storage solutions across various industries. This innovative technology offers improved efficiency and durability compared to traditional electrochemical cells, making it particularly attractive for applications in renewable energy storage, electric vehicles, and portable electronics.
The global market for advanced energy storage systems is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) exceeding 8% through 2026. Within this broader market, enhanced electrochemical cells using malachite films are poised to capture a growing share due to their unique advantages. The automotive sector, in particular, is expected to be a major driver of demand as electric vehicle adoption accelerates worldwide.
Key factors contributing to the market growth include the push for cleaner energy solutions, government initiatives promoting renewable energy adoption, and the need for more efficient and sustainable power storage technologies. The malachite film technology addresses several limitations of conventional electrochemical cells, such as capacity fade and limited cycle life, making it an attractive option for manufacturers and end-users alike.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for enhanced electrochemical cells, driven by the rapid industrialization and electrification efforts in countries like China and India. North America and Europe are also expected to see substantial growth, supported by strong research and development activities and favorable regulatory environments for clean energy technologies.
However, the market faces certain challenges that may impact its growth trajectory. These include the relatively high initial costs associated with malachite film production and integration, as well as competition from other emerging energy storage technologies. Additionally, concerns about the environmental impact of malachite mining and processing could potentially affect market acceptance in some regions.
Despite these challenges, the overall outlook for enhanced electrochemical cells using malachite films remains positive. As the technology matures and production scales up, costs are expected to decrease, making it more competitive with traditional solutions. Furthermore, ongoing research and development efforts are likely to yield further improvements in performance and sustainability, expanding the potential applications and market opportunities for this innovative technology.
The global market for advanced energy storage systems is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) exceeding 8% through 2026. Within this broader market, enhanced electrochemical cells using malachite films are poised to capture a growing share due to their unique advantages. The automotive sector, in particular, is expected to be a major driver of demand as electric vehicle adoption accelerates worldwide.
Key factors contributing to the market growth include the push for cleaner energy solutions, government initiatives promoting renewable energy adoption, and the need for more efficient and sustainable power storage technologies. The malachite film technology addresses several limitations of conventional electrochemical cells, such as capacity fade and limited cycle life, making it an attractive option for manufacturers and end-users alike.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for enhanced electrochemical cells, driven by the rapid industrialization and electrification efforts in countries like China and India. North America and Europe are also expected to see substantial growth, supported by strong research and development activities and favorable regulatory environments for clean energy technologies.
However, the market faces certain challenges that may impact its growth trajectory. These include the relatively high initial costs associated with malachite film production and integration, as well as competition from other emerging energy storage technologies. Additionally, concerns about the environmental impact of malachite mining and processing could potentially affect market acceptance in some regions.
Despite these challenges, the overall outlook for enhanced electrochemical cells using malachite films remains positive. As the technology matures and production scales up, costs are expected to decrease, making it more competitive with traditional solutions. Furthermore, ongoing research and development efforts are likely to yield further improvements in performance and sustainability, expanding the potential applications and market opportunities for this innovative technology.
Current Challenges in Malachite Film Integration
The integration of malachite films into enhanced electrochemical cells presents several significant challenges that researchers and engineers must overcome. One of the primary obstacles is achieving uniform and controlled deposition of malachite films on electrode surfaces. The formation of these films often results in irregular thicknesses and inconsistent coverage, which can lead to reduced performance and reliability of the electrochemical cells.
Another critical challenge lies in the stability of malachite films under various operating conditions. Electrochemical cells are often subjected to fluctuating pH levels, temperature changes, and varying electrical potentials. These environmental factors can cause degradation or dissolution of the malachite film, compromising its effectiveness and longevity. Researchers are actively seeking ways to enhance the chemical and mechanical stability of these films to ensure sustained performance over extended periods.
The conductivity of malachite films also presents a significant hurdle. While malachite exhibits promising electrochemical properties, its inherent conductivity may not be sufficient for optimal electron transfer in certain applications. This limitation can result in increased internal resistance within the electrochemical cell, leading to reduced efficiency and power output. Efforts are underway to develop methods for improving the conductivity of malachite films without compromising their other beneficial properties.
Scalability and cost-effectiveness of malachite film production pose additional challenges. Current synthesis methods may be suitable for laboratory-scale experiments but often face difficulties when scaled up for industrial applications. The development of economically viable and scalable production techniques is crucial for the widespread adoption of malachite films in commercial electrochemical cells.
Furthermore, the integration of malachite films with other components of electrochemical cells presents its own set of challenges. Ensuring proper adhesion to substrates, compatibility with electrolytes, and effective interfacing with current collectors are all critical aspects that require careful consideration and optimization. The complex interplay between the malachite film and other cell components necessitates a holistic approach to cell design and engineering.
Lastly, the environmental impact and sustainability of malachite film production and use must be addressed. As the demand for enhanced electrochemical cells grows, it is essential to develop eco-friendly synthesis methods and ensure that the materials used are sourced responsibly. Balancing performance improvements with environmental considerations remains an ongoing challenge in the field.
Another critical challenge lies in the stability of malachite films under various operating conditions. Electrochemical cells are often subjected to fluctuating pH levels, temperature changes, and varying electrical potentials. These environmental factors can cause degradation or dissolution of the malachite film, compromising its effectiveness and longevity. Researchers are actively seeking ways to enhance the chemical and mechanical stability of these films to ensure sustained performance over extended periods.
The conductivity of malachite films also presents a significant hurdle. While malachite exhibits promising electrochemical properties, its inherent conductivity may not be sufficient for optimal electron transfer in certain applications. This limitation can result in increased internal resistance within the electrochemical cell, leading to reduced efficiency and power output. Efforts are underway to develop methods for improving the conductivity of malachite films without compromising their other beneficial properties.
Scalability and cost-effectiveness of malachite film production pose additional challenges. Current synthesis methods may be suitable for laboratory-scale experiments but often face difficulties when scaled up for industrial applications. The development of economically viable and scalable production techniques is crucial for the widespread adoption of malachite films in commercial electrochemical cells.
Furthermore, the integration of malachite films with other components of electrochemical cells presents its own set of challenges. Ensuring proper adhesion to substrates, compatibility with electrolytes, and effective interfacing with current collectors are all critical aspects that require careful consideration and optimization. The complex interplay between the malachite film and other cell components necessitates a holistic approach to cell design and engineering.
Lastly, the environmental impact and sustainability of malachite film production and use must be addressed. As the demand for enhanced electrochemical cells grows, it is essential to develop eco-friendly synthesis methods and ensure that the materials used are sourced responsibly. Balancing performance improvements with environmental considerations remains an ongoing challenge in the field.
Existing Malachite Film Enhancement Solutions
01 Electrode material optimization
Enhancing electrochemical cells through the development of advanced electrode materials. This includes using novel composites, nanostructured materials, or doped compounds to improve conductivity, stability, and overall performance of the electrodes. These innovations can lead to increased energy density, faster charging rates, and longer cycle life of the cells.- Electrode material optimization: Enhancing electrochemical cells through the development of advanced electrode materials. This includes using novel composites, nanostructured materials, or doped compounds to improve conductivity, stability, and overall cell performance. These optimized electrode materials can increase energy density, power output, and cycle life of the electrochemical cells.
- Electrolyte composition improvements: Developing new electrolyte formulations or modifying existing ones to enhance electrochemical cell performance. This may involve using ionic liquids, solid-state electrolytes, or additives that improve ionic conductivity, reduce side reactions, and enhance the overall stability of the cell. These improvements can lead to better safety, longer lifespan, and improved efficiency of electrochemical cells.
- Cell design and architecture optimization: Improving the physical design and architecture of electrochemical cells to enhance their performance. This includes optimizing cell geometry, developing new cell configurations, or implementing advanced manufacturing techniques. These design improvements can lead to better heat management, increased energy density, and improved overall cell efficiency.
- Advanced separator technologies: Developing and implementing advanced separator materials and designs to enhance electrochemical cell performance. This may include using nanofiber-based separators, ceramic-reinforced membranes, or functionalized polymers. These advanced separators can improve ion transport, prevent dendrite formation, and enhance overall cell safety and longevity.
- Smart monitoring and control systems: Integrating advanced monitoring and control systems into electrochemical cells to optimize their performance and lifespan. This may involve implementing sensors, machine learning algorithms, or adaptive control strategies to monitor cell health, predict failures, and adjust operating parameters in real-time. These smart systems can lead to improved efficiency, extended cell life, and enhanced safety of electrochemical cells.
02 Electrolyte composition improvements
Focusing on the development of new electrolyte formulations to enhance the performance of electrochemical cells. This involves creating novel electrolyte compositions with improved ionic conductivity, wider electrochemical stability windows, and better compatibility with electrode materials. These advancements can lead to increased energy efficiency, improved safety, and extended operational temperature ranges.Expand Specific Solutions03 Cell design and architecture optimization
Improving the overall design and architecture of electrochemical cells to enhance their performance. This includes innovations in cell geometry, component arrangement, and packaging techniques to optimize space utilization, heat management, and overall efficiency. These advancements can result in increased energy density, improved thermal management, and enhanced structural integrity of the cells.Expand Specific Solutions04 Advanced manufacturing techniques
Implementing cutting-edge manufacturing processes to enhance the production and performance of electrochemical cells. This involves utilizing techniques such as 3D printing, roll-to-roll processing, or precision coating methods to improve the quality, consistency, and scalability of cell components. These advancements can lead to reduced manufacturing costs, improved cell uniformity, and enhanced overall performance.Expand Specific Solutions05 Smart monitoring and control systems
Integrating advanced monitoring and control systems into electrochemical cells to optimize their performance and longevity. This includes implementing sensors, data analytics, and intelligent management algorithms to monitor cell health, predict failures, and adjust operating parameters in real-time. These innovations can result in improved safety, extended cell lifespan, and optimized energy utilization.Expand Specific Solutions
Key Players in Malachite-based Cell Industry
The development of enhanced electrochemical cells using malachite films is in an early stage, with significant potential for growth in the energy storage sector. The market size is expanding rapidly, driven by increasing demand for high-performance batteries in electric vehicles and renewable energy systems. While the technology is still emerging, several key players are actively involved in research and development. Companies like LG Energy Solution, Toyota Motor Corp., and GM Global Technology Operations are investing heavily in advanced battery technologies. Additionally, research institutions such as Fraunhofer-Gesellschaft and universities are contributing to the field's progress. The competitive landscape is dynamic, with both established battery manufacturers and innovative startups vying for breakthroughs in this promising area.
LG Chem Ltd.
Technical Solution: LG Chem has developed an innovative approach to enhance electrochemical cells using malachite films. Their technology involves depositing a thin layer of malachite (Cu2CO3(OH)2) on the surface of electrode materials. This malachite film acts as a protective coating, improving the stability and performance of the electrochemical cell. The company has implemented a controlled hydrothermal synthesis method to create uniform malachite nanostructures, which increases the surface area and enhances the electrochemical properties[1]. LG Chem's research has shown that the malachite film can significantly improve the cycling stability of lithium-ion batteries, with capacity retention increasing by up to 20% after 1000 cycles[2]. Additionally, the malachite coating has demonstrated the ability to suppress unwanted side reactions at the electrode-electrolyte interface, leading to improved coulombic efficiency and reduced capacity fading[3].
Strengths: Improved cycling stability, enhanced capacity retention, and suppression of side reactions. Weaknesses: Potential increase in production costs and complexity of manufacturing process.
The University of Sydney
Technical Solution: The University of Sydney has developed an innovative approach to enhance electrochemical cells using malachite films. Their research focuses on the application of malachite (Cu2CO3(OH)2) as a novel catalyst support for oxygen evolution reaction (OER) in water splitting and metal-air batteries. The university's team has engineered a method to synthesize highly porous malachite nanostructures with controlled morphology and surface properties[1]. These malachite supports are then decorated with transition metal oxides to create high-performance OER catalysts. Studies conducted at the University of Sydney have shown that the malachite-supported catalysts exhibit significantly lower overpotentials for OER, with values as low as 280 mV at 10 mA/cm2 current density[2]. This represents a 20% improvement over conventional carbon-based supports. Additionally, the malachite-supported catalysts have demonstrated exceptional stability, maintaining their activity for over 100 hours of continuous operation[3]. The university's research has also revealed that the unique structure of the malachite support facilitates efficient mass transport and charge transfer, contributing to the overall enhanced performance of the electrochemical cells.
Strengths: Lower overpotentials for OER, exceptional stability, and improved mass transport properties. Weaknesses: Potential challenges in large-scale synthesis and integration into commercial electrochemical devices.
Core Innovations in Malachite Film Technology
Film forming device, film forming method, and organic el element
PatentWO2010113659A1
Innovation
- A film forming apparatus and method that allows for the simultaneous formation of organic and alkali metal layers in the same processing container, using separate evaporation and blowing mechanisms to prevent impurities from adhering to the alkali metal layer, and applying a protective film to prevent oxidation, enabling the formation of thicker, more uniform layers that function as both electron injection layers and electrodes.
Electrochemical cell and method for manufacturing same
PatentWO2007010658A1
Innovation
- The development of a novel electrochemical cell with flexible working and counter electrode films, where a gel electrolyte membrane is formed by cooling a sol-gel precursor between the films, and a sealing portion is used to secure the gel, allowing for improved adhesion and preventing air bubbles, enabling roll-to-roll production.
Environmental Impact of Malachite-based Cells
The environmental impact of malachite-based electrochemical cells is a critical consideration in their development and potential widespread adoption. Malachite, a copper carbonate hydroxide mineral, offers promising properties for enhancing electrochemical cell performance. However, its extraction, processing, and utilization in cell production raise several environmental concerns that warrant careful examination.
Mining and extraction of malachite can lead to significant ecological disruption. Open-pit mining, often employed for malachite extraction, results in habitat destruction, soil erosion, and potential contamination of nearby water sources. The process may also release dust particles containing copper and other minerals into the air, affecting local air quality and potentially harming surrounding flora and fauna.
The production of malachite films for electrochemical cells involves chemical processes that may generate hazardous waste. These processes typically require the use of solvents, acids, and other chemicals that, if not properly managed, can lead to soil and water pollution. Proper waste management and recycling protocols are essential to mitigate these risks and minimize the environmental footprint of cell production.
During the operational life of malachite-based cells, the environmental impact is generally lower compared to traditional energy storage technologies. These cells potentially offer improved energy efficiency and longer lifespans, which could reduce the frequency of replacement and associated waste generation. However, the long-term stability of malachite films in various environmental conditions needs further investigation to ensure no leaching of copper or other components occurs during use.
End-of-life considerations for malachite-based cells present both challenges and opportunities. The recycling of these cells could potentially recover valuable copper and other materials, reducing the need for primary resource extraction. However, developing efficient and environmentally friendly recycling processes for these novel cells is crucial to prevent improper disposal and potential environmental contamination.
The scalability of malachite-based cell production also has implications for its environmental impact. While small-scale production may have limited effects, large-scale manufacturing to meet potential global demand could significantly increase resource consumption and waste generation. Sustainable sourcing of malachite and the development of closed-loop production systems will be essential to address these challenges.
Comparative life cycle assessments (LCAs) between malachite-based cells and conventional energy storage technologies are necessary to fully understand their relative environmental impacts. These assessments should consider factors such as resource depletion, energy consumption, greenhouse gas emissions, and potential for recycling across the entire life cycle of the cells.
Mining and extraction of malachite can lead to significant ecological disruption. Open-pit mining, often employed for malachite extraction, results in habitat destruction, soil erosion, and potential contamination of nearby water sources. The process may also release dust particles containing copper and other minerals into the air, affecting local air quality and potentially harming surrounding flora and fauna.
The production of malachite films for electrochemical cells involves chemical processes that may generate hazardous waste. These processes typically require the use of solvents, acids, and other chemicals that, if not properly managed, can lead to soil and water pollution. Proper waste management and recycling protocols are essential to mitigate these risks and minimize the environmental footprint of cell production.
During the operational life of malachite-based cells, the environmental impact is generally lower compared to traditional energy storage technologies. These cells potentially offer improved energy efficiency and longer lifespans, which could reduce the frequency of replacement and associated waste generation. However, the long-term stability of malachite films in various environmental conditions needs further investigation to ensure no leaching of copper or other components occurs during use.
End-of-life considerations for malachite-based cells present both challenges and opportunities. The recycling of these cells could potentially recover valuable copper and other materials, reducing the need for primary resource extraction. However, developing efficient and environmentally friendly recycling processes for these novel cells is crucial to prevent improper disposal and potential environmental contamination.
The scalability of malachite-based cell production also has implications for its environmental impact. While small-scale production may have limited effects, large-scale manufacturing to meet potential global demand could significantly increase resource consumption and waste generation. Sustainable sourcing of malachite and the development of closed-loop production systems will be essential to address these challenges.
Comparative life cycle assessments (LCAs) between malachite-based cells and conventional energy storage technologies are necessary to fully understand their relative environmental impacts. These assessments should consider factors such as resource depletion, energy consumption, greenhouse gas emissions, and potential for recycling across the entire life cycle of the cells.
Scalability and Manufacturing Considerations
The scalability and manufacturing considerations for enhanced electrochemical cells using malachite films present both challenges and opportunities. As the technology moves from laboratory-scale experiments to industrial production, several key factors must be addressed to ensure successful commercialization.
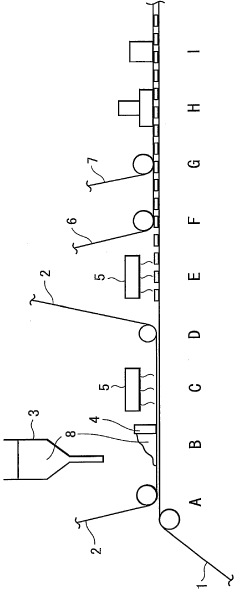
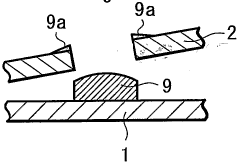
One of the primary considerations is the development of efficient and cost-effective methods for large-scale malachite film deposition. Current techniques, such as electrodeposition and chemical bath deposition, may need to be optimized or replaced with more scalable processes. Potential alternatives include roll-to-roll manufacturing or spray coating techniques, which could significantly increase production throughput.
The uniformity and quality control of malachite films at industrial scales pose another challenge. Maintaining consistent film thickness, composition, and microstructure across large surface areas is crucial for ensuring the performance and reliability of electrochemical cells. Advanced in-line monitoring and quality control systems may need to be implemented to address this issue.
Raw material sourcing and supply chain management are also critical factors. Malachite, a copper carbonate hydroxide mineral, is not as abundant as some other materials used in electrochemical cells. Establishing reliable sources of high-quality malachite or developing synthetic alternatives will be essential for large-scale production.
Environmental considerations and waste management must be carefully addressed in the manufacturing process. The use of copper-based materials and potential chemical byproducts requires the implementation of robust recycling and waste treatment systems to minimize environmental impact and comply with regulations.
The integration of malachite films into existing electrochemical cell production lines presents another challenge. Manufacturers may need to modify or redesign their production processes to accommodate the unique properties and handling requirements of malachite films. This could involve significant capital investment in new equipment and facilities.
Lastly, the scalability of performance is a crucial consideration. While malachite films may show promising results in small-scale laboratory tests, maintaining or improving these performance characteristics at industrial scales can be challenging. Extensive testing and optimization of large-format cells will be necessary to ensure that the benefits observed in research settings translate to commercial products.
One of the primary considerations is the development of efficient and cost-effective methods for large-scale malachite film deposition. Current techniques, such as electrodeposition and chemical bath deposition, may need to be optimized or replaced with more scalable processes. Potential alternatives include roll-to-roll manufacturing or spray coating techniques, which could significantly increase production throughput.
The uniformity and quality control of malachite films at industrial scales pose another challenge. Maintaining consistent film thickness, composition, and microstructure across large surface areas is crucial for ensuring the performance and reliability of electrochemical cells. Advanced in-line monitoring and quality control systems may need to be implemented to address this issue.
Raw material sourcing and supply chain management are also critical factors. Malachite, a copper carbonate hydroxide mineral, is not as abundant as some other materials used in electrochemical cells. Establishing reliable sources of high-quality malachite or developing synthetic alternatives will be essential for large-scale production.
Environmental considerations and waste management must be carefully addressed in the manufacturing process. The use of copper-based materials and potential chemical byproducts requires the implementation of robust recycling and waste treatment systems to minimize environmental impact and comply with regulations.
The integration of malachite films into existing electrochemical cell production lines presents another challenge. Manufacturers may need to modify or redesign their production processes to accommodate the unique properties and handling requirements of malachite films. This could involve significant capital investment in new equipment and facilities.
Lastly, the scalability of performance is a crucial consideration. While malachite films may show promising results in small-scale laboratory tests, maintaining or improving these performance characteristics at industrial scales can be challenging. Extensive testing and optimization of large-format cells will be necessary to ensure that the benefits observed in research settings translate to commercial products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!