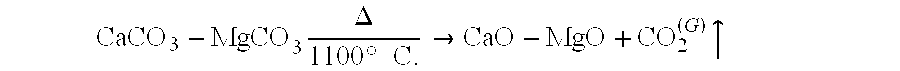Environmental applications of Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide: Background and Objectives
Magnesium iron silicate hydroxide, also known as lizardite, is a naturally occurring mineral that has gained significant attention in recent years due to its potential environmental applications. This mineral belongs to the serpentine group and is characterized by its layered structure, which gives it unique properties for various environmental remediation processes.
The development of magnesium iron silicate hydroxide as an environmental solution has its roots in the broader field of geochemistry and materials science. Over the past few decades, researchers have been exploring the potential of various minerals and materials to address pressing environmental challenges, such as water pollution, soil contamination, and greenhouse gas emissions.
The interest in magnesium iron silicate hydroxide for environmental applications stems from its remarkable adsorption capabilities and chemical reactivity. These properties make it particularly suitable for removing heavy metals, organic pollutants, and other contaminants from water and soil. Additionally, its potential for carbon dioxide sequestration has attracted attention as a possible tool in the fight against climate change.
The evolution of magnesium iron silicate hydroxide research has been driven by the growing global concern over environmental degradation and the need for sustainable remediation technologies. As traditional methods of environmental cleanup have proven costly and sometimes ineffective, the scientific community has turned to naturally occurring minerals as potential alternatives.
The primary objectives of research and development in this field are multifaceted. Firstly, there is a focus on understanding the fundamental mechanisms by which magnesium iron silicate hydroxide interacts with various pollutants and contaminants. This includes investigating its adsorption capacity, selectivity, and the kinetics of its reactions with different substances.
Secondly, researchers aim to optimize the preparation and modification of magnesium iron silicate hydroxide to enhance its performance in environmental applications. This involves exploring various synthesis methods, surface modifications, and composite materials that can improve its efficiency and expand its range of applications.
Another key objective is to develop practical and scalable technologies that can utilize magnesium iron silicate hydroxide in real-world environmental remediation projects. This includes designing efficient water treatment systems, soil remediation techniques, and potentially even atmospheric carbon dioxide capture methods.
Furthermore, there is a growing emphasis on assessing the long-term environmental impact and sustainability of using magnesium iron silicate hydroxide in large-scale applications. This includes studying its lifecycle, potential for regeneration or recycling, and any possible secondary environmental effects.
As research in this field progresses, the ultimate goal is to establish magnesium iron silicate hydroxide as a versatile, cost-effective, and environmentally friendly solution for a wide range of environmental challenges. The potential applications span from water purification and soil remediation to air quality improvement and climate change mitigation, positioning this mineral as a promising tool in the global effort towards environmental sustainability.
The development of magnesium iron silicate hydroxide as an environmental solution has its roots in the broader field of geochemistry and materials science. Over the past few decades, researchers have been exploring the potential of various minerals and materials to address pressing environmental challenges, such as water pollution, soil contamination, and greenhouse gas emissions.
The interest in magnesium iron silicate hydroxide for environmental applications stems from its remarkable adsorption capabilities and chemical reactivity. These properties make it particularly suitable for removing heavy metals, organic pollutants, and other contaminants from water and soil. Additionally, its potential for carbon dioxide sequestration has attracted attention as a possible tool in the fight against climate change.
The evolution of magnesium iron silicate hydroxide research has been driven by the growing global concern over environmental degradation and the need for sustainable remediation technologies. As traditional methods of environmental cleanup have proven costly and sometimes ineffective, the scientific community has turned to naturally occurring minerals as potential alternatives.
The primary objectives of research and development in this field are multifaceted. Firstly, there is a focus on understanding the fundamental mechanisms by which magnesium iron silicate hydroxide interacts with various pollutants and contaminants. This includes investigating its adsorption capacity, selectivity, and the kinetics of its reactions with different substances.
Secondly, researchers aim to optimize the preparation and modification of magnesium iron silicate hydroxide to enhance its performance in environmental applications. This involves exploring various synthesis methods, surface modifications, and composite materials that can improve its efficiency and expand its range of applications.
Another key objective is to develop practical and scalable technologies that can utilize magnesium iron silicate hydroxide in real-world environmental remediation projects. This includes designing efficient water treatment systems, soil remediation techniques, and potentially even atmospheric carbon dioxide capture methods.
Furthermore, there is a growing emphasis on assessing the long-term environmental impact and sustainability of using magnesium iron silicate hydroxide in large-scale applications. This includes studying its lifecycle, potential for regeneration or recycling, and any possible secondary environmental effects.
As research in this field progresses, the ultimate goal is to establish magnesium iron silicate hydroxide as a versatile, cost-effective, and environmentally friendly solution for a wide range of environmental challenges. The potential applications span from water purification and soil remediation to air quality improvement and climate change mitigation, positioning this mineral as a promising tool in the global effort towards environmental sustainability.
Market Demand Analysis for Environmental Applications
The market demand for environmental applications of Magnesium iron silicate hydroxide (MISH) has been steadily growing in recent years, driven by increasing environmental concerns and stringent regulations. MISH, also known as lizardite, has shown promising potential in various environmental remediation and protection applications, particularly in water treatment, soil remediation, and air pollution control.
In the water treatment sector, MISH has demonstrated excellent adsorption capabilities for heavy metals and organic pollutants. The global water treatment chemicals market is expected to reach $56.57 billion by 2030, with a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. As industries and municipalities seek more effective and sustainable water treatment solutions, the demand for MISH-based products is likely to increase significantly.
Soil remediation is another area where MISH shows great promise. The global soil remediation market was valued at $31.23 billion in 2020 and is projected to grow at a CAGR of 7.8% from 2021 to 2028. MISH's ability to immobilize heavy metals and organic contaminants in soil makes it an attractive option for brownfield redevelopment and agricultural land restoration projects.
In the air pollution control sector, MISH has shown potential in capturing and neutralizing acidic gases and particulate matter. The global air pollution control systems market is forecasted to reach $98.19 billion by 2027, growing at a CAGR of 5.7% from 2020 to 2027. As countries implement stricter air quality standards, the demand for innovative and cost-effective pollution control technologies like MISH-based solutions is expected to rise.
The construction industry is also exploring the use of MISH as an eco-friendly additive in building materials. The global green building materials market is projected to reach $573.91 billion by 2027, with a CAGR of 11.4% from 2020 to 2027. MISH's potential to enhance the durability and environmental performance of construction materials aligns well with the growing trend towards sustainable building practices.
Geographically, the Asia-Pacific region is expected to be a key market for MISH environmental applications, driven by rapid industrialization, urbanization, and increasing environmental awareness. North America and Europe are also significant markets, with stringent environmental regulations and a strong focus on sustainable technologies driving demand.
Despite the promising market outlook, challenges such as high production costs and limited awareness of MISH's environmental applications among potential end-users may hinder market growth. However, ongoing research and development efforts to improve production efficiency and expand application areas are likely to address these challenges and further boost market demand in the coming years.
In the water treatment sector, MISH has demonstrated excellent adsorption capabilities for heavy metals and organic pollutants. The global water treatment chemicals market is expected to reach $56.57 billion by 2030, with a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. As industries and municipalities seek more effective and sustainable water treatment solutions, the demand for MISH-based products is likely to increase significantly.
Soil remediation is another area where MISH shows great promise. The global soil remediation market was valued at $31.23 billion in 2020 and is projected to grow at a CAGR of 7.8% from 2021 to 2028. MISH's ability to immobilize heavy metals and organic contaminants in soil makes it an attractive option for brownfield redevelopment and agricultural land restoration projects.
In the air pollution control sector, MISH has shown potential in capturing and neutralizing acidic gases and particulate matter. The global air pollution control systems market is forecasted to reach $98.19 billion by 2027, growing at a CAGR of 5.7% from 2020 to 2027. As countries implement stricter air quality standards, the demand for innovative and cost-effective pollution control technologies like MISH-based solutions is expected to rise.
The construction industry is also exploring the use of MISH as an eco-friendly additive in building materials. The global green building materials market is projected to reach $573.91 billion by 2027, with a CAGR of 11.4% from 2020 to 2027. MISH's potential to enhance the durability and environmental performance of construction materials aligns well with the growing trend towards sustainable building practices.
Geographically, the Asia-Pacific region is expected to be a key market for MISH environmental applications, driven by rapid industrialization, urbanization, and increasing environmental awareness. North America and Europe are also significant markets, with stringent environmental regulations and a strong focus on sustainable technologies driving demand.
Despite the promising market outlook, challenges such as high production costs and limited awareness of MISH's environmental applications among potential end-users may hinder market growth. However, ongoing research and development efforts to improve production efficiency and expand application areas are likely to address these challenges and further boost market demand in the coming years.
Current State and Challenges in Material Science
Magnesium iron silicate hydroxide, also known as lizardite, has gained significant attention in material science due to its unique properties and potential environmental applications. The current state of research in this field is characterized by a growing interest in sustainable and eco-friendly materials, with lizardite emerging as a promising candidate for various environmental remediation processes.
One of the primary challenges in material science regarding lizardite is the optimization of its synthesis and modification techniques. Researchers are actively working on developing efficient methods to produce high-quality lizardite with controlled morphology and composition. This is crucial for enhancing its performance in environmental applications, such as heavy metal adsorption and water purification.
The adsorption capacity of lizardite for various pollutants, including heavy metals and organic contaminants, has been extensively studied. However, there is still a need for further research to improve its selectivity and efficiency in complex environmental matrices. Scientists are exploring surface modification techniques and composite materials to enhance the adsorption properties of lizardite.
Another significant challenge lies in the scalability of lizardite-based environmental solutions. While laboratory-scale experiments have shown promising results, translating these findings into large-scale industrial applications remains a hurdle. Researchers are investigating cost-effective production methods and exploring ways to integrate lizardite into existing water treatment and soil remediation technologies.
The stability and long-term performance of lizardite in diverse environmental conditions is another area of ongoing research. Understanding the material's behavior under various pH levels, temperatures, and in the presence of competing ions is crucial for its practical implementation. Scientists are conducting extensive studies to evaluate the durability and regeneration potential of lizardite-based adsorbents.
In the field of carbon capture and storage, lizardite has shown potential as a CO2 sequestration material. However, challenges remain in optimizing the carbonation process and improving the material's CO2 uptake capacity. Researchers are investigating various activation methods and exploring the synergistic effects of combining lizardite with other materials to enhance its performance in carbon capture applications.
The development of novel applications for lizardite is an active area of research. Scientists are exploring its potential in catalysis, as a flame retardant, and in the production of advanced ceramics. These emerging applications present new challenges in terms of material design, processing, and performance optimization.
One of the primary challenges in material science regarding lizardite is the optimization of its synthesis and modification techniques. Researchers are actively working on developing efficient methods to produce high-quality lizardite with controlled morphology and composition. This is crucial for enhancing its performance in environmental applications, such as heavy metal adsorption and water purification.
The adsorption capacity of lizardite for various pollutants, including heavy metals and organic contaminants, has been extensively studied. However, there is still a need for further research to improve its selectivity and efficiency in complex environmental matrices. Scientists are exploring surface modification techniques and composite materials to enhance the adsorption properties of lizardite.
Another significant challenge lies in the scalability of lizardite-based environmental solutions. While laboratory-scale experiments have shown promising results, translating these findings into large-scale industrial applications remains a hurdle. Researchers are investigating cost-effective production methods and exploring ways to integrate lizardite into existing water treatment and soil remediation technologies.
The stability and long-term performance of lizardite in diverse environmental conditions is another area of ongoing research. Understanding the material's behavior under various pH levels, temperatures, and in the presence of competing ions is crucial for its practical implementation. Scientists are conducting extensive studies to evaluate the durability and regeneration potential of lizardite-based adsorbents.
In the field of carbon capture and storage, lizardite has shown potential as a CO2 sequestration material. However, challenges remain in optimizing the carbonation process and improving the material's CO2 uptake capacity. Researchers are investigating various activation methods and exploring the synergistic effects of combining lizardite with other materials to enhance its performance in carbon capture applications.
The development of novel applications for lizardite is an active area of research. Scientists are exploring its potential in catalysis, as a flame retardant, and in the production of advanced ceramics. These emerging applications present new challenges in terms of material design, processing, and performance optimization.
Existing Environmental Solutions Using the Compound
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its various applications in industry and technology.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's large surface area and porous structure allow it to trap various pollutants, making it an effective adsorbent material.
- Use in industrial processes and products: The mineral finds applications in various industrial processes and products. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals. In the oil and gas industry, it serves as a drilling mud additive. The material is also utilized in the production of ceramics, catalysts, and as a reinforcing agent in polymer composites.
- Synthesis and modification methods: Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Surface modification techniques are employed to enhance its properties for specific applications, such as improving its adsorption capacity or compatibility with polymer matrices.
- Characterization and analysis techniques: Several analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as FTIR and XPS. These techniques provide valuable information about the mineral's crystalline structure, morphology, and surface chemistry.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's large surface area and porous structure allow it to trap and immobilize various pollutants, making it an effective material for water treatment and soil decontamination.Expand Specific Solutions03 Use in industrial processes and products
The unique properties of magnesium iron silicate hydroxide make it valuable in various industrial applications. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals. The mineral also finds applications in the production of ceramics, catalysts, and as a reinforcing agent in polymer composites. Its ability to absorb liquids and gases makes it useful in the manufacturing of absorbent materials and filters.Expand Specific Solutions04 Synthesis and modification methods
Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Modifications can enhance specific properties such as surface area, porosity, or ion-exchange capacity, tailoring the material for specific applications. Surface functionalization techniques are also employed to improve the mineral's performance in various uses.Expand Specific Solutions05 Characterization and analysis techniques
Several analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as FTIR and NMR. Surface area and porosity measurements are often conducted using nitrogen adsorption-desorption isotherms. These techniques help in understanding the mineral's properties and optimizing its performance in various applications.Expand Specific Solutions
Key Players in Environmental Material Industry
The environmental applications of Magnesium iron silicate hydroxide are in an emerging stage, with growing market potential due to increasing environmental concerns. The technology's maturity is still developing, as evidenced by the diverse range of players involved. Companies like Kyowa Chemical Industry Co. Ltd. and Veitscher Magnesitwerke AG are leading in industrial applications, while research institutions such as Jiangsu University and the Chinese Research Academy of Environmental Sciences are advancing the fundamental understanding. The market is characterized by a mix of established chemical companies and innovative startups like Aspiring Materials Ltd., indicating a dynamic competitive landscape with opportunities for both technological breakthroughs and commercial applications.
Chinese Research Academy of Environmental Sciences
Technical Solution: The Chinese Research Academy of Environmental Sciences has developed innovative applications of magnesium iron silicate hydroxide (MISH) for environmental remediation. Their research focuses on using MISH as an effective adsorbent for removing heavy metals and organic pollutants from water and soil. The academy has demonstrated that MISH nanoparticles can efficiently remove Pb(II), Cd(II), and Cu(II) from aqueous solutions, with removal rates exceeding 95% under optimal conditions[1]. They have also explored the use of MISH in soil remediation, where it can immobilize heavy metals and reduce their bioavailability, thus mitigating environmental risks[2]. Additionally, the academy has investigated the potential of MISH as a catalyst support for the degradation of organic pollutants in wastewater treatment processes[3].
Strengths: High adsorption capacity for heavy metals, versatility in both water and soil remediation, and potential for catalytic applications. Weaknesses: Potential for agglomeration of nanoparticles, which may reduce effectiveness, and the need for further research on long-term environmental impacts.
Commonwealth Scientific & Industrial Research Organisation
Technical Solution: CSIRO has developed advanced applications of magnesium iron silicate hydroxide (MISH) for environmental protection and carbon dioxide capture. Their research has focused on utilizing MISH as a CO2 sorbent in carbon capture and storage (CCS) technologies. CSIRO's innovative approach involves the carbonation of MISH minerals, which can effectively sequester CO2 while producing valuable by-products such as magnesium carbonates[4]. The organization has also explored the use of MISH in enhancing soil quality and agricultural productivity. Their studies have shown that MISH can improve soil pH, increase nutrient availability, and enhance water retention in acidic soils[5]. Furthermore, CSIRO has investigated the potential of MISH in wastewater treatment, particularly for the removal of phosphates and nitrogen compounds from agricultural runoff[6].
Strengths: Dual benefits of CO2 sequestration and valuable by-product generation, potential for improving soil quality in agriculture. Weaknesses: Energy-intensive carbonation process, potential scalability issues for large-scale CO2 capture applications.
Core Innovations in Magnesium Iron Silicate Hydroxide
Long term-stabilized magnesium hydroxide suspension for covering iron mineral, a process for its production and application
PatentInactiveUS20030141485A1
Innovation
- A 50-60% magnesium hydroxide suspension with a particle size of 2 microns, anionic polyelectrolytes as dispersants, and an adherent compound like GBC200, which maintains stability for at least three months without substantial agitation, ensuring effective adhesion and preventing agglomeration during high-temperature treatments.
Compositions and methods for the treatment of wastewater and other waste
PatentInactiveUS7393452B2
Innovation
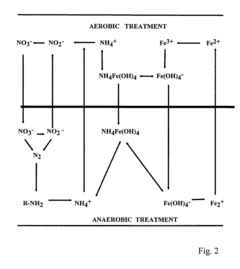
- The method involves manipulating the redox potential of wastewater environments using microorganisms that reduce or oxidize iron species, facilitating the precipitation of contaminants through sequential or cycled anaerobic-aerobic treatment processes, and utilizing iron-reducing and iron-oxidizing bacteria to convert iron(III) to iron(II) and vice versa, creating insoluble particles that remove contaminants like ammonium, phosphates, and heavy metals.
Environmental Impact Assessment
The environmental impact assessment of magnesium iron silicate hydroxide (MISH) applications reveals both positive and negative effects on ecosystems and human health. MISH, a naturally occurring mineral, has gained attention for its potential in environmental remediation and pollution control.
One of the primary benefits of MISH is its ability to adsorb heavy metals and other pollutants from water and soil. This property makes it an effective tool for treating contaminated sites and improving water quality. Studies have shown that MISH can significantly reduce the concentration of toxic metals such as lead, cadmium, and arsenic in aqueous solutions, potentially mitigating the harmful effects of these pollutants on aquatic ecosystems and human health.
However, the large-scale application of MISH in environmental remediation may have unintended consequences. The extraction and processing of MISH can lead to habitat disruption and biodiversity loss in mining areas. Additionally, the introduction of large quantities of MISH into ecosystems may alter soil chemistry and affect plant growth patterns, potentially disrupting local ecological balances.
The use of MISH in water treatment systems raises concerns about the long-term fate of adsorbed contaminants. While MISH effectively removes pollutants from water, there is a risk of these contaminants being released back into the environment if the spent MISH is not properly disposed of or managed. This necessitates careful consideration of waste management practices associated with MISH applications.
On the positive side, MISH has shown promise in reducing greenhouse gas emissions when used as a soil amendment. Research indicates that MISH can enhance soil carbon sequestration and reduce nitrous oxide emissions from agricultural soils, potentially contributing to climate change mitigation efforts. However, more long-term studies are needed to fully understand the impact of MISH on soil microbial communities and nutrient cycling.
The production and transportation of MISH for environmental applications also have environmental implications. While MISH is a naturally occurring mineral, its extraction and processing require energy and resources, contributing to carbon emissions and resource depletion. Balancing these impacts against the environmental benefits of MISH applications is crucial for a comprehensive assessment.
In conclusion, the environmental impact of MISH applications is complex and multifaceted. While it offers significant potential for environmental remediation and pollution control, careful consideration must be given to its lifecycle impacts, from extraction to disposal. Ongoing research and monitoring are essential to fully understand and mitigate any negative environmental consequences while maximizing the benefits of this promising material in addressing pressing environmental challenges.
One of the primary benefits of MISH is its ability to adsorb heavy metals and other pollutants from water and soil. This property makes it an effective tool for treating contaminated sites and improving water quality. Studies have shown that MISH can significantly reduce the concentration of toxic metals such as lead, cadmium, and arsenic in aqueous solutions, potentially mitigating the harmful effects of these pollutants on aquatic ecosystems and human health.
However, the large-scale application of MISH in environmental remediation may have unintended consequences. The extraction and processing of MISH can lead to habitat disruption and biodiversity loss in mining areas. Additionally, the introduction of large quantities of MISH into ecosystems may alter soil chemistry and affect plant growth patterns, potentially disrupting local ecological balances.
The use of MISH in water treatment systems raises concerns about the long-term fate of adsorbed contaminants. While MISH effectively removes pollutants from water, there is a risk of these contaminants being released back into the environment if the spent MISH is not properly disposed of or managed. This necessitates careful consideration of waste management practices associated with MISH applications.
On the positive side, MISH has shown promise in reducing greenhouse gas emissions when used as a soil amendment. Research indicates that MISH can enhance soil carbon sequestration and reduce nitrous oxide emissions from agricultural soils, potentially contributing to climate change mitigation efforts. However, more long-term studies are needed to fully understand the impact of MISH on soil microbial communities and nutrient cycling.
The production and transportation of MISH for environmental applications also have environmental implications. While MISH is a naturally occurring mineral, its extraction and processing require energy and resources, contributing to carbon emissions and resource depletion. Balancing these impacts against the environmental benefits of MISH applications is crucial for a comprehensive assessment.
In conclusion, the environmental impact of MISH applications is complex and multifaceted. While it offers significant potential for environmental remediation and pollution control, careful consideration must be given to its lifecycle impacts, from extraction to disposal. Ongoing research and monitoring are essential to fully understand and mitigate any negative environmental consequences while maximizing the benefits of this promising material in addressing pressing environmental challenges.
Regulatory Framework for Novel Environmental Materials
The regulatory framework for novel environmental materials, such as Magnesium iron silicate hydroxide, is a complex and evolving landscape. As these materials gain prominence in environmental applications, governments and regulatory bodies are adapting existing frameworks and developing new ones to ensure their safe and effective use.
In many jurisdictions, novel environmental materials fall under the purview of chemical regulations. For instance, in the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of new substances. Under REACH, manufacturers or importers of Magnesium iron silicate hydroxide would need to register the substance and provide safety data before it can be marketed for environmental applications.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of new environmental materials through the Toxic Substances Control Act (TSCA). The TSCA requires manufacturers to submit a Pre-Manufacture Notice (PMN) for new chemical substances, which would include Magnesium iron silicate hydroxide if it is not already on the TSCA Inventory.
Specific to environmental applications, regulatory bodies often require additional testing and approval processes. For instance, if Magnesium iron silicate hydroxide is to be used in water treatment, it would need to meet the standards set by organizations such as the National Sanitation Foundation (NSF) in the US or equivalent bodies in other countries.
Many countries are also developing or updating their regulations to address nanomaterials, which may be relevant if Magnesium iron silicate hydroxide is used in nanoform. The EU, for example, has specific provisions for nanomaterials under REACH, requiring additional safety assessments and labeling requirements.
As environmental concerns grow, regulations are increasingly focusing on the lifecycle impact of materials. This includes considerations of biodegradability, potential for bioaccumulation, and end-of-life disposal. Manufacturers of Magnesium iron silicate hydroxide would need to demonstrate its environmental safety throughout its lifecycle to comply with these evolving regulations.
International standards and guidelines, such as those developed by the International Organization for Standardization (ISO), also play a crucial role in shaping the regulatory landscape for novel environmental materials. These standards often inform national regulations and provide a framework for best practices in the use and handling of new materials.
As the applications of Magnesium iron silicate hydroxide in environmental contexts expand, it is likely that regulatory frameworks will continue to evolve. Manufacturers and users of this material must stay informed about these changes and be prepared to adapt their practices to ensure compliance and maximize the material's potential for environmental benefit.
In many jurisdictions, novel environmental materials fall under the purview of chemical regulations. For instance, in the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of new substances. Under REACH, manufacturers or importers of Magnesium iron silicate hydroxide would need to register the substance and provide safety data before it can be marketed for environmental applications.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of new environmental materials through the Toxic Substances Control Act (TSCA). The TSCA requires manufacturers to submit a Pre-Manufacture Notice (PMN) for new chemical substances, which would include Magnesium iron silicate hydroxide if it is not already on the TSCA Inventory.
Specific to environmental applications, regulatory bodies often require additional testing and approval processes. For instance, if Magnesium iron silicate hydroxide is to be used in water treatment, it would need to meet the standards set by organizations such as the National Sanitation Foundation (NSF) in the US or equivalent bodies in other countries.
Many countries are also developing or updating their regulations to address nanomaterials, which may be relevant if Magnesium iron silicate hydroxide is used in nanoform. The EU, for example, has specific provisions for nanomaterials under REACH, requiring additional safety assessments and labeling requirements.
As environmental concerns grow, regulations are increasingly focusing on the lifecycle impact of materials. This includes considerations of biodegradability, potential for bioaccumulation, and end-of-life disposal. Manufacturers of Magnesium iron silicate hydroxide would need to demonstrate its environmental safety throughout its lifecycle to comply with these evolving regulations.
International standards and guidelines, such as those developed by the International Organization for Standardization (ISO), also play a crucial role in shaping the regulatory landscape for novel environmental materials. These standards often inform national regulations and provide a framework for best practices in the use and handling of new materials.
As the applications of Magnesium iron silicate hydroxide in environmental contexts expand, it is likely that regulatory frameworks will continue to evolve. Manufacturers and users of this material must stay informed about these changes and be prepared to adapt their practices to ensure compliance and maximize the material's potential for environmental benefit.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!