Evaluating malachite's role in chemical pathways of biogeochemical cycles
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite in Biogeochemistry
Malachite, a copper carbonate hydroxide mineral, plays a significant role in the chemical pathways of biogeochemical cycles. This green mineral, with its chemical formula Cu2CO3(OH)2, is not only aesthetically pleasing but also serves as an important component in various environmental processes. Its presence in the Earth's crust and its interactions with biological systems make it a crucial element in the study of biogeochemistry.
In the context of biogeochemical cycles, malachite acts as both a source and a sink for copper and carbonate ions. The mineral's formation and dissolution processes are intricately linked to the carbon and copper cycles, influencing the distribution and availability of these elements in different environmental compartments. Malachite's solubility in water, albeit limited, allows for the slow release of copper ions into aqueous systems, thereby affecting the bioavailability of this essential micronutrient for various organisms.
The weathering of malachite contributes to the natural copper flux in terrestrial and aquatic ecosystems. As rainwater and other environmental factors interact with exposed malachite deposits, copper is gradually released into soils and water bodies. This process plays a crucial role in maintaining the copper balance in ecosystems, supporting the growth and development of plants and microorganisms that require copper for their metabolic functions.
Furthermore, malachite participates in the carbon cycle through its carbonate component. The formation of malachite serves as a carbon sink, temporarily sequestering atmospheric carbon dioxide in a solid mineral form. Conversely, the dissolution of malachite releases carbonate ions back into the environment, potentially influencing local pH levels and carbonate equilibria in aquatic systems.
In soil environments, malachite can act as a buffer against extreme pH changes due to its basic nature. This buffering capacity can have significant implications for soil chemistry and microbial communities, indirectly affecting nutrient cycling and plant growth. Additionally, the presence of malachite in soils can influence the mobility and bioavailability of other metal ions through adsorption and co-precipitation processes.
The role of malachite in biogeochemical cycles extends to its interactions with microorganisms. Some bacteria and fungi have developed mechanisms to solubilize copper from malachite, incorporating it into their cellular processes or using it as an electron acceptor in anaerobic respiration. These microbial interactions can accelerate the weathering of malachite and influence the local geochemistry of their habitats.
In the context of biogeochemical cycles, malachite acts as both a source and a sink for copper and carbonate ions. The mineral's formation and dissolution processes are intricately linked to the carbon and copper cycles, influencing the distribution and availability of these elements in different environmental compartments. Malachite's solubility in water, albeit limited, allows for the slow release of copper ions into aqueous systems, thereby affecting the bioavailability of this essential micronutrient for various organisms.
The weathering of malachite contributes to the natural copper flux in terrestrial and aquatic ecosystems. As rainwater and other environmental factors interact with exposed malachite deposits, copper is gradually released into soils and water bodies. This process plays a crucial role in maintaining the copper balance in ecosystems, supporting the growth and development of plants and microorganisms that require copper for their metabolic functions.
Furthermore, malachite participates in the carbon cycle through its carbonate component. The formation of malachite serves as a carbon sink, temporarily sequestering atmospheric carbon dioxide in a solid mineral form. Conversely, the dissolution of malachite releases carbonate ions back into the environment, potentially influencing local pH levels and carbonate equilibria in aquatic systems.
In soil environments, malachite can act as a buffer against extreme pH changes due to its basic nature. This buffering capacity can have significant implications for soil chemistry and microbial communities, indirectly affecting nutrient cycling and plant growth. Additionally, the presence of malachite in soils can influence the mobility and bioavailability of other metal ions through adsorption and co-precipitation processes.
The role of malachite in biogeochemical cycles extends to its interactions with microorganisms. Some bacteria and fungi have developed mechanisms to solubilize copper from malachite, incorporating it into their cellular processes or using it as an electron acceptor in anaerobic respiration. These microbial interactions can accelerate the weathering of malachite and influence the local geochemistry of their habitats.
Market Demand Analysis
The market demand for understanding malachite's role in biogeochemical cycles has been steadily increasing in recent years, driven by several key factors. Environmental concerns, particularly related to climate change and pollution, have heightened interest in comprehending the intricate chemical pathways within natural systems. Malachite, as a copper carbonate hydroxide mineral, plays a significant role in these cycles, making it a focal point for research and industrial applications.
In the mining and mineral exploration sector, there is a growing need for advanced knowledge of malachite's behavior in geological processes. This information is crucial for improving extraction techniques and assessing the environmental impact of mining activities. The global copper mining market, valued at over $100 billion annually, is particularly interested in malachite research due to its importance as a copper ore.
The environmental remediation industry has shown increased demand for insights into malachite's role in biogeochemical cycles. As regulations on pollution control and site cleanup become more stringent, understanding how malachite interacts with other elements in soil and water systems is essential for developing effective remediation strategies. This market segment is expected to grow significantly, with projections suggesting a compound annual growth rate of over 7% in the coming years.
Agricultural sectors are also expressing interest in malachite research, particularly in relation to soil health and crop nutrition. As sustainable farming practices gain traction, farmers and agronomists are seeking deeper understanding of mineral interactions in soil ecosystems. This knowledge can lead to improved fertilizer formulations and soil management techniques, addressing a market valued at tens of billions of dollars globally.
In the field of materials science and nanotechnology, there is a burgeoning demand for research on malachite's properties and its potential applications in advanced materials. Industries ranging from electronics to energy storage are exploring how malachite's unique characteristics can be leveraged for innovative products and processes.
The academic and research community represents another significant market for malachite-related studies. Universities, government laboratories, and private research institutions are allocating substantial resources to projects focused on biogeochemical cycles. This demand is reflected in the increasing number of publications and research grants dedicated to this area of study.
Lastly, the growing emphasis on circular economy principles and sustainable resource management is driving interest in understanding mineral cycles, including malachite's role. Industries across various sectors are seeking ways to minimize waste and maximize resource efficiency, creating a demand for comprehensive knowledge of material flows in natural and industrial systems.
In the mining and mineral exploration sector, there is a growing need for advanced knowledge of malachite's behavior in geological processes. This information is crucial for improving extraction techniques and assessing the environmental impact of mining activities. The global copper mining market, valued at over $100 billion annually, is particularly interested in malachite research due to its importance as a copper ore.
The environmental remediation industry has shown increased demand for insights into malachite's role in biogeochemical cycles. As regulations on pollution control and site cleanup become more stringent, understanding how malachite interacts with other elements in soil and water systems is essential for developing effective remediation strategies. This market segment is expected to grow significantly, with projections suggesting a compound annual growth rate of over 7% in the coming years.
Agricultural sectors are also expressing interest in malachite research, particularly in relation to soil health and crop nutrition. As sustainable farming practices gain traction, farmers and agronomists are seeking deeper understanding of mineral interactions in soil ecosystems. This knowledge can lead to improved fertilizer formulations and soil management techniques, addressing a market valued at tens of billions of dollars globally.
In the field of materials science and nanotechnology, there is a burgeoning demand for research on malachite's properties and its potential applications in advanced materials. Industries ranging from electronics to energy storage are exploring how malachite's unique characteristics can be leveraged for innovative products and processes.
The academic and research community represents another significant market for malachite-related studies. Universities, government laboratories, and private research institutions are allocating substantial resources to projects focused on biogeochemical cycles. This demand is reflected in the increasing number of publications and research grants dedicated to this area of study.
Lastly, the growing emphasis on circular economy principles and sustainable resource management is driving interest in understanding mineral cycles, including malachite's role. Industries across various sectors are seeking ways to minimize waste and maximize resource efficiency, creating a demand for comprehensive knowledge of material flows in natural and industrial systems.
Current Research Status
Recent research on malachite's role in biogeochemical cycles has revealed its significant impact on various chemical pathways. Malachite, a copper carbonate hydroxide mineral, has been found to play a crucial role in carbon, copper, and oxygen cycles within terrestrial and aquatic ecosystems.
In the carbon cycle, malachite acts as a carbon sink by sequestering atmospheric CO2 through its formation process. Studies have shown that malachite precipitation can occur in both natural and anthropogenic environments, contributing to carbon fixation. This process has implications for climate change mitigation strategies and carbon capture technologies.
Regarding the copper cycle, malachite serves as an important source and sink for copper in the environment. Research has demonstrated that malachite dissolution can release bioavailable copper into soil and water systems, influencing microbial activity and plant growth. Conversely, malachite formation can immobilize copper, potentially reducing its toxicity in contaminated sites.
The oxygen cycle is also affected by malachite through its involvement in redox reactions. Recent studies have explored malachite's role in oxygen evolution reactions, particularly in the context of water splitting for hydrogen production. This research has implications for renewable energy technologies and artificial photosynthesis.
Investigations into malachite's interaction with microorganisms have revealed its potential as a microbial habitat and electron donor. Some bacteria have been found to utilize malachite as an energy source, highlighting its importance in microbial ecology and biogeochemical processes.
Current research is also focusing on the use of malachite in environmental remediation. Its ability to adsorb heavy metals and organic pollutants has been demonstrated in laboratory and field studies, suggesting potential applications in water and soil treatment technologies.
The role of malachite in phosphorus cycling has gained attention, with studies showing its capacity to adsorb and release phosphate ions. This property has implications for nutrient dynamics in aquatic ecosystems and potential applications in phosphorus recovery from wastewater.
Advances in analytical techniques have enabled more detailed investigations of malachite's structure and reactivity. High-resolution microscopy and spectroscopy methods are providing new insights into the mineral's surface properties and its interactions with various chemical species in the environment.
In the carbon cycle, malachite acts as a carbon sink by sequestering atmospheric CO2 through its formation process. Studies have shown that malachite precipitation can occur in both natural and anthropogenic environments, contributing to carbon fixation. This process has implications for climate change mitigation strategies and carbon capture technologies.
Regarding the copper cycle, malachite serves as an important source and sink for copper in the environment. Research has demonstrated that malachite dissolution can release bioavailable copper into soil and water systems, influencing microbial activity and plant growth. Conversely, malachite formation can immobilize copper, potentially reducing its toxicity in contaminated sites.
The oxygen cycle is also affected by malachite through its involvement in redox reactions. Recent studies have explored malachite's role in oxygen evolution reactions, particularly in the context of water splitting for hydrogen production. This research has implications for renewable energy technologies and artificial photosynthesis.
Investigations into malachite's interaction with microorganisms have revealed its potential as a microbial habitat and electron donor. Some bacteria have been found to utilize malachite as an energy source, highlighting its importance in microbial ecology and biogeochemical processes.
Current research is also focusing on the use of malachite in environmental remediation. Its ability to adsorb heavy metals and organic pollutants has been demonstrated in laboratory and field studies, suggesting potential applications in water and soil treatment technologies.
The role of malachite in phosphorus cycling has gained attention, with studies showing its capacity to adsorb and release phosphate ions. This property has implications for nutrient dynamics in aquatic ecosystems and potential applications in phosphorus recovery from wastewater.
Advances in analytical techniques have enabled more detailed investigations of malachite's structure and reactivity. High-resolution microscopy and spectroscopy methods are providing new insights into the mineral's surface properties and its interactions with various chemical species in the environment.
Analytical Techniques
01 Synthesis of malachite nanoparticles
Chemical pathways for synthesizing malachite nanoparticles involve controlled precipitation methods. These processes typically use copper salts and carbonate sources under specific pH and temperature conditions to form malachite nanostructures with desired morphologies and properties.- Synthesis of malachite nanostructures: Chemical pathways for synthesizing malachite nanostructures, including nanoparticles and nanorods. These methods often involve controlled precipitation reactions and hydrothermal processes, allowing for the manipulation of size, shape, and properties of the resulting malachite structures.
- Malachite in catalytic applications: Exploration of malachite's chemical pathways in catalytic processes, particularly in organic synthesis and environmental remediation. The unique structure and composition of malachite enable it to act as an efficient catalyst or catalyst precursor in various chemical reactions.
- Malachite in electrochemical systems: Investigation of malachite's chemical pathways in electrochemical applications, including its use in electrodes, sensors, and energy storage devices. The material's redox properties and ion exchange capabilities make it suitable for various electrochemical processes.
- Malachite in pigment production: Chemical pathways involved in the production and application of malachite as a pigment. This includes methods for extracting, processing, and stabilizing malachite for use in paints, inks, and other coloring applications, as well as techniques for enhancing its color properties.
- Malachite in environmental remediation: Exploration of malachite's chemical pathways in environmental applications, particularly in the removal of heavy metals and other pollutants from water and soil. The material's adsorption properties and ion exchange capabilities make it effective in various remediation processes.
02 Malachite as a catalyst precursor
Malachite serves as an important precursor in the preparation of copper-based catalysts. Chemical pathways involve the thermal decomposition of malachite to form copper oxides, which are then reduced to active catalytic species. These catalysts find applications in various chemical reactions and industrial processes.Expand Specific Solutions03 Malachite in corrosion inhibition
Chemical pathways for utilizing malachite in corrosion inhibition involve the formation of protective layers on metal surfaces. Malachite can be incorporated into coatings or directly applied to form a barrier against corrosive agents, exploiting its chemical stability and adherence properties.Expand Specific Solutions04 Malachite in environmental remediation
Chemical pathways for using malachite in environmental remediation focus on its ability to adsorb and immobilize heavy metals and other pollutants. The process involves the interaction between malachite's surface functional groups and contaminants, leading to their removal from water or soil.Expand Specific Solutions05 Conversion of malachite to other copper compounds
Chemical pathways for converting malachite to other copper compounds involve various reactions such as acid treatment, thermal decomposition, or redox processes. These transformations can produce copper oxides, hydroxides, or other copper salts with specific properties for different applications.Expand Specific Solutions
Key Research Institutions
The evaluation of malachite's role in chemical pathways of biogeochemical cycles is in an early stage of research, with a growing market potential as environmental concerns increase. The technology is still emerging, with varying levels of maturity across different research institutions and companies. Key players like The Regents of the University of California, Ajinomoto Co., Inc., and the National University of Singapore are leading academic research efforts, while companies such as Metabolon, Inc. and Novo Nordisk A/S are exploring potential commercial applications. The field is characterized by interdisciplinary collaboration between geochemistry, environmental science, and biotechnology sectors, indicating a complex and evolving competitive landscape.
The Regents of the University of California
Technical Solution: The University of California has developed advanced spectroscopic techniques to study malachite's role in biogeochemical cycles. Their research utilizes synchrotron-based X-ray absorption spectroscopy (XAS) and X-ray fluorescence (XRF) imaging to analyze malachite's interactions with organic matter and microorganisms in soil and aquatic environments[1]. This approach allows for in-situ characterization of malachite's chemical transformations and its influence on carbon and copper cycles. The university has also pioneered the use of isotope tracing methods to track malachite-derived copper through various environmental compartments, providing insights into its mobility and bioavailability[2].
Strengths: Access to state-of-the-art analytical facilities and multidisciplinary expertise. Weaknesses: Research may be primarily focused on fundamental science rather than applied solutions.
Kunming University of Science & Technology
Technical Solution: Kunming University has developed a novel approach to studying malachite's role in biogeochemical cycles using advanced geomicrobiological techniques. Their research combines high-throughput sequencing of microbial communities with geochemical analysis to understand how malachite influences microbial ecology and vice versa. They have identified specific bacterial strains capable of accelerating malachite dissolution, potentially affecting copper mobility in the environment[3]. Additionally, the university has developed a unique bioreactor system that simulates various environmental conditions to study malachite's behavior under different pH, redox, and microbial community compositions[4].
Strengths: Strong focus on microbial-mineral interactions and innovative experimental setups. Weaknesses: May have limited resources compared to larger international institutions.
Malachite Reaction Mechanisms
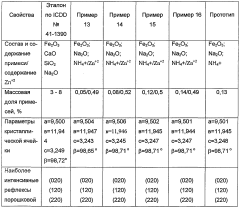
Malachite and method for the production thereof
PatentWO2004076354A1
Innovation
- The process involves evaporating a solution of basic copper carbonate and ammonium carbonate with controlled zinc content, forming polycrystalline malachite with alternating light and dark green layers, and condensing vapor to achieve malachite with enhanced mechanical properties and reduced impurities.
Methotrexate adjuvants to reduce toxicity and methods for using the same
PatentActiveUS7998967B2
Innovation
- Administering an MTX active agent in conjunction with a 2,2′-anhydropyrimidine or uridine phosphorylase inhibitor adjuvant to reduce MTX toxicity, which can be given sequentially, simultaneously, or as a combination, to mitigate mucositis and other toxicities while maintaining the cytotoxic effects of MTX.
Environmental Impact Assessment
The environmental impact assessment of malachite's role in chemical pathways of biogeochemical cycles reveals significant implications for ecosystems and human health. Malachite, a copper carbonate hydroxide mineral, plays a crucial role in the cycling of copper and carbon in natural systems. Its dissolution and precipitation processes influence the availability of copper in soils and water bodies, affecting microbial communities and plant growth.
In aquatic environments, malachite can act as both a source and sink for copper ions. When dissolved, it releases copper, which can be beneficial in trace amounts for aquatic organisms but toxic at higher concentrations. The release of copper from malachite can lead to bioaccumulation in aquatic food chains, potentially impacting fish populations and other higher-level consumers. Conversely, malachite formation can sequester excess copper from water, potentially mitigating toxicity in contaminated environments.
Terrestrial ecosystems are also affected by malachite's presence in soils. The mineral can influence soil pH and copper availability, which in turn affects plant growth and microbial activity. In copper-deficient soils, malachite weathering can provide essential micronutrients for plants. However, in areas with high malachite concentrations, excessive copper release may lead to phytotoxicity and reduced biodiversity.
The carbon dioxide sequestration potential of malachite formation is an important consideration in the context of climate change mitigation. As a carbonate mineral, malachite can act as a long-term carbon sink, potentially contributing to the natural regulation of atmospheric CO2 levels. However, the scale of this effect in natural systems requires further investigation to determine its significance in global carbon cycling.
Human activities, such as mining and industrial processes, can significantly alter the natural cycling of malachite and its components. Increased weathering rates due to excavation and exposure of malachite-bearing rocks can lead to accelerated copper release into the environment. This anthropogenic influence may disrupt local ecosystems and potentially contaminate water resources, necessitating careful management and remediation strategies.
The assessment also highlights the potential for malachite to serve as an indicator of environmental change. Its formation and dissolution patterns can provide insights into local geochemical conditions, including pH, redox potential, and metal ion concentrations. This information can be valuable for monitoring ecosystem health and identifying areas of concern for environmental management.
In aquatic environments, malachite can act as both a source and sink for copper ions. When dissolved, it releases copper, which can be beneficial in trace amounts for aquatic organisms but toxic at higher concentrations. The release of copper from malachite can lead to bioaccumulation in aquatic food chains, potentially impacting fish populations and other higher-level consumers. Conversely, malachite formation can sequester excess copper from water, potentially mitigating toxicity in contaminated environments.
Terrestrial ecosystems are also affected by malachite's presence in soils. The mineral can influence soil pH and copper availability, which in turn affects plant growth and microbial activity. In copper-deficient soils, malachite weathering can provide essential micronutrients for plants. However, in areas with high malachite concentrations, excessive copper release may lead to phytotoxicity and reduced biodiversity.
The carbon dioxide sequestration potential of malachite formation is an important consideration in the context of climate change mitigation. As a carbonate mineral, malachite can act as a long-term carbon sink, potentially contributing to the natural regulation of atmospheric CO2 levels. However, the scale of this effect in natural systems requires further investigation to determine its significance in global carbon cycling.
Human activities, such as mining and industrial processes, can significantly alter the natural cycling of malachite and its components. Increased weathering rates due to excavation and exposure of malachite-bearing rocks can lead to accelerated copper release into the environment. This anthropogenic influence may disrupt local ecosystems and potentially contaminate water resources, necessitating careful management and remediation strategies.
The assessment also highlights the potential for malachite to serve as an indicator of environmental change. Its formation and dissolution patterns can provide insights into local geochemical conditions, including pH, redox potential, and metal ion concentrations. This information can be valuable for monitoring ecosystem health and identifying areas of concern for environmental management.
Geochemical Modeling Approaches
Geochemical modeling approaches play a crucial role in evaluating malachite's involvement in the chemical pathways of biogeochemical cycles. These approaches utilize mathematical representations of complex geochemical systems to simulate and predict the behavior of malachite in various environmental conditions.
One of the primary modeling techniques employed is equilibrium modeling, which focuses on the thermodynamic stability of malachite in different geochemical settings. This approach helps researchers understand the conditions under which malachite forms, dissolves, or transforms into other mineral phases. By incorporating parameters such as pH, temperature, and ion concentrations, equilibrium models can predict the stability fields of malachite and its interactions with other minerals and aqueous species.
Kinetic modeling is another essential approach used to assess the rate-dependent processes involving malachite in biogeochemical cycles. These models consider the time-dependent aspects of malachite formation, dissolution, and transformation. By incorporating reaction rate laws and transport mechanisms, kinetic models provide insights into the temporal evolution of malachite-bearing systems and the factors controlling its reactivity in natural environments.
Reactive transport modeling combines geochemical reactions with fluid flow and mass transport processes. This integrated approach is particularly valuable for evaluating malachite's role in dynamic systems, such as groundwater aquifers or soil profiles. These models can simulate the spatial and temporal distribution of malachite and associated chemical species, accounting for advection, dispersion, and diffusion processes.
Surface complexation modeling is employed to investigate the interactions between malachite surfaces and dissolved species in aqueous environments. This approach is crucial for understanding the adsorption and desorption processes of various elements on malachite surfaces, which can significantly influence the mobility and bioavailability of trace metals in natural systems.
Isotope fractionation modeling is utilized to trace the sources and sinks of elements associated with malachite in biogeochemical cycles. By simulating the isotopic signatures of elements such as copper and carbon during malachite formation and dissolution, researchers can gain insights into the origin and fate of these elements in natural systems.
Advanced computational techniques, including machine learning and artificial intelligence, are increasingly being integrated into geochemical modeling approaches. These methods enhance the predictive capabilities of models and allow for the analysis of large, complex datasets related to malachite's behavior in biogeochemical cycles.
By employing these diverse geochemical modeling approaches, researchers can gain a comprehensive understanding of malachite's role in chemical pathways of biogeochemical cycles, enabling more accurate predictions of element cycling and environmental impacts in various geological settings.
One of the primary modeling techniques employed is equilibrium modeling, which focuses on the thermodynamic stability of malachite in different geochemical settings. This approach helps researchers understand the conditions under which malachite forms, dissolves, or transforms into other mineral phases. By incorporating parameters such as pH, temperature, and ion concentrations, equilibrium models can predict the stability fields of malachite and its interactions with other minerals and aqueous species.
Kinetic modeling is another essential approach used to assess the rate-dependent processes involving malachite in biogeochemical cycles. These models consider the time-dependent aspects of malachite formation, dissolution, and transformation. By incorporating reaction rate laws and transport mechanisms, kinetic models provide insights into the temporal evolution of malachite-bearing systems and the factors controlling its reactivity in natural environments.
Reactive transport modeling combines geochemical reactions with fluid flow and mass transport processes. This integrated approach is particularly valuable for evaluating malachite's role in dynamic systems, such as groundwater aquifers or soil profiles. These models can simulate the spatial and temporal distribution of malachite and associated chemical species, accounting for advection, dispersion, and diffusion processes.
Surface complexation modeling is employed to investigate the interactions between malachite surfaces and dissolved species in aqueous environments. This approach is crucial for understanding the adsorption and desorption processes of various elements on malachite surfaces, which can significantly influence the mobility and bioavailability of trace metals in natural systems.
Isotope fractionation modeling is utilized to trace the sources and sinks of elements associated with malachite in biogeochemical cycles. By simulating the isotopic signatures of elements such as copper and carbon during malachite formation and dissolution, researchers can gain insights into the origin and fate of these elements in natural systems.
Advanced computational techniques, including machine learning and artificial intelligence, are increasingly being integrated into geochemical modeling approaches. These methods enhance the predictive capabilities of models and allow for the analysis of large, complex datasets related to malachite's behavior in biogeochemical cycles.
By employing these diverse geochemical modeling approaches, researchers can gain a comprehensive understanding of malachite's role in chemical pathways of biogeochemical cycles, enabling more accurate predictions of element cycling and environmental impacts in various geological settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!