Techniques for quantifying malachite's dissolution rates in acid media
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Dissolution Background and Objectives
Malachite, a copper carbonate hydroxide mineral with the chemical formula Cu2(CO3)(OH)2, has been a subject of significant interest in various fields, including geology, environmental science, and industrial applications. The dissolution of malachite in acidic media is a crucial process that has implications for both natural systems and human activities. Understanding and quantifying the dissolution rates of malachite in acid media is essential for addressing environmental concerns, optimizing industrial processes, and advancing our knowledge of geochemical cycles.
The study of malachite dissolution has evolved over the past decades, with researchers employing increasingly sophisticated techniques to measure and model the process. Early investigations relied on simple batch experiments, while more recent studies have utilized advanced spectroscopic and electrochemical methods. The progression of analytical techniques has allowed for more precise measurements of dissolution rates and a better understanding of the mechanisms involved.
The primary objective of research in this area is to develop accurate and reliable methods for quantifying malachite's dissolution rates in various acidic environments. This goal encompasses several key aspects, including the identification of factors influencing dissolution kinetics, the development of standardized experimental protocols, and the creation of predictive models that can be applied across different acid media and environmental conditions.
One of the main challenges in this field is the complexity of the dissolution process, which is influenced by numerous factors such as pH, temperature, solution composition, and the presence of other minerals or organic compounds. Researchers aim to isolate and quantify the effects of these variables to build comprehensive models of malachite dissolution behavior. Additionally, there is a growing emphasis on understanding the role of surface reactions and the formation of secondary minerals during the dissolution process.
The technological evolution in this area is closely linked to advancements in analytical instrumentation and computational capabilities. Modern research often employs a combination of in-situ monitoring techniques, such as atomic force microscopy (AFM) and X-ray absorption spectroscopy (XAS), alongside traditional chemical analysis methods. These approaches allow for real-time observation of dissolution processes at the molecular level, providing unprecedented insights into reaction mechanisms and kinetics.
As we look to the future, the objectives of malachite dissolution research are expanding to include broader environmental and industrial applications. There is an increasing focus on developing techniques that can be applied in field settings, enabling real-time monitoring of copper release in natural water systems. Furthermore, the integration of machine learning and artificial intelligence in data analysis and modeling is expected to enhance our ability to predict and control malachite dissolution processes across various scenarios.
The study of malachite dissolution has evolved over the past decades, with researchers employing increasingly sophisticated techniques to measure and model the process. Early investigations relied on simple batch experiments, while more recent studies have utilized advanced spectroscopic and electrochemical methods. The progression of analytical techniques has allowed for more precise measurements of dissolution rates and a better understanding of the mechanisms involved.
The primary objective of research in this area is to develop accurate and reliable methods for quantifying malachite's dissolution rates in various acidic environments. This goal encompasses several key aspects, including the identification of factors influencing dissolution kinetics, the development of standardized experimental protocols, and the creation of predictive models that can be applied across different acid media and environmental conditions.
One of the main challenges in this field is the complexity of the dissolution process, which is influenced by numerous factors such as pH, temperature, solution composition, and the presence of other minerals or organic compounds. Researchers aim to isolate and quantify the effects of these variables to build comprehensive models of malachite dissolution behavior. Additionally, there is a growing emphasis on understanding the role of surface reactions and the formation of secondary minerals during the dissolution process.
The technological evolution in this area is closely linked to advancements in analytical instrumentation and computational capabilities. Modern research often employs a combination of in-situ monitoring techniques, such as atomic force microscopy (AFM) and X-ray absorption spectroscopy (XAS), alongside traditional chemical analysis methods. These approaches allow for real-time observation of dissolution processes at the molecular level, providing unprecedented insights into reaction mechanisms and kinetics.
As we look to the future, the objectives of malachite dissolution research are expanding to include broader environmental and industrial applications. There is an increasing focus on developing techniques that can be applied in field settings, enabling real-time monitoring of copper release in natural water systems. Furthermore, the integration of machine learning and artificial intelligence in data analysis and modeling is expected to enhance our ability to predict and control malachite dissolution processes across various scenarios.
Industrial Applications and Market Analysis
The dissolution of malachite in acidic media has significant industrial applications, particularly in the fields of mining, metallurgy, and environmental remediation. The copper mining industry heavily relies on understanding and optimizing malachite dissolution rates to improve extraction efficiency and reduce operational costs. As global demand for copper continues to rise, driven by the growth of renewable energy technologies and electric vehicles, the market for malachite processing is expected to expand substantially.
In the mining sector, acid leaching is a common method for extracting copper from malachite ores. Accurate quantification of dissolution rates enables mining companies to optimize their leaching processes, leading to higher copper recovery rates and reduced environmental impact. This optimization can result in significant cost savings and increased productivity for mining operations worldwide.
The metallurgical industry also benefits from precise malachite dissolution rate data. Hydrometallurgical processes for copper production often involve the dissolution of malachite-containing ores or concentrates. By understanding the kinetics of malachite dissolution, metallurgists can design more efficient and cost-effective extraction circuits, potentially leading to improvements in overall copper production capacity.
Environmental remediation represents another crucial application area for malachite dissolution techniques. Contaminated sites with elevated copper levels, often resulting from historical mining or industrial activities, require effective cleanup strategies. Quantifying malachite dissolution rates in various acidic media aids in developing targeted remediation approaches, potentially reducing treatment times and associated costs.
The market for technologies and services related to malachite dissolution is closely tied to the global copper market. As copper demand is projected to grow steadily in the coming years, driven by infrastructure development and the transition to green technologies, the need for advanced dissolution rate quantification techniques is expected to increase correspondingly.
Furthermore, the push for more sustainable mining practices is likely to drive innovation in this field. Technologies that can accurately measure and control malachite dissolution rates may contribute to reducing the environmental footprint of copper extraction processes, aligning with increasingly stringent regulatory requirements and corporate sustainability goals.
In conclusion, the industrial applications of techniques for quantifying malachite's dissolution rates in acid media span multiple sectors, with significant market potential. As the global focus on resource efficiency and environmental protection intensifies, the demand for advanced dissolution rate quantification methods is expected to grow, offering opportunities for technology providers and research institutions to develop innovative solutions in this space.
In the mining sector, acid leaching is a common method for extracting copper from malachite ores. Accurate quantification of dissolution rates enables mining companies to optimize their leaching processes, leading to higher copper recovery rates and reduced environmental impact. This optimization can result in significant cost savings and increased productivity for mining operations worldwide.
The metallurgical industry also benefits from precise malachite dissolution rate data. Hydrometallurgical processes for copper production often involve the dissolution of malachite-containing ores or concentrates. By understanding the kinetics of malachite dissolution, metallurgists can design more efficient and cost-effective extraction circuits, potentially leading to improvements in overall copper production capacity.
Environmental remediation represents another crucial application area for malachite dissolution techniques. Contaminated sites with elevated copper levels, often resulting from historical mining or industrial activities, require effective cleanup strategies. Quantifying malachite dissolution rates in various acidic media aids in developing targeted remediation approaches, potentially reducing treatment times and associated costs.
The market for technologies and services related to malachite dissolution is closely tied to the global copper market. As copper demand is projected to grow steadily in the coming years, driven by infrastructure development and the transition to green technologies, the need for advanced dissolution rate quantification techniques is expected to increase correspondingly.
Furthermore, the push for more sustainable mining practices is likely to drive innovation in this field. Technologies that can accurately measure and control malachite dissolution rates may contribute to reducing the environmental footprint of copper extraction processes, aligning with increasingly stringent regulatory requirements and corporate sustainability goals.
In conclusion, the industrial applications of techniques for quantifying malachite's dissolution rates in acid media span multiple sectors, with significant market potential. As the global focus on resource efficiency and environmental protection intensifies, the demand for advanced dissolution rate quantification methods is expected to grow, offering opportunities for technology providers and research institutions to develop innovative solutions in this space.
Current Challenges in Quantifying Dissolution Rates
Quantifying malachite's dissolution rates in acid media presents several significant challenges that researchers and industry professionals must address. One of the primary difficulties lies in the complex nature of malachite's dissolution process, which is influenced by multiple factors simultaneously. These factors include pH levels, temperature, pressure, and the presence of other minerals or impurities in the acid media.
The heterogeneous nature of malachite samples further complicates the quantification process. Natural malachite deposits often contain varying compositions and impurities, leading to inconsistencies in dissolution rates even within the same sample. This variability makes it challenging to establish standardized methods for measuring and comparing dissolution rates across different studies or applications.
Another critical challenge is the dynamic nature of the dissolution process itself. As malachite dissolves, the composition of the acid media changes, potentially altering the dissolution rate over time. This continuous evolution of the system requires sophisticated monitoring techniques and real-time data collection to accurately capture the dissolution kinetics throughout the entire process.
The selection of appropriate analytical techniques poses another hurdle in quantifying malachite dissolution rates. Traditional methods such as atomic absorption spectroscopy (AAS) or inductively coupled plasma mass spectrometry (ICP-MS) provide accurate measurements of dissolved copper concentrations but may not capture the full complexity of the dissolution process. Advanced techniques like in-situ X-ray diffraction or synchrotron-based methods offer more comprehensive insights but are often costly and less accessible for routine analyses.
Scale-up issues present additional challenges when translating laboratory findings to industrial applications. Dissolution rates observed in small-scale experiments may not accurately reflect the behavior of malachite in large-scale acid leaching operations. Factors such as mass transfer limitations, fluid dynamics, and heat transfer become increasingly important at industrial scales, necessitating careful consideration and modeling of these effects.
The development of accurate mathematical models to describe malachite dissolution kinetics remains a significant challenge. Existing models often struggle to account for the complex interplay of factors influencing the dissolution process, leading to discrepancies between predicted and observed rates. Improving these models requires extensive experimental data and sophisticated computational approaches to capture the multifaceted nature of malachite dissolution.
Lastly, ensuring the reproducibility and comparability of dissolution rate measurements across different studies and laboratories poses a persistent challenge. Variations in experimental setups, sample preparation methods, and data analysis techniques can lead to inconsistent results, hindering the development of a unified understanding of malachite dissolution behavior in acid media.
The heterogeneous nature of malachite samples further complicates the quantification process. Natural malachite deposits often contain varying compositions and impurities, leading to inconsistencies in dissolution rates even within the same sample. This variability makes it challenging to establish standardized methods for measuring and comparing dissolution rates across different studies or applications.
Another critical challenge is the dynamic nature of the dissolution process itself. As malachite dissolves, the composition of the acid media changes, potentially altering the dissolution rate over time. This continuous evolution of the system requires sophisticated monitoring techniques and real-time data collection to accurately capture the dissolution kinetics throughout the entire process.
The selection of appropriate analytical techniques poses another hurdle in quantifying malachite dissolution rates. Traditional methods such as atomic absorption spectroscopy (AAS) or inductively coupled plasma mass spectrometry (ICP-MS) provide accurate measurements of dissolved copper concentrations but may not capture the full complexity of the dissolution process. Advanced techniques like in-situ X-ray diffraction or synchrotron-based methods offer more comprehensive insights but are often costly and less accessible for routine analyses.
Scale-up issues present additional challenges when translating laboratory findings to industrial applications. Dissolution rates observed in small-scale experiments may not accurately reflect the behavior of malachite in large-scale acid leaching operations. Factors such as mass transfer limitations, fluid dynamics, and heat transfer become increasingly important at industrial scales, necessitating careful consideration and modeling of these effects.
The development of accurate mathematical models to describe malachite dissolution kinetics remains a significant challenge. Existing models often struggle to account for the complex interplay of factors influencing the dissolution process, leading to discrepancies between predicted and observed rates. Improving these models requires extensive experimental data and sophisticated computational approaches to capture the multifaceted nature of malachite dissolution.
Lastly, ensuring the reproducibility and comparability of dissolution rate measurements across different studies and laboratories poses a persistent challenge. Variations in experimental setups, sample preparation methods, and data analysis techniques can lead to inconsistent results, hindering the development of a unified understanding of malachite dissolution behavior in acid media.
Existing Methodologies for Acid Dissolution Quantification
01 Dissolution rate measurement techniques
Various techniques are employed to measure malachite dissolution rates, including spectrophotometric methods, electrochemical analysis, and real-time monitoring systems. These methods allow for accurate determination of dissolution kinetics under different conditions, providing valuable data for geological and industrial applications.- Dissolution rate measurement techniques: Various techniques are employed to measure malachite dissolution rates, including spectrophotometric methods, electrochemical analysis, and real-time monitoring systems. These methods allow for accurate determination of dissolution kinetics under different conditions, providing valuable data for geological and industrial applications.
- Factors affecting malachite dissolution: The dissolution rate of malachite is influenced by several factors, including pH, temperature, particle size, and the presence of other minerals or organic compounds. Understanding these factors is crucial for optimizing dissolution processes in various applications, such as mineral processing and environmental remediation.
- Malachite dissolution in acid environments: Malachite dissolution is particularly significant in acidic environments, such as those found in acid mine drainage or certain industrial processes. The dissolution kinetics and mechanisms in these conditions are studied to develop effective strategies for environmental protection and resource recovery.
- Modeling and simulation of malachite dissolution: Computational models and simulations are developed to predict malachite dissolution rates under various conditions. These tools incorporate thermodynamic and kinetic data to provide insights into dissolution processes, aiding in the design of more efficient extraction and remediation techniques.
- Applications of malachite dissolution studies: Research on malachite dissolution rates has important applications in fields such as geochemistry, mineral processing, and environmental science. The knowledge gained from these studies is used to improve copper extraction methods, develop more effective remediation strategies for contaminated sites, and enhance our understanding of natural weathering processes.
02 Factors affecting malachite dissolution
The dissolution rate of malachite is influenced by several factors, including pH, temperature, particle size, and the presence of other minerals or organic compounds. Understanding these factors is crucial for optimizing dissolution processes in various applications, such as mineral processing and environmental remediation.Expand Specific Solutions03 Malachite dissolution in acid environments
Malachite dissolution is particularly rapid in acidic environments, making it relevant for acid mine drainage studies and mineral processing. Research focuses on the kinetics of dissolution under various acid concentrations and types, as well as the potential for metal recovery from acidic solutions.Expand Specific Solutions04 Modeling and simulation of malachite dissolution
Computational models and simulations are developed to predict malachite dissolution rates under various conditions. These models incorporate factors such as surface area, solution chemistry, and mass transfer, enabling more efficient process design and optimization in industrial applications.Expand Specific Solutions05 Applications of malachite dissolution studies
Understanding malachite dissolution rates has important applications in fields such as geochemistry, environmental science, and materials engineering. This knowledge is applied in areas including copper ore processing, environmental remediation of contaminated sites, and the development of novel materials based on controlled dissolution of malachite.Expand Specific Solutions
Key Players in Mineral Dissolution Research
The competitive landscape for quantifying malachite's dissolution rates in acid media is in an early development stage, with a relatively small market size but growing interest from both academic and industrial sectors. The technology is still evolving, with varying levels of maturity across different research groups and companies. Key players like PetroChina, China Petroleum & Chemical Corp., and Petróleo Brasileiro SA are likely investing in this area due to its relevance to mineral processing and environmental remediation. Academic institutions such as Southwest Petroleum University, Chongqing University, and Nankai University are contributing to fundamental research, while companies like IFP Energies Nouvelles and Oil Plus Ltd. may be developing practical applications for the oil and gas industry.
PetroChina Co., Ltd.
Technical Solution: PetroChina has developed advanced techniques for quantifying malachite's dissolution rates in acid media, crucial for their oil and gas extraction processes. Their method involves using high-precision spectrophotometry combined with in-situ pH monitoring to measure real-time dissolution kinetics[1]. This approach allows for accurate determination of dissolution rates under various acidic conditions, temperatures, and pressures, mimicking reservoir environments. PetroChina's researchers have also implemented machine learning algorithms to predict dissolution behavior based on historical data, enhancing the efficiency of their extraction planning[3].
Strengths: Highly accurate real-time measurements, adaptable to various reservoir conditions, and predictive capabilities. Weaknesses: Potentially high equipment costs and complexity in implementation for field operations.
IFP Energies Nouvelles
Technical Solution: IFP Energies Nouvelles has pioneered a microfluidic approach to quantify malachite dissolution rates in acid media. Their technique utilizes custom-designed microfluidic chips with integrated sensors to monitor dissolution processes at the microscale[2]. This method allows for precise control of fluid flow rates, temperature, and pressure, enabling the study of dissolution kinetics under conditions that closely simulate pore-scale phenomena in reservoir rocks. IFP's researchers have also developed a novel image analysis algorithm that can track the evolution of malachite particle size and morphology during dissolution, providing insights into the mechanisms of acid-induced mineral breakdown[4].
Strengths: High-resolution data at pore scale, minimal sample requirements, and ability to simulate complex reservoir conditions. Weaknesses: Potential scaling issues when extrapolating results to field-scale applications.
Innovative Approaches in Dissolution Rate Analysis
Compositions, apparatus, and methods for determining phosphate content of water
PatentPendingUS20230273164A1
Innovation
- A colorimetric assay using lyophilized compositions in microwell plates with a molybdate salt, buffer, reaction accelerant, and excipients, including (2-hydroxylpropyl)-β-cyclodextrin, which reduces acid requirements, minimizes interference from anions, and accelerates assay development to less than 180 seconds, allowing for quick and accurate phosphate concentration measurement.
The measuring method of malachite green concentration in aqueous solution by adjusting the ph
PatentInactiveKR1020130098679A
Innovation
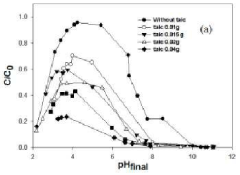
- Adjusting the pH of the malachite green solution with an acetate buffer at pH 4.6 to convert malachite green into a chromatic form for accurate concentration measurement and using talc for removal.
Environmental Impact of Acid Dissolution Processes
The acid dissolution of malachite, a copper carbonate hydroxide mineral, can have significant environmental implications. This process, often employed in mining and mineral processing, releases copper ions and carbonate species into the surrounding ecosystem. The environmental impact is multifaceted, affecting soil, water, and biological systems.
In aquatic environments, the increased acidity resulting from the dissolution process can lead to pH changes in water bodies. This alteration in pH can disrupt aquatic ecosystems, affecting the survival and reproduction of various species. Additionally, the release of copper ions can be toxic to aquatic organisms, even at relatively low concentrations. Fish, invertebrates, and algae are particularly susceptible to copper toxicity, which can cause physiological stress, reduced growth rates, and mortality.
Soil ecosystems are also affected by the acid dissolution of malachite. The increased acidity can alter soil chemistry, potentially leading to the mobilization of other metal ions present in the soil. This can result in the contamination of groundwater and surface water sources. Furthermore, the acidification of soil can negatively impact plant growth and microbial communities, disrupting nutrient cycles and reducing soil fertility.
The dissolution process can contribute to the formation of acid mine drainage (AMD) when occurring in mining environments. AMD is characterized by low pH and high concentrations of dissolved metals, which can persist long after mining activities have ceased. This long-term environmental liability poses challenges for ecosystem restoration and water resource management.
Atmospheric impacts should also be considered. The release of carbonate species during malachite dissolution can contribute to carbon dioxide emissions, albeit on a relatively small scale compared to other industrial processes. However, in large-scale operations, this could become a non-negligible factor in greenhouse gas inventories.
Mitigation strategies are crucial to minimize the environmental impact of malachite dissolution in acid media. These may include containment measures to prevent the spread of acidic solutions, neutralization techniques to adjust pH levels, and water treatment processes to remove dissolved copper and other contaminants. Additionally, the development of more environmentally friendly extraction methods and the implementation of stringent regulatory frameworks are essential for sustainable mineral processing practices.
In aquatic environments, the increased acidity resulting from the dissolution process can lead to pH changes in water bodies. This alteration in pH can disrupt aquatic ecosystems, affecting the survival and reproduction of various species. Additionally, the release of copper ions can be toxic to aquatic organisms, even at relatively low concentrations. Fish, invertebrates, and algae are particularly susceptible to copper toxicity, which can cause physiological stress, reduced growth rates, and mortality.
Soil ecosystems are also affected by the acid dissolution of malachite. The increased acidity can alter soil chemistry, potentially leading to the mobilization of other metal ions present in the soil. This can result in the contamination of groundwater and surface water sources. Furthermore, the acidification of soil can negatively impact plant growth and microbial communities, disrupting nutrient cycles and reducing soil fertility.
The dissolution process can contribute to the formation of acid mine drainage (AMD) when occurring in mining environments. AMD is characterized by low pH and high concentrations of dissolved metals, which can persist long after mining activities have ceased. This long-term environmental liability poses challenges for ecosystem restoration and water resource management.
Atmospheric impacts should also be considered. The release of carbonate species during malachite dissolution can contribute to carbon dioxide emissions, albeit on a relatively small scale compared to other industrial processes. However, in large-scale operations, this could become a non-negligible factor in greenhouse gas inventories.
Mitigation strategies are crucial to minimize the environmental impact of malachite dissolution in acid media. These may include containment measures to prevent the spread of acidic solutions, neutralization techniques to adjust pH levels, and water treatment processes to remove dissolved copper and other contaminants. Additionally, the development of more environmentally friendly extraction methods and the implementation of stringent regulatory frameworks are essential for sustainable mineral processing practices.
Standardization of Dissolution Rate Measurement Techniques
The standardization of dissolution rate measurement techniques for malachite in acid media is crucial for ensuring consistent and comparable results across different studies and applications. This process involves establishing uniform protocols for sample preparation, experimental setup, and data analysis. A key aspect of standardization is the selection of appropriate acid media, which typically includes sulfuric acid, hydrochloric acid, or nitric acid, depending on the specific research objectives and industrial applications.
Standardized sample preparation methods are essential to minimize variability in results. This includes specifying the particle size distribution of malachite samples, surface area measurements, and pre-treatment procedures such as washing and drying. The use of certified reference materials can help calibrate instruments and validate measurement techniques across different laboratories.
Experimental setup standardization encompasses factors such as temperature control, stirring rate, and solid-to-liquid ratio. These parameters significantly influence dissolution kinetics and must be precisely defined and controlled. Additionally, standardized methods for measuring and maintaining pH levels throughout the experiment are critical, as pH changes can dramatically affect dissolution rates.
Data collection and analysis procedures also require standardization. This includes specifying the frequency of sampling, analytical methods for quantifying dissolved copper and carbonate species, and data processing techniques. Advanced spectroscopic methods, such as in-situ ATR-FTIR or Raman spectroscopy, may be incorporated to provide real-time monitoring of dissolution processes.
Standardized reporting formats are equally important to ensure that all relevant experimental details and results are consistently documented. This includes reporting uncertainties in measurements, providing detailed descriptions of experimental conditions, and presenting dissolution rate data in a uniform manner, such as mol/m²/s or mg/cm²/h.
Interlaboratory comparison studies play a vital role in validating and refining standardized techniques. These studies help identify sources of variability between different laboratories and equipment, leading to improvements in measurement protocols. Regular proficiency testing programs can also be implemented to maintain high standards of measurement accuracy and precision across the scientific community.
The development of standardized dissolution rate measurement techniques for malachite in acid media is an ongoing process that requires collaboration between academic institutions, industry partners, and regulatory bodies. As new technologies and analytical methods emerge, these standards must be periodically reviewed and updated to incorporate best practices and ensure their continued relevance and effectiveness in both research and industrial applications.
Standardized sample preparation methods are essential to minimize variability in results. This includes specifying the particle size distribution of malachite samples, surface area measurements, and pre-treatment procedures such as washing and drying. The use of certified reference materials can help calibrate instruments and validate measurement techniques across different laboratories.
Experimental setup standardization encompasses factors such as temperature control, stirring rate, and solid-to-liquid ratio. These parameters significantly influence dissolution kinetics and must be precisely defined and controlled. Additionally, standardized methods for measuring and maintaining pH levels throughout the experiment are critical, as pH changes can dramatically affect dissolution rates.
Data collection and analysis procedures also require standardization. This includes specifying the frequency of sampling, analytical methods for quantifying dissolved copper and carbonate species, and data processing techniques. Advanced spectroscopic methods, such as in-situ ATR-FTIR or Raman spectroscopy, may be incorporated to provide real-time monitoring of dissolution processes.
Standardized reporting formats are equally important to ensure that all relevant experimental details and results are consistently documented. This includes reporting uncertainties in measurements, providing detailed descriptions of experimental conditions, and presenting dissolution rate data in a uniform manner, such as mol/m²/s or mg/cm²/h.
Interlaboratory comparison studies play a vital role in validating and refining standardized techniques. These studies help identify sources of variability between different laboratories and equipment, leading to improvements in measurement protocols. Regular proficiency testing programs can also be implemented to maintain high standards of measurement accuracy and precision across the scientific community.
The development of standardized dissolution rate measurement techniques for malachite in acid media is an ongoing process that requires collaboration between academic institutions, industry partners, and regulatory bodies. As new technologies and analytical methods emerge, these standards must be periodically reviewed and updated to incorporate best practices and ensure their continued relevance and effectiveness in both research and industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!