Malachite's interaction effects with sulfur-rich mineral phases
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite-Sulfur Interaction Background
Malachite, a copper carbonate hydroxide mineral with the chemical formula Cu2CO3(OH)2, has been a subject of interest in mineralogy and geochemistry for decades. Its interaction with sulfur-rich mineral phases has garnered significant attention due to the implications for both natural geological processes and industrial applications. This interaction is particularly relevant in the context of ore deposits, environmental remediation, and mineral processing.
The study of malachite's interaction with sulfur-rich minerals dates back to the early 20th century when researchers began to investigate the formation and alteration of copper deposits. These early studies laid the groundwork for understanding the complex relationships between copper carbonates and sulfides in various geological settings. As analytical techniques advanced, so did the depth of knowledge regarding these interactions.
In natural environments, malachite often forms as a secondary mineral through the weathering of primary copper sulfides such as chalcopyrite (CuFeS2) and bornite (Cu5FeS4). This process involves the oxidation of sulfides and subsequent reaction with carbonate-rich groundwater or atmospheric carbon dioxide. The reverse process, where malachite interacts with sulfur-rich environments to form sulfides, is also of great interest in understanding mineral deposit evolution and geochemical cycling.
The interaction between malachite and sulfur-rich phases is not limited to natural systems. In industrial contexts, particularly in hydrometallurgy and mineral processing, these interactions play a crucial role in copper extraction and recovery processes. The behavior of malachite in the presence of sulfur-bearing reagents or minerals can significantly impact the efficiency of leaching, flotation, and precipitation operations.
Environmental concerns have also driven research into malachite-sulfur interactions. As mine waste and acid mine drainage continue to pose challenges, understanding how malachite behaves in sulfur-rich environments becomes critical for developing effective remediation strategies. This includes studying the potential for malachite to act as a natural buffer against acid generation or its role in the sequestration of harmful elements.
Recent advancements in analytical techniques, such as synchrotron-based X-ray spectroscopy and high-resolution electron microscopy, have enabled researchers to probe these interactions at the molecular level. These tools have revealed intricate details about the surface reactions, dissolution mechanisms, and transformation pathways that occur when malachite encounters sulfur-rich phases.
As we delve deeper into the complexities of malachite-sulfur interactions, it becomes clear that this field of study intersects with various disciplines, including geochemistry, materials science, and environmental engineering. The ongoing research in this area continues to uncover new insights that have far-reaching implications for our understanding of mineral behavior and our ability to manipulate these interactions for technological and environmental benefits.
The study of malachite's interaction with sulfur-rich minerals dates back to the early 20th century when researchers began to investigate the formation and alteration of copper deposits. These early studies laid the groundwork for understanding the complex relationships between copper carbonates and sulfides in various geological settings. As analytical techniques advanced, so did the depth of knowledge regarding these interactions.
In natural environments, malachite often forms as a secondary mineral through the weathering of primary copper sulfides such as chalcopyrite (CuFeS2) and bornite (Cu5FeS4). This process involves the oxidation of sulfides and subsequent reaction with carbonate-rich groundwater or atmospheric carbon dioxide. The reverse process, where malachite interacts with sulfur-rich environments to form sulfides, is also of great interest in understanding mineral deposit evolution and geochemical cycling.
The interaction between malachite and sulfur-rich phases is not limited to natural systems. In industrial contexts, particularly in hydrometallurgy and mineral processing, these interactions play a crucial role in copper extraction and recovery processes. The behavior of malachite in the presence of sulfur-bearing reagents or minerals can significantly impact the efficiency of leaching, flotation, and precipitation operations.
Environmental concerns have also driven research into malachite-sulfur interactions. As mine waste and acid mine drainage continue to pose challenges, understanding how malachite behaves in sulfur-rich environments becomes critical for developing effective remediation strategies. This includes studying the potential for malachite to act as a natural buffer against acid generation or its role in the sequestration of harmful elements.
Recent advancements in analytical techniques, such as synchrotron-based X-ray spectroscopy and high-resolution electron microscopy, have enabled researchers to probe these interactions at the molecular level. These tools have revealed intricate details about the surface reactions, dissolution mechanisms, and transformation pathways that occur when malachite encounters sulfur-rich phases.
As we delve deeper into the complexities of malachite-sulfur interactions, it becomes clear that this field of study intersects with various disciplines, including geochemistry, materials science, and environmental engineering. The ongoing research in this area continues to uncover new insights that have far-reaching implications for our understanding of mineral behavior and our ability to manipulate these interactions for technological and environmental benefits.
Market Demand Analysis
The market demand for understanding malachite's interaction effects with sulfur-rich mineral phases is driven by several key factors in the mining and environmental sectors. As global mineral exploration and extraction activities continue to expand, there is an increasing need to optimize ore processing techniques and manage environmental impacts effectively.
In the mining industry, malachite, a copper carbonate hydroxide mineral, is an important ore of copper. Its interaction with sulfur-rich minerals can significantly affect extraction efficiency and product quality. Mining companies are seeking advanced knowledge in this area to improve their operational processes, reduce costs, and maximize copper recovery rates. This demand is particularly strong in regions with abundant copper deposits, such as Chile, Peru, and the Democratic Republic of Congo.
Environmental concerns also play a crucial role in driving market demand for this research. The interaction between malachite and sulfur-rich minerals can impact acid mine drainage, a significant environmental issue in mining operations. Understanding these interactions is essential for developing more effective strategies to mitigate environmental risks and comply with increasingly stringent regulations worldwide.
The growing focus on sustainable mining practices has further intensified the need for this research. Companies are investing in technologies and methodologies that can minimize environmental impact while maintaining profitability. This trend is expected to continue, with the global mining waste management market projected to grow substantially in the coming years.
In the realm of materials science and nanotechnology, there is emerging interest in leveraging the unique properties of malachite and its interactions with sulfur-rich phases for developing novel materials and applications. This could potentially open up new market opportunities in sectors such as electronics, energy storage, and catalysis.
The academic and research community also contributes to the market demand, as understanding these mineral interactions is crucial for advancing geological and environmental sciences. Universities and research institutions are actively seeking funding and partnerships to conduct studies in this field, creating a market for specialized equipment, analytical services, and software tools.
Geographically, the demand for this research is global but particularly strong in countries with significant mining activities or environmental challenges related to mineral extraction. North America, South America, Australia, and parts of Africa and Asia are key regions driving this market demand.
As the world transitions towards a low-carbon economy, the demand for copper and other minerals critical for renewable energy technologies is expected to rise. This trend will likely sustain and potentially increase the market demand for research into malachite's interactions with sulfur-rich mineral phases in the foreseeable future.
In the mining industry, malachite, a copper carbonate hydroxide mineral, is an important ore of copper. Its interaction with sulfur-rich minerals can significantly affect extraction efficiency and product quality. Mining companies are seeking advanced knowledge in this area to improve their operational processes, reduce costs, and maximize copper recovery rates. This demand is particularly strong in regions with abundant copper deposits, such as Chile, Peru, and the Democratic Republic of Congo.
Environmental concerns also play a crucial role in driving market demand for this research. The interaction between malachite and sulfur-rich minerals can impact acid mine drainage, a significant environmental issue in mining operations. Understanding these interactions is essential for developing more effective strategies to mitigate environmental risks and comply with increasingly stringent regulations worldwide.
The growing focus on sustainable mining practices has further intensified the need for this research. Companies are investing in technologies and methodologies that can minimize environmental impact while maintaining profitability. This trend is expected to continue, with the global mining waste management market projected to grow substantially in the coming years.
In the realm of materials science and nanotechnology, there is emerging interest in leveraging the unique properties of malachite and its interactions with sulfur-rich phases for developing novel materials and applications. This could potentially open up new market opportunities in sectors such as electronics, energy storage, and catalysis.
The academic and research community also contributes to the market demand, as understanding these mineral interactions is crucial for advancing geological and environmental sciences. Universities and research institutions are actively seeking funding and partnerships to conduct studies in this field, creating a market for specialized equipment, analytical services, and software tools.
Geographically, the demand for this research is global but particularly strong in countries with significant mining activities or environmental challenges related to mineral extraction. North America, South America, Australia, and parts of Africa and Asia are key regions driving this market demand.
As the world transitions towards a low-carbon economy, the demand for copper and other minerals critical for renewable energy technologies is expected to rise. This trend will likely sustain and potentially increase the market demand for research into malachite's interactions with sulfur-rich mineral phases in the foreseeable future.
Current Challenges
The interaction between malachite and sulfur-rich mineral phases presents several significant challenges in both geological and industrial contexts. One of the primary difficulties lies in the complex chemical reactions that occur when these materials come into contact. Malachite, a copper carbonate hydroxide mineral, can undergo rapid transformation when exposed to sulfur-rich environments, leading to the formation of secondary minerals and potentially altering the physical and chemical properties of the surrounding rock or ore.
A major challenge in studying these interactions is the variability of sulfur-rich mineral phases, which can include pyrite, marcasite, and various sulfates. Each of these sulfur-bearing minerals reacts differently with malachite, creating a multifaceted system that is difficult to predict and control. This variability complicates efforts to model and simulate these interactions accurately, hindering our ability to forecast the long-term stability of malachite in sulfur-rich geological formations.
In industrial applications, particularly in copper mining and processing, the presence of sulfur-rich minerals can significantly impact the efficiency of malachite extraction. The formation of copper sulfides or other sulfur-containing compounds can reduce the recovery rates of copper from malachite ores, leading to economic losses and increased processing costs. Additionally, the generation of acid mine drainage due to the oxidation of sulfide minerals in the presence of water and oxygen poses severe environmental risks, further complicated by the interaction with malachite and other carbonate minerals.
The kinetics of these interactions present another substantial challenge. The rate at which malachite reacts with sulfur-rich phases can vary greatly depending on environmental conditions such as temperature, pressure, and the presence of catalytic agents. This variability makes it difficult to develop standardized protocols for managing these interactions in both natural and industrial settings.
Furthermore, the microscale and nanoscale processes involved in these interactions are not fully understood. The surface chemistry at the interface between malachite and sulfur-rich minerals plays a crucial role in determining the nature and extent of the reactions. However, studying these processes at such small scales requires advanced analytical techniques and sophisticated experimental setups, which are not always readily available or easily implemented.
Lastly, the environmental implications of these interactions pose significant challenges for remediation efforts in contaminated sites. The potential for malachite to act as a sink or source of heavy metals when interacting with sulfur-rich phases complicates the development of effective strategies for environmental cleanup and long-term site management.
A major challenge in studying these interactions is the variability of sulfur-rich mineral phases, which can include pyrite, marcasite, and various sulfates. Each of these sulfur-bearing minerals reacts differently with malachite, creating a multifaceted system that is difficult to predict and control. This variability complicates efforts to model and simulate these interactions accurately, hindering our ability to forecast the long-term stability of malachite in sulfur-rich geological formations.
In industrial applications, particularly in copper mining and processing, the presence of sulfur-rich minerals can significantly impact the efficiency of malachite extraction. The formation of copper sulfides or other sulfur-containing compounds can reduce the recovery rates of copper from malachite ores, leading to economic losses and increased processing costs. Additionally, the generation of acid mine drainage due to the oxidation of sulfide minerals in the presence of water and oxygen poses severe environmental risks, further complicated by the interaction with malachite and other carbonate minerals.
The kinetics of these interactions present another substantial challenge. The rate at which malachite reacts with sulfur-rich phases can vary greatly depending on environmental conditions such as temperature, pressure, and the presence of catalytic agents. This variability makes it difficult to develop standardized protocols for managing these interactions in both natural and industrial settings.
Furthermore, the microscale and nanoscale processes involved in these interactions are not fully understood. The surface chemistry at the interface between malachite and sulfur-rich minerals plays a crucial role in determining the nature and extent of the reactions. However, studying these processes at such small scales requires advanced analytical techniques and sophisticated experimental setups, which are not always readily available or easily implemented.
Lastly, the environmental implications of these interactions pose significant challenges for remediation efforts in contaminated sites. The potential for malachite to act as a sink or source of heavy metals when interacting with sulfur-rich phases complicates the development of effective strategies for environmental cleanup and long-term site management.
Existing Research Methods
01 Malachite in catalytic reactions
Malachite exhibits catalytic properties in various chemical reactions. It can be used as a catalyst or catalyst support in processes such as oxidation, reduction, and organic synthesis. The unique structure and composition of malachite contribute to its catalytic activity, making it valuable in industrial applications and research.- Malachite in catalytic reactions: Malachite exhibits catalytic properties in various chemical reactions. It can be used as a catalyst or catalyst support in processes such as oxidation, reduction, and organic synthesis. The unique structure and composition of malachite contribute to its catalytic activity, making it valuable in industrial applications and research.
- Malachite in environmental remediation: Malachite demonstrates effectiveness in environmental remediation processes. It can be used for the adsorption and removal of pollutants, heavy metals, and organic contaminants from water and soil. The interaction between malachite and various pollutants is exploited in developing eco-friendly treatment methods for contaminated environments.
- Malachite in material science: Malachite has applications in material science due to its unique properties. It can be used in the synthesis of novel materials, nanocomposites, and functional coatings. The interaction of malachite with other substances leads to the development of materials with enhanced mechanical, thermal, or electrical properties.
- Malachite in biological systems: Malachite exhibits various interactions in biological systems. It can be used in biomedical applications, such as antimicrobial agents, drug delivery systems, or biosensors. The interaction of malachite with biological molecules and cellular components is studied for potential therapeutic and diagnostic purposes.
- Malachite in analytical chemistry: Malachite plays a role in analytical chemistry applications. It can be used as a colorimetric indicator, in spectrophotometric methods, or as part of sensor systems. The interaction of malachite with specific analytes or reagents enables the development of sensitive and selective analytical techniques for various substances.
02 Malachite in environmental remediation
Malachite demonstrates effectiveness in environmental remediation processes, particularly in the removal of heavy metals and organic pollutants from water and soil. Its adsorption properties and ion exchange capabilities make it suitable for wastewater treatment and soil decontamination applications.Expand Specific Solutions03 Malachite in antimicrobial applications
Malachite exhibits antimicrobial properties, making it useful in various applications such as medical devices, textiles, and coatings. Its ability to inhibit the growth of bacteria and fungi has led to its incorporation in products aimed at preventing infections and promoting hygiene.Expand Specific Solutions04 Malachite in electronic and optical devices
The unique optical and electronic properties of malachite make it suitable for use in various devices. It can be incorporated into sensors, displays, and other electronic components to enhance their performance or provide specific functionalities. The interaction of malachite with light and electrical signals is exploited in these applications.Expand Specific Solutions05 Malachite in composite materials
Malachite can be incorporated into composite materials to enhance their properties or impart new functionalities. These composites find applications in areas such as construction, aerospace, and automotive industries. The interaction between malachite and the matrix material can lead to improved mechanical, thermal, or electrical properties of the resulting composite.Expand Specific Solutions
Key Industry Players
The interaction effects of malachite with sulfur-rich mineral phases represent a complex technological challenge in the mining and materials science sectors. This field is currently in a growth phase, with increasing market demand driven by the need for more efficient mineral processing and environmental remediation techniques. The global market for related technologies is expanding, estimated to reach several billion dollars by 2025. Technologically, the area is moderately mature, with ongoing research to improve understanding and applications. Key players like Freeport-McMoRan, Inc. and Merichem Co. are investing in R&D to develop advanced solutions, while academic institutions such as Kunming University of Science & Technology and Central South University are contributing fundamental research to advance the field.
Freeport-McMoRan, Inc.
Technical Solution: Freeport-McMoRan has developed advanced techniques for managing malachite interactions with sulfur-rich mineral phases in copper mining operations. Their approach involves selective flotation and leaching processes that minimize the formation of copper sulfides. The company utilizes a proprietary pre-treatment method to passivate reactive sulfide surfaces, reducing their interaction with malachite during mineral processing[1]. This is combined with carefully controlled pH levels and the addition of specific reagents to optimize copper recovery while mitigating the negative effects of sulfur-rich minerals[3].
Strengths: Extensive practical experience in large-scale copper mining operations; proprietary technologies for mineral separation. Weaknesses: May be limited to applications in copper mining; potential environmental concerns related to chemical usage.
Central South University
Technical Solution: Central South University has conducted extensive research on the interaction between malachite and sulfur-rich mineral phases. Their approach focuses on the molecular-level understanding of surface interactions and the development of novel separation techniques. The university has pioneered the use of advanced spectroscopic methods, including in-situ ATR-FTIR and XPS, to study the adsorption mechanisms of malachite on pyrite surfaces under various conditions[2]. They have also developed a selective flotation process using modified collectors that can effectively separate malachite from sulfide minerals, even in complex ore systems[4].
Strengths: Strong fundamental research capabilities; innovative analytical techniques. Weaknesses: May require further development for industrial-scale applications; limited field testing compared to industry players.
Core Innovations
Compositions and method for treating out hydrogen sulfide and preventing settling of precipitate in an environmentally responsible drilling and packer fluid
PatentActiveUS20170152431A1
Innovation
- The use of iron chelating agents such as ferrous lactate, ferrous gluconate, and other ferrous compounds, which form stable complexes with hydrogen sulfide at high pH, preventing precipitation of iron hydroxide and maintaining fluid stability, along with a viscosifier like sepiolite to prevent settling of the precipitate.
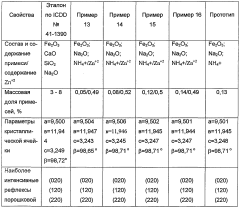
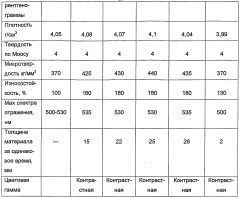
Malachite and method for the production thereof
PatentWO2004076354A1
Innovation
- The process involves evaporating a solution of basic copper carbonate and ammonium carbonate with controlled zinc content, forming polycrystalline malachite with alternating light and dark green layers, and condensing vapor to achieve malachite with enhanced mechanical properties and reduced impurities.
Environmental Impact Assessment
The environmental impact assessment of malachite's interaction effects with sulfur-rich mineral phases is crucial for understanding the potential consequences on ecosystems and human health. Malachite, a copper carbonate hydroxide mineral, can undergo significant chemical transformations when exposed to sulfur-rich environments, leading to various environmental concerns.
One of the primary environmental impacts is the potential release of copper ions into surrounding water bodies. As malachite interacts with sulfur-rich minerals, it may dissolve and release copper, which can be toxic to aquatic organisms at elevated concentrations. This can lead to disruptions in aquatic ecosystems, affecting fish populations, algae growth, and overall water quality. The extent of copper release depends on factors such as pH, temperature, and the presence of other minerals.
Soil contamination is another significant concern arising from these interactions. As malachite breaks down in the presence of sulfur-rich minerals, it can increase the copper content in soils. This may result in phytotoxicity, inhibiting plant growth and reducing agricultural productivity in affected areas. Additionally, the altered soil chemistry can impact microbial communities, potentially disrupting essential nutrient cycles and soil fertility.
The formation of secondary minerals during these interactions can also have environmental implications. For instance, the reaction between malachite and sulfur-rich minerals may lead to the formation of copper sulfides or other sulfate minerals. These secondary minerals can alter the physical and chemical properties of the soil, affecting water retention, nutrient availability, and overall soil structure.
Atmospheric impacts should also be considered, particularly in mining or industrial areas where malachite and sulfur-rich minerals are present in large quantities. The interaction between these minerals can potentially release sulfur dioxide or other sulfur-containing gases, contributing to air pollution and acid rain formation. This can have far-reaching effects on both terrestrial and aquatic ecosystems beyond the immediate area of interaction.
The mobilization of trace elements is another environmental concern. As malachite interacts with sulfur-rich minerals, it may facilitate the release of other potentially harmful elements that are often associated with copper deposits, such as arsenic, lead, or cadmium. These trace elements can accumulate in soils and water bodies, posing risks to wildlife and human health through bioaccumulation in the food chain.
Long-term environmental monitoring is essential to fully assess the impact of these mineral interactions. This includes regular testing of water quality, soil composition, and air quality in affected areas. Additionally, biomonitoring of plant and animal species can provide valuable insights into the ecological effects of these mineral interactions over time.
One of the primary environmental impacts is the potential release of copper ions into surrounding water bodies. As malachite interacts with sulfur-rich minerals, it may dissolve and release copper, which can be toxic to aquatic organisms at elevated concentrations. This can lead to disruptions in aquatic ecosystems, affecting fish populations, algae growth, and overall water quality. The extent of copper release depends on factors such as pH, temperature, and the presence of other minerals.
Soil contamination is another significant concern arising from these interactions. As malachite breaks down in the presence of sulfur-rich minerals, it can increase the copper content in soils. This may result in phytotoxicity, inhibiting plant growth and reducing agricultural productivity in affected areas. Additionally, the altered soil chemistry can impact microbial communities, potentially disrupting essential nutrient cycles and soil fertility.
The formation of secondary minerals during these interactions can also have environmental implications. For instance, the reaction between malachite and sulfur-rich minerals may lead to the formation of copper sulfides or other sulfate minerals. These secondary minerals can alter the physical and chemical properties of the soil, affecting water retention, nutrient availability, and overall soil structure.
Atmospheric impacts should also be considered, particularly in mining or industrial areas where malachite and sulfur-rich minerals are present in large quantities. The interaction between these minerals can potentially release sulfur dioxide or other sulfur-containing gases, contributing to air pollution and acid rain formation. This can have far-reaching effects on both terrestrial and aquatic ecosystems beyond the immediate area of interaction.
The mobilization of trace elements is another environmental concern. As malachite interacts with sulfur-rich minerals, it may facilitate the release of other potentially harmful elements that are often associated with copper deposits, such as arsenic, lead, or cadmium. These trace elements can accumulate in soils and water bodies, posing risks to wildlife and human health through bioaccumulation in the food chain.
Long-term environmental monitoring is essential to fully assess the impact of these mineral interactions. This includes regular testing of water quality, soil composition, and air quality in affected areas. Additionally, biomonitoring of plant and animal species can provide valuable insights into the ecological effects of these mineral interactions over time.
Geochemical Modeling Approaches
Geochemical modeling approaches play a crucial role in understanding the complex interactions between malachite and sulfur-rich mineral phases. These approaches utilize sophisticated computational techniques to simulate and predict the behavior of chemical species in various geological environments.
One of the primary modeling approaches employed in this context is thermodynamic equilibrium modeling. This method relies on extensive databases of thermodynamic properties for minerals and aqueous species to calculate the equilibrium state of a system. Software packages such as PHREEQC and Geochemist's Workbench are commonly used to perform these calculations, allowing researchers to predict the stability of malachite in the presence of sulfur-rich minerals under different environmental conditions.
Kinetic modeling is another essential approach that complements equilibrium modeling. While equilibrium models provide insights into the final state of a system, kinetic models focus on the rates of reactions and the evolution of the system over time. This is particularly important when studying the interaction of malachite with sulfur-rich minerals, as these processes often involve complex dissolution-precipitation reactions that may not reach equilibrium on observable timescales.
Reactive transport modeling combines geochemical reactions with fluid flow and mass transport processes. This approach is invaluable for understanding how malachite interacts with sulfur-rich minerals in dynamic geological systems, such as groundwater aquifers or ore deposits. Software packages like TOUGHREACT and OpenGeoSys are frequently used for these simulations, enabling researchers to predict the spatial and temporal evolution of mineral assemblages and fluid compositions.
Surface complexation modeling is another critical tool in the geochemist's arsenal. This approach focuses on the adsorption and desorption processes occurring at mineral-water interfaces, which are particularly relevant when studying the interaction of malachite with sulfur-bearing minerals. By incorporating surface complexation models into larger geochemical simulations, researchers can better account for the role of mineral surfaces in controlling the mobility and speciation of copper and sulfur in natural systems.
Molecular dynamics simulations and ab initio calculations represent the cutting edge of geochemical modeling approaches. These techniques operate at the atomic and molecular scales, providing insights into the fundamental mechanisms governing malachite-sulfur mineral interactions. While computationally intensive, these methods offer unparalleled detail and can inform the development of more accurate macroscale models.
One of the primary modeling approaches employed in this context is thermodynamic equilibrium modeling. This method relies on extensive databases of thermodynamic properties for minerals and aqueous species to calculate the equilibrium state of a system. Software packages such as PHREEQC and Geochemist's Workbench are commonly used to perform these calculations, allowing researchers to predict the stability of malachite in the presence of sulfur-rich minerals under different environmental conditions.
Kinetic modeling is another essential approach that complements equilibrium modeling. While equilibrium models provide insights into the final state of a system, kinetic models focus on the rates of reactions and the evolution of the system over time. This is particularly important when studying the interaction of malachite with sulfur-rich minerals, as these processes often involve complex dissolution-precipitation reactions that may not reach equilibrium on observable timescales.
Reactive transport modeling combines geochemical reactions with fluid flow and mass transport processes. This approach is invaluable for understanding how malachite interacts with sulfur-rich minerals in dynamic geological systems, such as groundwater aquifers or ore deposits. Software packages like TOUGHREACT and OpenGeoSys are frequently used for these simulations, enabling researchers to predict the spatial and temporal evolution of mineral assemblages and fluid compositions.
Surface complexation modeling is another critical tool in the geochemist's arsenal. This approach focuses on the adsorption and desorption processes occurring at mineral-water interfaces, which are particularly relevant when studying the interaction of malachite with sulfur-bearing minerals. By incorporating surface complexation models into larger geochemical simulations, researchers can better account for the role of mineral surfaces in controlling the mobility and speciation of copper and sulfur in natural systems.
Molecular dynamics simulations and ab initio calculations represent the cutting edge of geochemical modeling approaches. These techniques operate at the atomic and molecular scales, providing insights into the fundamental mechanisms governing malachite-sulfur mineral interactions. While computationally intensive, these methods offer unparalleled detail and can inform the development of more accurate macroscale models.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!