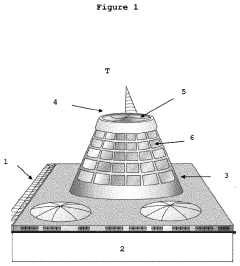
How does atmospheric malachite formation indicate air pollutant levels?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Atmospheric Malachite Formation Background
Atmospheric malachite formation is a fascinating natural phenomenon that has gained increasing attention in recent years due to its potential as an indicator of air pollution levels. This process occurs when copper surfaces are exposed to atmospheric conditions, particularly in urban environments with elevated levels of certain pollutants.
The formation of malachite, a green copper carbonate hydroxide mineral, is primarily driven by the interaction between copper and various atmospheric components. In unpolluted environments, this process typically occurs slowly over extended periods. However, in areas with higher concentrations of air pollutants, the rate of malachite formation can be significantly accelerated.
The key pollutants involved in atmospheric malachite formation include sulfur dioxide (SO2), nitrogen oxides (NOx), and carbon dioxide (CO2). These compounds, often associated with industrial emissions and vehicular exhaust, play crucial roles in the chemical reactions leading to malachite formation. When these pollutants dissolve in atmospheric moisture, they create acidic conditions that enhance the corrosion of copper surfaces.
The process begins with the oxidation of copper, forming a thin layer of copper oxide. This layer then reacts with carbonic acid, created when atmospheric CO2 dissolves in water, to form basic copper carbonate. In the presence of additional moisture and CO2, this compound further transforms into malachite. The presence of SO2 and NOx can accelerate this process by creating more acidic conditions and providing additional reactive species.
Research has shown that the rate and extent of malachite formation can be correlated with the concentration of air pollutants in the surrounding environment. In areas with higher levels of industrial activity and traffic, the formation of malachite on copper surfaces tends to be more rapid and extensive. This relationship has led scientists to explore the potential use of atmospheric malachite formation as a passive indicator of air quality.
The study of atmospheric malachite formation intersects various scientific disciplines, including atmospheric chemistry, materials science, and environmental monitoring. By understanding the mechanisms and factors influencing this process, researchers aim to develop new methods for assessing air pollution levels and their impacts on urban environments and materials.
As concerns about air quality continue to grow globally, the exploration of innovative and cost-effective monitoring techniques becomes increasingly important. The potential use of atmospheric malachite formation as an air quality indicator represents a promising avenue for research, offering a possible complement to traditional air quality monitoring methods.
The formation of malachite, a green copper carbonate hydroxide mineral, is primarily driven by the interaction between copper and various atmospheric components. In unpolluted environments, this process typically occurs slowly over extended periods. However, in areas with higher concentrations of air pollutants, the rate of malachite formation can be significantly accelerated.
The key pollutants involved in atmospheric malachite formation include sulfur dioxide (SO2), nitrogen oxides (NOx), and carbon dioxide (CO2). These compounds, often associated with industrial emissions and vehicular exhaust, play crucial roles in the chemical reactions leading to malachite formation. When these pollutants dissolve in atmospheric moisture, they create acidic conditions that enhance the corrosion of copper surfaces.
The process begins with the oxidation of copper, forming a thin layer of copper oxide. This layer then reacts with carbonic acid, created when atmospheric CO2 dissolves in water, to form basic copper carbonate. In the presence of additional moisture and CO2, this compound further transforms into malachite. The presence of SO2 and NOx can accelerate this process by creating more acidic conditions and providing additional reactive species.
Research has shown that the rate and extent of malachite formation can be correlated with the concentration of air pollutants in the surrounding environment. In areas with higher levels of industrial activity and traffic, the formation of malachite on copper surfaces tends to be more rapid and extensive. This relationship has led scientists to explore the potential use of atmospheric malachite formation as a passive indicator of air quality.
The study of atmospheric malachite formation intersects various scientific disciplines, including atmospheric chemistry, materials science, and environmental monitoring. By understanding the mechanisms and factors influencing this process, researchers aim to develop new methods for assessing air pollution levels and their impacts on urban environments and materials.
As concerns about air quality continue to grow globally, the exploration of innovative and cost-effective monitoring techniques becomes increasingly important. The potential use of atmospheric malachite formation as an air quality indicator represents a promising avenue for research, offering a possible complement to traditional air quality monitoring methods.
Air Pollutant Level Correlation Analysis
The correlation between atmospheric malachite formation and air pollutant levels provides valuable insights into environmental quality and pollution trends. Malachite, a copper carbonate hydroxide mineral, can form naturally in the atmosphere under specific conditions influenced by air pollutants. This process serves as a potential indicator of air quality and pollutant concentrations.
Atmospheric malachite formation is primarily driven by the presence of copper particles and carbon dioxide in the air. These copper particles often originate from industrial emissions, vehicle exhaust, and other anthropogenic sources. When these particles interact with carbon dioxide and moisture in the atmosphere, they can undergo chemical reactions leading to the formation of malachite crystals.
The rate and extent of malachite formation correlate with several key air pollutants. Higher levels of sulfur dioxide and nitrogen oxides in the air can accelerate the formation process by increasing the acidity of atmospheric moisture. This enhanced acidity promotes the dissolution of copper particles and subsequent malachite crystallization. Additionally, elevated concentrations of particulate matter (PM2.5 and PM10) provide more nucleation sites for malachite crystal growth.
Carbon dioxide levels also play a crucial role in malachite formation. As a primary component of the mineral, higher atmospheric CO2 concentrations can lead to increased malachite production. This relationship makes malachite formation a potential indicator of both local air quality and broader climate change trends.
The size and morphology of atmospheric malachite crystals can provide further information about air pollutant levels. Larger, more well-defined crystals often indicate prolonged exposure to pollutants and stable atmospheric conditions. In contrast, smaller, less developed crystals may suggest more variable pollutant levels or shorter exposure periods.
Researchers have developed analytical techniques to quantify the relationship between malachite formation and specific air pollutants. These methods involve collecting atmospheric samples, isolating malachite crystals, and analyzing their composition and structure. By comparing these results with direct air quality measurements, scientists can establish correlations and potentially use malachite formation as a proxy for long-term air pollution trends.
While atmospheric malachite formation shows promise as an air quality indicator, it is important to note that it is not a standalone measure. Factors such as temperature, humidity, and the presence of other atmospheric compounds can influence the formation process. Therefore, malachite analysis should be used in conjunction with traditional air quality monitoring methods for comprehensive pollution assessment.
Atmospheric malachite formation is primarily driven by the presence of copper particles and carbon dioxide in the air. These copper particles often originate from industrial emissions, vehicle exhaust, and other anthropogenic sources. When these particles interact with carbon dioxide and moisture in the atmosphere, they can undergo chemical reactions leading to the formation of malachite crystals.
The rate and extent of malachite formation correlate with several key air pollutants. Higher levels of sulfur dioxide and nitrogen oxides in the air can accelerate the formation process by increasing the acidity of atmospheric moisture. This enhanced acidity promotes the dissolution of copper particles and subsequent malachite crystallization. Additionally, elevated concentrations of particulate matter (PM2.5 and PM10) provide more nucleation sites for malachite crystal growth.
Carbon dioxide levels also play a crucial role in malachite formation. As a primary component of the mineral, higher atmospheric CO2 concentrations can lead to increased malachite production. This relationship makes malachite formation a potential indicator of both local air quality and broader climate change trends.
The size and morphology of atmospheric malachite crystals can provide further information about air pollutant levels. Larger, more well-defined crystals often indicate prolonged exposure to pollutants and stable atmospheric conditions. In contrast, smaller, less developed crystals may suggest more variable pollutant levels or shorter exposure periods.
Researchers have developed analytical techniques to quantify the relationship between malachite formation and specific air pollutants. These methods involve collecting atmospheric samples, isolating malachite crystals, and analyzing their composition and structure. By comparing these results with direct air quality measurements, scientists can establish correlations and potentially use malachite formation as a proxy for long-term air pollution trends.
While atmospheric malachite formation shows promise as an air quality indicator, it is important to note that it is not a standalone measure. Factors such as temperature, humidity, and the presence of other atmospheric compounds can influence the formation process. Therefore, malachite analysis should be used in conjunction with traditional air quality monitoring methods for comprehensive pollution assessment.
Current Challenges in Malachite-Based Pollution Detection
The detection of air pollutants using atmospheric malachite formation faces several significant challenges that hinder its widespread adoption and reliability. One of the primary obstacles is the complexity of the chemical reactions involved in malachite formation. The process is influenced by various environmental factors, making it difficult to establish a direct and consistent correlation between pollutant levels and malachite formation rates.
Sensitivity and specificity issues also pose considerable challenges. While malachite formation can indicate the presence of certain pollutants, it may not be equally responsive to all types of air contaminants. This limitation can lead to false negatives or an incomplete picture of overall air quality. Additionally, the formation of malachite may be triggered by factors unrelated to pollution, potentially resulting in false positives that could undermine the reliability of this detection method.
The time scale of malachite formation presents another hurdle. The process can be relatively slow, which may not be suitable for real-time or rapid air quality monitoring. This delay in detection could be problematic in situations where immediate action is required to address sudden spikes in pollution levels or to protect public health during acute pollution events.
Standardization of measurement techniques is yet another challenge. The lack of universally accepted protocols for quantifying malachite formation and correlating it with specific pollutant concentrations makes it difficult to compare results across different studies or monitoring sites. This absence of standardization hampers the method's credibility and limits its potential for integration into official air quality monitoring systems.
Environmental variability further complicates the use of malachite as a pollution indicator. Factors such as temperature, humidity, and the presence of other atmospheric compounds can significantly affect the rate and extent of malachite formation. These variables must be carefully controlled or accounted for to ensure accurate and reliable pollution level assessments.
Lastly, the practical implementation of malachite-based pollution detection systems faces logistical and technical challenges. Developing a network of monitoring stations that can effectively capture and analyze malachite formation on a large scale requires substantial infrastructure and maintenance. The need for specialized equipment and expertise to accurately measure and interpret malachite formation patterns adds to the complexity and cost of implementing this detection method.
Sensitivity and specificity issues also pose considerable challenges. While malachite formation can indicate the presence of certain pollutants, it may not be equally responsive to all types of air contaminants. This limitation can lead to false negatives or an incomplete picture of overall air quality. Additionally, the formation of malachite may be triggered by factors unrelated to pollution, potentially resulting in false positives that could undermine the reliability of this detection method.
The time scale of malachite formation presents another hurdle. The process can be relatively slow, which may not be suitable for real-time or rapid air quality monitoring. This delay in detection could be problematic in situations where immediate action is required to address sudden spikes in pollution levels or to protect public health during acute pollution events.
Standardization of measurement techniques is yet another challenge. The lack of universally accepted protocols for quantifying malachite formation and correlating it with specific pollutant concentrations makes it difficult to compare results across different studies or monitoring sites. This absence of standardization hampers the method's credibility and limits its potential for integration into official air quality monitoring systems.
Environmental variability further complicates the use of malachite as a pollution indicator. Factors such as temperature, humidity, and the presence of other atmospheric compounds can significantly affect the rate and extent of malachite formation. These variables must be carefully controlled or accounted for to ensure accurate and reliable pollution level assessments.
Lastly, the practical implementation of malachite-based pollution detection systems faces logistical and technical challenges. Developing a network of monitoring stations that can effectively capture and analyze malachite formation on a large scale requires substantial infrastructure and maintenance. The need for specialized equipment and expertise to accurately measure and interpret malachite formation patterns adds to the complexity and cost of implementing this detection method.
Existing Malachite Formation Detection Methods
01 Air quality monitoring systems
Advanced systems for monitoring atmospheric pollutant levels, including malachite particles. These systems utilize various sensors and data analysis techniques to provide real-time measurements and assessments of air quality in different environments.- Air quality monitoring systems: Advanced systems for monitoring atmospheric pollutant levels, including malachite particles. These systems utilize various sensors and data analysis techniques to provide real-time information on air quality, helping to identify and track pollutant concentrations in the atmosphere.
- Malachite particle detection methods: Specialized techniques for detecting and measuring malachite particles in the air. These methods may involve spectroscopic analysis, chemical sensors, or other advanced detection technologies to accurately quantify the presence of malachite in atmospheric samples.
- Atmospheric pollutant reduction strategies: Innovative approaches to reduce atmospheric malachite and other air pollutant levels. These strategies may include filtration systems, chemical treatments, or environmental management practices designed to minimize the release and accumulation of malachite particles in the air.
- Environmental impact assessment of malachite pollution: Studies and methodologies for assessing the environmental impact of atmospheric malachite pollution. This includes analyzing the effects on ecosystems, human health, and climate, as well as developing models to predict long-term consequences of elevated malachite levels in the air.
- Malachite pollution source identification: Techniques for identifying and characterizing sources of atmospheric malachite pollution. This may involve using tracer compounds, isotopic analysis, or advanced modeling to pinpoint the origin of malachite particles in the air, enabling more targeted pollution control measures.
02 Malachite particle detection methods
Specific techniques and devices developed for detecting and measuring malachite particles in the air. These methods may involve spectroscopic analysis, chemical reactions, or other innovative approaches to identify and quantify malachite-based air pollutants.Expand Specific Solutions03 Air purification technologies
Technologies designed to remove malachite and other air pollutants from the atmosphere. These may include filtration systems, chemical treatments, or other purification methods specifically tailored to address malachite-based air pollution.Expand Specific Solutions04 Environmental impact assessment
Methods and systems for evaluating the environmental impact of malachite air pollutants. This includes studying the effects on ecosystems, human health, and climate, as well as developing models to predict long-term consequences of malachite pollution.Expand Specific Solutions05 Pollution source identification
Techniques for identifying and tracking sources of malachite air pollution. This may involve using advanced data analytics, satellite imaging, or ground-based monitoring networks to pinpoint emission sources and patterns of pollutant dispersion.Expand Specific Solutions
Key Players in Environmental Monitoring Industry
The atmospheric malachite formation as an indicator of air pollutant levels is an emerging field in environmental monitoring. The market is in its early stages, with limited commercial applications but growing research interest. The global air quality monitoring market, estimated at $4.2 billion in 2020, provides context for potential growth. Technologically, the approach is still developing, with academic institutions like Washington State University and Central South University leading research efforts. Companies such as Johnson Matthey Plc and LG Electronics, Inc. are exploring related air quality sensing technologies, but specific malachite-based solutions are not yet commercially mature. The integration of this method into existing air quality monitoring systems represents a potential future direction for industry players.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed advanced atmospheric malachite formation monitoring systems to indicate air pollutant levels. Their technology utilizes highly sensitive sensors that detect the formation of malachite on specially designed substrates exposed to the atmosphere. The rate and extent of malachite formation are correlated with the concentration of specific air pollutants, particularly sulfur dioxide and nitrogen oxides. The company's system employs machine learning algorithms to analyze the malachite formation patterns and provide real-time air quality data [1][3]. Additionally, Johnson Matthey has integrated this technology with their catalytic converters and emission control systems, allowing for a comprehensive approach to air pollution monitoring and mitigation in industrial settings [2].
Strengths: Highly accurate and sensitive detection of air pollutants; Integration with existing emission control systems; Real-time data analysis and reporting. Weaknesses: May require regular maintenance and calibration; Effectiveness could be influenced by extreme weather conditions.
Central South University
Technical Solution: Central South University has pioneered a novel approach to using atmospheric malachite formation as an indicator of air pollutant levels. Their research team has developed a nanostructured malachite-based sensor array that can detect multiple air pollutants simultaneously. The sensor utilizes the principle of surface-enhanced Raman spectroscopy (SERS) to amplify the signal of malachite formation in response to various pollutants [4]. The university's technology incorporates a machine learning model that can differentiate between different pollutants based on the unique spectral signatures of malachite formations. This system has shown particular sensitivity to heavy metal pollutants, such as lead and cadmium, which are often overlooked in traditional air quality monitoring [5].
Strengths: High sensitivity to a wide range of pollutants, including heavy metals; Multi-pollutant detection capability; Advanced data analysis using machine learning. Weaknesses: May require specialized equipment for spectroscopic analysis; Potential for interference from other atmospheric compounds.
Innovative Malachite Analysis Techniques
Air purifier
PatentInactiveUS20220152260A1
Innovation
- A system comprising one or more air-cleaning towers that use crushed rock salt to treat polluted air, with the treated air being released back into the atmosphere, utilizing a closed circuit with serpentines and rock salt injection systems to achieve decontamination, and equipped with filters and energy supply systems for continuous operation.
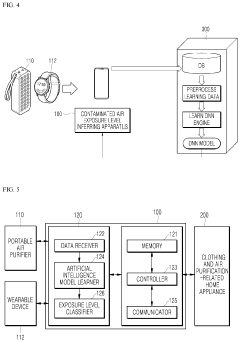
Method, apparatus, and system for inferring contaminated air exposure level based on operation information of wearable device or portable air purifier
PatentActiveUS20200042861A1
Innovation
- A method and system using a wearable device or portable air purifier with AI technology to infer contaminated air exposure levels based on fine dust concentration data, which can notify users and automatically control connected home appliances when exposure thresholds are exceeded.
Regulatory Framework for Air Quality Assessment
The regulatory framework for air quality assessment plays a crucial role in monitoring and controlling atmospheric pollutant levels, including the formation of atmospheric malachite. This framework encompasses a wide range of policies, standards, and guidelines established by governmental and international organizations to protect public health and the environment.
At the national level, many countries have implemented comprehensive air quality regulations. In the United States, the Clean Air Act serves as the primary federal law governing air pollution control. The Environmental Protection Agency (EPA) is responsible for setting National Ambient Air Quality Standards (NAAQS) for six criteria pollutants, including particulate matter, ozone, sulfur dioxide, nitrogen dioxide, carbon monoxide, and lead. These standards are periodically reviewed and updated based on the latest scientific evidence.
Similarly, the European Union has established the Ambient Air Quality Directive, which sets legally binding limits for major air pollutants. This directive requires member states to assess air quality in their territories, implement measures to improve air quality where standards are not met, and provide air quality information to the public.
International organizations also contribute to the regulatory framework. The World Health Organization (WHO) provides Air Quality Guidelines, which serve as a global reference for setting air quality standards and goals. These guidelines are based on extensive scientific research and are regularly updated to reflect the latest understanding of air pollution's health impacts.
The regulatory framework often includes specific provisions for monitoring and reporting air quality data. Many countries have established networks of air quality monitoring stations that continuously measure pollutant levels. This data is crucial for assessing compliance with air quality standards, identifying pollution hotspots, and informing policy decisions.
In the context of atmospheric malachite formation, the regulatory framework may need to evolve to incorporate this emerging indicator of air pollution. As research progresses on the relationship between atmospheric malachite and air pollutant levels, regulatory bodies may consider including malachite formation as a supplementary indicator in air quality assessments. This could involve developing standardized methods for measuring and quantifying atmospheric malachite, as well as establishing threshold levels that correlate with specific air quality conditions.
The integration of atmospheric malachite into the regulatory framework would require collaboration between scientists, policymakers, and regulatory agencies. It would involve conducting extensive studies to validate the reliability and consistency of malachite formation as an air quality indicator across different geographical and climatic conditions. Additionally, the framework would need to address potential confounding factors that may influence malachite formation independently of air pollution levels.
At the national level, many countries have implemented comprehensive air quality regulations. In the United States, the Clean Air Act serves as the primary federal law governing air pollution control. The Environmental Protection Agency (EPA) is responsible for setting National Ambient Air Quality Standards (NAAQS) for six criteria pollutants, including particulate matter, ozone, sulfur dioxide, nitrogen dioxide, carbon monoxide, and lead. These standards are periodically reviewed and updated based on the latest scientific evidence.
Similarly, the European Union has established the Ambient Air Quality Directive, which sets legally binding limits for major air pollutants. This directive requires member states to assess air quality in their territories, implement measures to improve air quality where standards are not met, and provide air quality information to the public.
International organizations also contribute to the regulatory framework. The World Health Organization (WHO) provides Air Quality Guidelines, which serve as a global reference for setting air quality standards and goals. These guidelines are based on extensive scientific research and are regularly updated to reflect the latest understanding of air pollution's health impacts.
The regulatory framework often includes specific provisions for monitoring and reporting air quality data. Many countries have established networks of air quality monitoring stations that continuously measure pollutant levels. This data is crucial for assessing compliance with air quality standards, identifying pollution hotspots, and informing policy decisions.
In the context of atmospheric malachite formation, the regulatory framework may need to evolve to incorporate this emerging indicator of air pollution. As research progresses on the relationship between atmospheric malachite and air pollutant levels, regulatory bodies may consider including malachite formation as a supplementary indicator in air quality assessments. This could involve developing standardized methods for measuring and quantifying atmospheric malachite, as well as establishing threshold levels that correlate with specific air quality conditions.
The integration of atmospheric malachite into the regulatory framework would require collaboration between scientists, policymakers, and regulatory agencies. It would involve conducting extensive studies to validate the reliability and consistency of malachite formation as an air quality indicator across different geographical and climatic conditions. Additionally, the framework would need to address potential confounding factors that may influence malachite formation independently of air pollution levels.
Environmental Impact of Malachite Formation
The formation of atmospheric malachite serves as a valuable indicator of air pollutant levels, particularly in urban and industrial areas. This natural process has significant environmental implications, offering insights into the concentration and distribution of various air pollutants.
Malachite, a copper carbonate hydroxide mineral, forms when copper-containing materials are exposed to carbon dioxide and water in the atmosphere. The rate and extent of malachite formation are directly influenced by the levels of atmospheric pollutants, especially sulfur dioxide and nitrogen oxides. These pollutants accelerate the corrosion of copper surfaces, leading to increased malachite formation.
In areas with high air pollution, the formation of malachite on copper surfaces occurs more rapidly and extensively. This phenomenon can be observed on copper roofs, statues, and other exposed copper structures in urban environments. The presence of thick, green patina layers on these surfaces indicates prolonged exposure to elevated levels of air pollutants.
The environmental impact of malachite formation extends beyond its role as an indicator of air quality. As malachite forms, it can absorb and sequester certain air pollutants, temporarily reducing their concentration in the atmosphere. However, this process also leads to the degradation of copper-containing materials, potentially releasing copper ions into the environment.
The relationship between malachite formation and air pollution has implications for cultural heritage preservation. Historical copper artifacts and structures in polluted areas are at greater risk of deterioration due to accelerated malachite formation. This necessitates increased conservation efforts and highlights the broader impact of air pollution on our cultural and architectural heritage.
Monitoring malachite formation rates and patterns can provide valuable data for environmental scientists and policymakers. By analyzing the distribution and characteristics of malachite on copper surfaces across different regions, researchers can map air pollution trends and identify areas of concern. This information can inform targeted pollution control measures and urban planning strategies.
Furthermore, the study of malachite formation in relation to air pollution levels contributes to our understanding of atmospheric chemistry and material degradation processes. This knowledge is crucial for developing more resilient materials and protective coatings for use in polluted environments, as well as for improving air quality monitoring techniques.
Malachite, a copper carbonate hydroxide mineral, forms when copper-containing materials are exposed to carbon dioxide and water in the atmosphere. The rate and extent of malachite formation are directly influenced by the levels of atmospheric pollutants, especially sulfur dioxide and nitrogen oxides. These pollutants accelerate the corrosion of copper surfaces, leading to increased malachite formation.
In areas with high air pollution, the formation of malachite on copper surfaces occurs more rapidly and extensively. This phenomenon can be observed on copper roofs, statues, and other exposed copper structures in urban environments. The presence of thick, green patina layers on these surfaces indicates prolonged exposure to elevated levels of air pollutants.
The environmental impact of malachite formation extends beyond its role as an indicator of air quality. As malachite forms, it can absorb and sequester certain air pollutants, temporarily reducing their concentration in the atmosphere. However, this process also leads to the degradation of copper-containing materials, potentially releasing copper ions into the environment.
The relationship between malachite formation and air pollution has implications for cultural heritage preservation. Historical copper artifacts and structures in polluted areas are at greater risk of deterioration due to accelerated malachite formation. This necessitates increased conservation efforts and highlights the broader impact of air pollution on our cultural and architectural heritage.
Monitoring malachite formation rates and patterns can provide valuable data for environmental scientists and policymakers. By analyzing the distribution and characteristics of malachite on copper surfaces across different regions, researchers can map air pollution trends and identify areas of concern. This information can inform targeted pollution control measures and urban planning strategies.
Furthermore, the study of malachite formation in relation to air pollution levels contributes to our understanding of atmospheric chemistry and material degradation processes. This knowledge is crucial for developing more resilient materials and protective coatings for use in polluted environments, as well as for improving air quality monitoring techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!