Enovix Batteries in Medical Devices Meeting Reliable and Safe Power Requirements
Enovix Battery Tech Evolution and Objectives
Enovix Corporation has emerged as a pioneering force in the development of advanced lithium-ion battery technology, with a particular focus on meeting the stringent requirements of medical devices. The evolution of Enovix battery technology represents a significant leap forward in addressing the critical needs of reliable and safe power sources for medical applications.
The journey of Enovix battery technology began with the recognition of the limitations inherent in traditional lithium-ion battery designs. Conventional batteries often struggled to provide the necessary energy density, longevity, and safety features required for medical devices. Enovix set out to revolutionize this landscape by introducing a novel 3D silicon anode architecture, which marked a departure from the planar electrode designs of conventional batteries.
This innovative approach allowed for a substantial increase in energy density while maintaining a compact form factor, a crucial consideration for medical devices where space is often at a premium. The 3D silicon anode technology enabled Enovix to pack more active material into a given volume, resulting in batteries that could store more energy without increasing in size.
As the technology progressed, Enovix focused on enhancing the safety features of their batteries, a paramount concern for medical device applications. They developed advanced separator technologies and implemented robust thermal management systems to mitigate the risks associated with thermal runaway and other potential failure modes.
Concurrent with these developments, Enovix worked on improving the cycling performance and longevity of their batteries. Medical devices often require power sources that can maintain consistent performance over extended periods, sometimes spanning several years. Enovix's research efforts were directed towards optimizing the electrode-electrolyte interface and developing novel electrolyte formulations to enhance the battery's cycle life and capacity retention.
The objectives of Enovix's battery technology evolution have been multifaceted. Primarily, they aim to provide medical device manufacturers with power solutions that offer higher energy density, improved safety, and extended operational lifetimes. These advancements are crucial for enabling the next generation of implantable medical devices, wearable health monitors, and portable diagnostic equipment.
Furthermore, Enovix has set ambitious goals for scalability and manufacturability. The company recognizes that to make a significant impact in the medical device sector, their battery technology must be amenable to large-scale production while maintaining consistent quality and performance. This has led to ongoing efforts to refine their manufacturing processes and develop automated production lines capable of high-volume output.
Looking ahead, Enovix continues to push the boundaries of battery technology for medical applications. Their research and development roadmap includes further improvements in energy density, the exploration of new materials for even safer operation, and the integration of smart features such as built-in diagnostics and predictive maintenance capabilities. These advancements aim to not only meet but exceed the evolving power requirements of future medical devices, potentially enabling new therapeutic and diagnostic modalities that were previously constrained by power limitations.
Medical Device Power Demand Analysis
The medical device industry is experiencing a significant surge in power demand, driven by the increasing complexity and functionality of modern medical devices. This trend is particularly evident in portable and implantable devices, where the need for reliable, long-lasting, and safe power sources is paramount. The power requirements for medical devices vary widely depending on their function, size, and intended use.
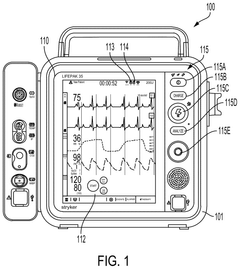
Portable medical devices, such as insulin pumps, continuous glucose monitors, and wearable ECG monitors, require compact power sources that can provide consistent energy over extended periods. These devices typically demand power in the range of microwatts to milliwatts, with a focus on energy density and longevity. The challenge lies in balancing the need for miniaturization with the requirement for sufficient power capacity to ensure uninterrupted operation between charging cycles.
Implantable medical devices, including pacemakers, neurostimulators, and drug delivery systems, present even more stringent power demands. These devices require ultra-low power consumption, often in the range of microwatts, coupled with an exceptionally long lifespan measured in years or even decades. The power source for these devices must be biocompatible, resistant to body fluids, and capable of maintaining stable performance under varying physiological conditions.
High-power medical equipment, such as surgical robots, imaging systems, and life support machines, have substantially different power requirements. These devices often need continuous, high-wattage power supplies, ranging from hundreds of watts to several kilowatts. Reliability and uninterrupted power delivery are critical for these applications, often necessitating backup power systems and advanced power management solutions.
The trend towards telemedicine and remote patient monitoring is introducing new power challenges. These devices must balance the need for continuous connectivity and data transmission with extended battery life. Power consumption in these devices is often dynamic, with periods of low-power standby interspersed with bursts of high-power data transmission.
As medical devices become more sophisticated, incorporating features like artificial intelligence, real-time data processing, and advanced sensors, their power demands are evolving. This evolution is driving the need for more efficient power management systems, advanced battery technologies, and innovative energy harvesting solutions. The industry is seeing a growing interest in technologies that can provide higher energy densities, faster charging capabilities, and improved safety profiles.
The regulatory landscape also plays a crucial role in shaping power requirements for medical devices. Stringent safety standards and the need for extensive testing and validation processes influence the design and selection of power sources. Manufacturers must ensure that their power solutions not only meet the functional requirements of the devices but also comply with regulatory standards for safety, reliability, and performance.
Enovix Battery Tech Challenges in Medical Applications
The integration of Enovix batteries into medical devices presents significant technical challenges that must be addressed to meet the stringent reliability and safety requirements of the healthcare industry. One of the primary hurdles is achieving the necessary energy density while maintaining a compact form factor suitable for implantable and wearable medical devices. Enovix's 3D silicon lithium-ion technology offers promising advancements in this area, but optimizing the battery architecture for medical-grade performance requires extensive research and development.
Safety considerations are paramount in medical applications, necessitating robust protection against thermal runaway, short circuits, and electrolyte leakage. Enovix must demonstrate that their battery design can withstand the unique stresses of in-vivo environments, including constant temperature fluctuations and potential exposure to bodily fluids. The challenge extends to ensuring long-term stability and predictable degradation patterns, as unexpected power failures in medical devices can have life-threatening consequences.
Biocompatibility is another critical factor that poses technical difficulties. The materials used in Enovix batteries must be thoroughly tested and potentially modified to ensure they do not elicit adverse biological responses when in close proximity to or in direct contact with human tissue. This may require the development of specialized encapsulation techniques or the exploration of alternative electrolyte compositions that are both high-performing and biologically inert.
The demand for extended battery life in medical devices adds another layer of complexity. Enovix must push the boundaries of their technology to achieve cycle lives that can support years of continuous operation without replacement. This challenge is compounded by the need to maintain consistent power output throughout the battery's lifespan, as medical devices often require precise and stable energy delivery for accurate diagnostics and therapies.
Lastly, the integration of smart battery management systems (BMS) tailored for medical applications presents a significant technical hurdle. These systems must be capable of real-time monitoring, predictive analytics, and fail-safe protocols specific to medical use cases. Developing a BMS that can interface seamlessly with various medical device platforms while adhering to regulatory standards for medical software is a complex undertaking that requires interdisciplinary expertise.
Current Enovix Battery Solutions for Medical Devices
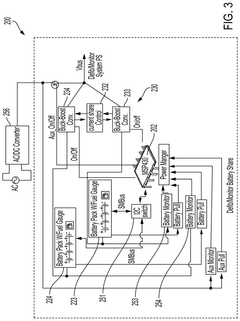
01 Battery Management System for Safety and Reliability
Advanced battery management systems are implemented to monitor and control various parameters such as temperature, voltage, and current. These systems enhance the safety and reliability of Enovix batteries by preventing overcharging, over-discharging, and thermal runaway. They also optimize battery performance and extend lifespan through intelligent charge and discharge management.- Battery Management System for Safety and Reliability: Advanced battery management systems are implemented to monitor and control various parameters such as temperature, voltage, and current. These systems enhance the safety and reliability of Enovix batteries by preventing overcharging, over-discharging, and thermal runaway. They also optimize battery performance and extend lifespan through intelligent charge and discharge management.
- Innovative Cell Design for Improved Safety: Enovix batteries utilize a unique cell design that incorporates advanced materials and structural elements to enhance safety. This design includes features such as improved thermal management, reinforced separators, and optimized electrode configurations. These innovations help prevent internal short circuits and contain potential failures, significantly improving the overall safety profile of the batteries.
- Enhanced Thermal Management Techniques: Specialized thermal management techniques are employed in Enovix batteries to maintain optimal operating temperatures and prevent thermal runaway. These may include advanced cooling systems, heat-dissipating materials, and thermal isolation strategies. Effective thermal management is crucial for ensuring the reliability and safety of the batteries under various operating conditions.
- Quality Control and Testing Protocols: Rigorous quality control measures and comprehensive testing protocols are implemented throughout the manufacturing process of Enovix batteries. These include automated inspection systems, accelerated life testing, and simulated abuse conditions. Such stringent quality assurance practices help identify potential defects and ensure the production of highly reliable and safe battery units.
- Advanced Materials for Improved Performance and Safety: Enovix batteries incorporate cutting-edge materials in their construction, such as high-performance electrolytes, advanced separator materials, and novel electrode compositions. These materials contribute to enhanced electrochemical stability, improved mechanical strength, and better overall safety characteristics, while also boosting the energy density and cycle life of the batteries.
02 Innovative Cell Design for Improved Safety
Enovix batteries utilize a unique cell design that incorporates advanced materials and structural elements to enhance safety. This design includes features such as improved thermal management, reinforced separators, and optimized electrode configurations. These innovations help prevent short circuits, reduce the risk of thermal runaway, and improve overall battery reliability.Expand Specific Solutions03 Enhanced Thermal Management Techniques
Enovix batteries employ sophisticated thermal management techniques to maintain optimal operating temperatures and prevent overheating. These may include advanced cooling systems, heat-dissipating materials, and thermal insulation layers. Effective thermal management contributes significantly to the safety and longevity of the batteries, especially in high-performance applications.Expand Specific Solutions04 Advanced Manufacturing Processes for Consistency
Enovix utilizes state-of-the-art manufacturing processes to ensure consistency and quality in battery production. These processes may include precision automation, rigorous quality control measures, and advanced testing protocols. By maintaining high manufacturing standards, Enovix enhances the reliability and safety of their batteries across production batches.Expand Specific Solutions05 Integration of Smart Diagnostics and Predictive Maintenance
Enovix batteries incorporate smart diagnostic features and predictive maintenance capabilities. These technologies allow for real-time monitoring of battery health, early detection of potential issues, and proactive maintenance scheduling. By anticipating and addressing problems before they escalate, these features significantly improve the long-term reliability and safety of the battery systems.Expand Specific Solutions
Key Players in Medical Device Battery Market
The market for Enovix batteries in medical devices is in an early growth stage, with increasing demand for reliable and safe power solutions in the healthcare sector. The global medical battery market is projected to expand significantly, driven by the growing adoption of portable and implantable medical devices. While the technology is promising, it is still evolving, with major players like Medtronic, Boston Scientific, and Stryker investing in advanced battery technologies. Smaller companies like Enovix are competing by offering innovative solutions that address the specific power requirements and safety standards of medical devices, potentially disrupting the established market dynamics.
Medtronic, Inc.
Stryker Corp.
Core Innovations in Enovix Battery Technology
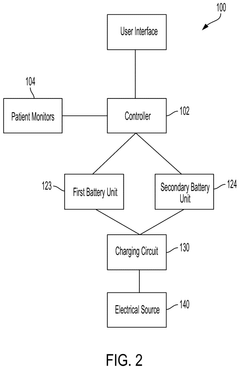
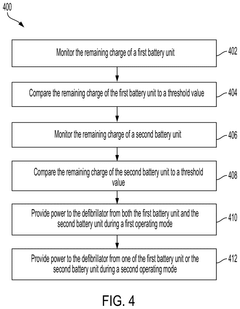
- Implementing a system with a first and second battery unit that powers the device in different modes, with a controller monitoring charge and useful life to adjust operation, allowing simultaneous power draw from both units during high demand or when either unit's charge or life falls below thresholds, and switching to single-unit power in low-power modes to extend usage.
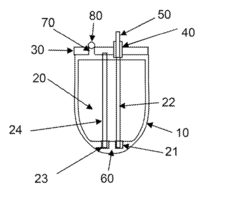
- A high energy density battery design with optimized placement of current carrying leads within the battery enclosure, allowing for a greater volume of active material and reduced size, featuring a case with a header assembly and electrode stacks connected via current collecting leads, and an electrolyte solution for efficient energy storage.
Regulatory Compliance for Medical Device Batteries
Regulatory compliance is a critical aspect of integrating Enovix batteries into medical devices to ensure reliable and safe power requirements are met. The medical device industry is heavily regulated, with stringent standards and guidelines set by various regulatory bodies worldwide. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the safety and efficacy of medical devices, including their power sources.
For Enovix batteries to be successfully implemented in medical devices, they must adhere to specific regulatory standards. The International Electrotechnical Commission (IEC) has established several standards relevant to batteries in medical devices, such as IEC 60601-1 for general safety and essential performance of medical electrical equipment. This standard includes requirements for battery-powered devices and their power sources.
Additionally, ISO 13485, which specifies requirements for quality management systems in the medical device industry, is crucial for manufacturers integrating Enovix batteries into their products. Compliance with this standard ensures that the entire production process, from design to distribution, meets regulatory requirements and maintains consistent quality.
Specific to batteries, IEC 62133 addresses safety requirements for portable sealed secondary cells and batteries containing alkaline or other non-acid electrolytes. This standard is particularly relevant for Enovix's lithium-ion technology and covers aspects such as safety during intended use and reasonably foreseeable misuse.
Regulatory bodies also require extensive documentation and testing to demonstrate compliance. This includes risk management processes as outlined in ISO 14971, which involves identifying potential hazards, estimating and evaluating risks, and implementing risk control measures. For Enovix batteries, this would encompass assessing risks related to thermal runaway, electrical safety, and long-term reliability in medical applications.
Furthermore, electromagnetic compatibility (EMC) is a crucial consideration, governed by standards such as IEC 60601-1-2. Enovix batteries must not interfere with the operation of other medical equipment and must themselves be immune to electromagnetic interference from surrounding devices.
Biocompatibility is another essential aspect of regulatory compliance, especially for implantable medical devices. Standards like ISO 10993 series guide the evaluation of biological responses to materials used in medical devices, which would apply to any components of Enovix batteries that may come into contact with the human body.
To ensure ongoing compliance, manufacturers using Enovix batteries in medical devices must implement post-market surveillance systems. This involves monitoring the performance and safety of devices in real-world use, reporting adverse events, and taking corrective actions when necessary. Regulatory bodies may require periodic safety updates and re-certification to maintain market approval.
Safety and Reliability Testing Protocols
Safety and reliability testing protocols for Enovix batteries in medical devices are crucial to ensure they meet the stringent requirements of the healthcare industry. These protocols are designed to evaluate the performance, durability, and safety of the batteries under various conditions that simulate real-world usage in medical applications.
The testing process typically begins with basic electrical characterization, including capacity measurements, charge-discharge cycles, and self-discharge rates. These tests provide baseline data on the battery's performance and help identify any initial deviations from expected specifications. Following this, environmental stress tests are conducted to assess the battery's resilience to temperature fluctuations, humidity, and atmospheric pressure changes, which are common in medical settings.
Mechanical integrity tests form another critical component of the safety protocols. These include vibration testing, shock testing, and drop tests to simulate the physical stresses that medical devices may encounter during transportation or daily use. The batteries must maintain their structural integrity and performance characteristics after exposure to these mechanical stresses.
Electromagnetic compatibility (EMC) testing is essential to ensure that the Enovix batteries do not interfere with other medical equipment and are not susceptible to electromagnetic interference themselves. This involves subjecting the batteries to various electromagnetic fields and measuring their response and potential emissions.
Long-term cycling tests are performed to evaluate the battery's lifespan and capacity retention over extended periods. These tests simulate years of use in medical devices, often involving thousands of charge-discharge cycles under controlled conditions. The results help predict the battery's long-term reliability and performance degradation over time.
Safety-specific tests are particularly critical for medical applications. These include overcharge protection, short-circuit protection, and thermal runaway prevention tests. The batteries must demonstrate robust safety features that prevent catastrophic failures even under extreme conditions. Additionally, biocompatibility tests are conducted to ensure that the battery materials are safe for use in close proximity to the human body.
Regulatory compliance testing is an integral part of the protocol, ensuring that the batteries meet industry standards such as IEC 62133 for safety requirements for portable batteries and FDA guidelines for medical devices. This often involves third-party certification to validate the test results and compliance with relevant regulations.
Finally, application-specific testing is tailored to the particular medical device in which the Enovix battery will be used. This may include simulating the device's power draw patterns, evaluating the battery's performance under specific medical procedures, and assessing its integration with the device's power management systems.
Through these comprehensive safety and reliability testing protocols, Enovix batteries can demonstrate their suitability for use in medical devices, providing the necessary assurance of performance, safety, and longevity required in critical healthcare applications.