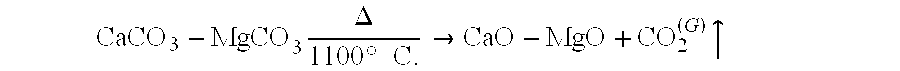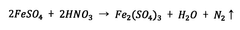Environmental safety of Magnesium iron silicate hydroxide applications.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide: Background and Objectives
Magnesium iron silicate hydroxide, also known as MISH, is a naturally occurring mineral with a complex chemical structure. Its unique properties have attracted significant attention in various industrial and environmental applications. The evolution of MISH technology can be traced back to the early 20th century when researchers first identified its potential as an adsorbent material. Over the decades, advancements in material science and environmental engineering have led to a deeper understanding of MISH's capabilities and expanded its potential uses.
The technological trajectory of MISH has been marked by several key milestones. Initially, it was primarily used in industrial processes for its adsorptive properties. However, as environmental concerns grew in the latter half of the 20th century, researchers began exploring MISH's potential in environmental remediation. This shift in focus led to the development of novel applications in water treatment, soil decontamination, and air purification.
Recent years have seen a surge in research activities aimed at enhancing the environmental safety of MISH applications. This renewed interest is driven by the growing global emphasis on sustainable technologies and the need for effective, eco-friendly solutions to environmental challenges. The current technological landscape is characterized by efforts to optimize MISH's performance while minimizing any potential negative impacts on ecosystems.
The primary objectives of current MISH research and development are multifaceted. Firstly, there is a strong focus on improving the efficiency of MISH in environmental applications, particularly in the removal of heavy metals and organic pollutants from water and soil. Secondly, researchers are working to enhance the stability and longevity of MISH-based materials to ensure their effectiveness over extended periods. Thirdly, there is a concerted effort to develop more sustainable production methods for MISH, aiming to reduce the environmental footprint of its manufacturing process.
Another critical objective is to gain a comprehensive understanding of MISH's long-term environmental impact. This includes studying its behavior in various ecosystems, its potential for bioaccumulation, and any possible effects on flora and fauna. The goal is to establish a robust scientific foundation that can inform regulatory frameworks and guide the safe implementation of MISH technologies.
As we look to the future, the technological trajectory of MISH is likely to be shaped by emerging environmental challenges and advances in nanotechnology and materials science. The integration of MISH with other innovative materials and technologies holds promise for creating more effective and versatile environmental solutions. Additionally, the development of smart, responsive MISH-based systems that can adapt to changing environmental conditions represents an exciting frontier in this field.
The technological trajectory of MISH has been marked by several key milestones. Initially, it was primarily used in industrial processes for its adsorptive properties. However, as environmental concerns grew in the latter half of the 20th century, researchers began exploring MISH's potential in environmental remediation. This shift in focus led to the development of novel applications in water treatment, soil decontamination, and air purification.
Recent years have seen a surge in research activities aimed at enhancing the environmental safety of MISH applications. This renewed interest is driven by the growing global emphasis on sustainable technologies and the need for effective, eco-friendly solutions to environmental challenges. The current technological landscape is characterized by efforts to optimize MISH's performance while minimizing any potential negative impacts on ecosystems.
The primary objectives of current MISH research and development are multifaceted. Firstly, there is a strong focus on improving the efficiency of MISH in environmental applications, particularly in the removal of heavy metals and organic pollutants from water and soil. Secondly, researchers are working to enhance the stability and longevity of MISH-based materials to ensure their effectiveness over extended periods. Thirdly, there is a concerted effort to develop more sustainable production methods for MISH, aiming to reduce the environmental footprint of its manufacturing process.
Another critical objective is to gain a comprehensive understanding of MISH's long-term environmental impact. This includes studying its behavior in various ecosystems, its potential for bioaccumulation, and any possible effects on flora and fauna. The goal is to establish a robust scientific foundation that can inform regulatory frameworks and guide the safe implementation of MISH technologies.
As we look to the future, the technological trajectory of MISH is likely to be shaped by emerging environmental challenges and advances in nanotechnology and materials science. The integration of MISH with other innovative materials and technologies holds promise for creating more effective and versatile environmental solutions. Additionally, the development of smart, responsive MISH-based systems that can adapt to changing environmental conditions represents an exciting frontier in this field.
Market Analysis for Eco-friendly Mineral Applications
The market for eco-friendly mineral applications, particularly those involving Magnesium iron silicate hydroxide (MISH), has shown significant growth in recent years. This trend is driven by increasing environmental concerns and stricter regulations across various industries. The global market for environmentally safe minerals is expected to continue its upward trajectory, with MISH applications playing a crucial role in this expansion.
In the construction sector, MISH has gained traction as a sustainable alternative to traditional materials. Its fire-resistant properties and low environmental impact have made it a preferred choice for green building projects. The construction industry's shift towards sustainable practices has created a substantial demand for MISH-based products, including insulation materials and fire-retardant coatings.
The automotive industry has also embraced MISH applications, particularly in the manufacturing of lightweight components. As automakers strive to reduce vehicle weight and improve fuel efficiency, MISH-based composites offer an eco-friendly solution that meets both performance and environmental requirements. This sector's adoption of MISH is expected to grow as electric vehicle production increases and environmental regulations tighten.
In the field of water treatment, MISH has shown promise as an effective and environmentally safe adsorbent for removing heavy metals and other pollutants from wastewater. The growing need for clean water solutions in both developed and developing countries has created a significant market opportunity for MISH-based water treatment technologies.
The personal care and cosmetics industry has also recognized the potential of MISH as a natural and safe ingredient. Its use in sunscreens, anti-aging products, and other skincare formulations has increased due to consumer demand for eco-friendly and non-toxic alternatives to synthetic chemicals.
Agricultural applications of MISH are emerging as another promising market segment. Its use as a soil amendment to improve nutrient retention and reduce the need for chemical fertilizers aligns with the growing trend towards sustainable farming practices.
Despite the positive market outlook, challenges remain in scaling up production and reducing costs to make MISH applications more competitive with traditional alternatives. However, ongoing research and development efforts are expected to address these issues, further expanding the market potential of MISH across various industries.
As environmental regulations become more stringent globally, the demand for eco-friendly mineral applications like MISH is projected to grow substantially. This market trend is likely to drive innovation in product development and manufacturing processes, creating new opportunities for businesses operating in this space.
In the construction sector, MISH has gained traction as a sustainable alternative to traditional materials. Its fire-resistant properties and low environmental impact have made it a preferred choice for green building projects. The construction industry's shift towards sustainable practices has created a substantial demand for MISH-based products, including insulation materials and fire-retardant coatings.
The automotive industry has also embraced MISH applications, particularly in the manufacturing of lightweight components. As automakers strive to reduce vehicle weight and improve fuel efficiency, MISH-based composites offer an eco-friendly solution that meets both performance and environmental requirements. This sector's adoption of MISH is expected to grow as electric vehicle production increases and environmental regulations tighten.
In the field of water treatment, MISH has shown promise as an effective and environmentally safe adsorbent for removing heavy metals and other pollutants from wastewater. The growing need for clean water solutions in both developed and developing countries has created a significant market opportunity for MISH-based water treatment technologies.
The personal care and cosmetics industry has also recognized the potential of MISH as a natural and safe ingredient. Its use in sunscreens, anti-aging products, and other skincare formulations has increased due to consumer demand for eco-friendly and non-toxic alternatives to synthetic chemicals.
Agricultural applications of MISH are emerging as another promising market segment. Its use as a soil amendment to improve nutrient retention and reduce the need for chemical fertilizers aligns with the growing trend towards sustainable farming practices.
Despite the positive market outlook, challenges remain in scaling up production and reducing costs to make MISH applications more competitive with traditional alternatives. However, ongoing research and development efforts are expected to address these issues, further expanding the market potential of MISH across various industries.
As environmental regulations become more stringent globally, the demand for eco-friendly mineral applications like MISH is projected to grow substantially. This market trend is likely to drive innovation in product development and manufacturing processes, creating new opportunities for businesses operating in this space.
Current Status and Environmental Challenges
Magnesium iron silicate hydroxide (MISH) has gained significant attention in recent years due to its potential applications in various industries. However, the current status of MISH applications and their environmental challenges require careful examination.
The use of MISH in industrial processes has shown promising results, particularly in areas such as wastewater treatment, catalysis, and environmental remediation. Its high adsorption capacity and unique physicochemical properties make it an attractive option for removing heavy metals and organic pollutants from water. Several studies have demonstrated its effectiveness in treating industrial effluents and contaminated groundwater.
Despite these positive developments, the widespread adoption of MISH technologies faces several environmental challenges. One of the primary concerns is the potential release of nanoparticles into the environment during production, use, or disposal of MISH-based products. The long-term effects of these nanoparticles on ecosystems and human health are not yet fully understood, necessitating further research and risk assessment studies.
Another significant challenge is the energy-intensive nature of MISH production processes. The synthesis of MISH often requires high temperatures and pressures, leading to substantial energy consumption and associated greenhouse gas emissions. This raises questions about the overall environmental sustainability of MISH applications, particularly when considering their life cycle impacts.
The disposal of MISH-containing products at the end of their useful life also presents environmental concerns. While MISH itself is generally considered non-toxic, the potential for it to accumulate in the environment and its interactions with other pollutants require careful consideration. Proper waste management strategies and recycling techniques for MISH-based materials are still in the early stages of development.
Water resource management is another critical aspect of MISH applications. While MISH shows promise in water treatment, its large-scale use could potentially alter local water chemistry and impact aquatic ecosystems. The long-term effects of MISH on water quality and biodiversity need to be thoroughly investigated to ensure sustainable implementation.
Regulatory frameworks governing the use and disposal of MISH-based products are still evolving. The lack of standardized guidelines for assessing the environmental safety of MISH applications poses challenges for both industry and regulatory bodies. Developing comprehensive risk assessment protocols and establishing clear regulatory standards are crucial steps in addressing these environmental challenges.
In conclusion, while MISH applications show great potential for environmental remediation and industrial processes, they also face significant environmental challenges. Addressing these challenges requires a multidisciplinary approach, combining advanced research, technological innovation, and robust regulatory frameworks to ensure the safe and sustainable use of MISH in various applications.
The use of MISH in industrial processes has shown promising results, particularly in areas such as wastewater treatment, catalysis, and environmental remediation. Its high adsorption capacity and unique physicochemical properties make it an attractive option for removing heavy metals and organic pollutants from water. Several studies have demonstrated its effectiveness in treating industrial effluents and contaminated groundwater.
Despite these positive developments, the widespread adoption of MISH technologies faces several environmental challenges. One of the primary concerns is the potential release of nanoparticles into the environment during production, use, or disposal of MISH-based products. The long-term effects of these nanoparticles on ecosystems and human health are not yet fully understood, necessitating further research and risk assessment studies.
Another significant challenge is the energy-intensive nature of MISH production processes. The synthesis of MISH often requires high temperatures and pressures, leading to substantial energy consumption and associated greenhouse gas emissions. This raises questions about the overall environmental sustainability of MISH applications, particularly when considering their life cycle impacts.
The disposal of MISH-containing products at the end of their useful life also presents environmental concerns. While MISH itself is generally considered non-toxic, the potential for it to accumulate in the environment and its interactions with other pollutants require careful consideration. Proper waste management strategies and recycling techniques for MISH-based materials are still in the early stages of development.
Water resource management is another critical aspect of MISH applications. While MISH shows promise in water treatment, its large-scale use could potentially alter local water chemistry and impact aquatic ecosystems. The long-term effects of MISH on water quality and biodiversity need to be thoroughly investigated to ensure sustainable implementation.
Regulatory frameworks governing the use and disposal of MISH-based products are still evolving. The lack of standardized guidelines for assessing the environmental safety of MISH applications poses challenges for both industry and regulatory bodies. Developing comprehensive risk assessment protocols and establishing clear regulatory standards are crucial steps in addressing these environmental challenges.
In conclusion, while MISH applications show great potential for environmental remediation and industrial processes, they also face significant environmental challenges. Addressing these challenges requires a multidisciplinary approach, combining advanced research, technological innovation, and robust regulatory frameworks to ensure the safe and sustainable use of MISH in various applications.
Existing Environmental Safety Protocols
01 Environmental safety assessment of magnesium iron silicate hydroxide
Studies have been conducted to evaluate the environmental safety of magnesium iron silicate hydroxide. These assessments typically include analysis of its biodegradability, potential for bioaccumulation, and effects on aquatic and terrestrial ecosystems. The results generally indicate that this compound has low environmental toxicity and does not persist in the environment.- Environmental safety assessment of magnesium iron silicate hydroxide: Studies have been conducted to evaluate the environmental safety of magnesium iron silicate hydroxide. These assessments typically include analysis of its biodegradability, potential for bioaccumulation, and effects on aquatic and terrestrial ecosystems. The results generally indicate that this compound has low environmental toxicity and does not persist in the environment.
- Use in water treatment and environmental remediation: Magnesium iron silicate hydroxide has been utilized in water treatment processes and environmental remediation projects. Its ability to adsorb heavy metals and other pollutants makes it an effective agent for cleaning contaminated water and soil. This application demonstrates its potential for improving environmental quality while maintaining safety.
- Eco-friendly alternatives in industrial processes: Research has focused on incorporating magnesium iron silicate hydroxide as an eco-friendly alternative in various industrial processes. Its use can reduce the environmental impact of certain manufacturing operations, particularly in industries where hazardous materials are traditionally employed. This shift towards greener processes contributes to overall environmental safety.
- Biodegradability and natural occurrence: Magnesium iron silicate hydroxide is found naturally in certain geological formations. Its natural occurrence and biodegradability contribute to its environmental safety profile. Studies have shown that it can break down into environmentally benign components over time, reducing long-term ecological impacts.
- Regulatory compliance and safety standards: Various regulatory bodies have established safety standards and guidelines for the use of magnesium iron silicate hydroxide in different applications. Compliance with these regulations ensures that its use meets environmental safety requirements. Ongoing research continues to refine these standards based on new scientific findings.
02 Use in water treatment and environmental remediation
Magnesium iron silicate hydroxide has been utilized in water treatment processes and environmental remediation projects. Its ability to adsorb heavy metals and other pollutants makes it an effective material for cleaning contaminated water and soil. This application demonstrates its potential for improving environmental quality.Expand Specific Solutions03 Sustainable production methods
Research has focused on developing sustainable production methods for magnesium iron silicate hydroxide. These methods aim to reduce energy consumption, minimize waste generation, and utilize eco-friendly raw materials. Such approaches contribute to the overall environmental safety profile of the compound.Expand Specific Solutions04 Ecotoxicological studies on aquatic organisms
Ecotoxicological studies have been conducted to assess the impact of magnesium iron silicate hydroxide on aquatic organisms. These studies typically involve exposing various species to different concentrations of the compound and observing effects on survival, growth, and reproduction. The results help determine safe concentration levels for aquatic ecosystems.Expand Specific Solutions05 Biodegradation and environmental fate
Research has been conducted on the biodegradation and environmental fate of magnesium iron silicate hydroxide. These studies examine how the compound breaks down in natural environments, its persistence in soil and water, and potential long-term effects on ecosystems. Understanding these aspects is crucial for assessing its overall environmental safety.Expand Specific Solutions
Key Industry Players and Stakeholders
The environmental safety of Magnesium iron silicate hydroxide applications is an emerging field with growing interest. The market is in its early stages, characterized by increasing research and development activities. While the market size is still relatively small, it shows potential for significant growth as environmental concerns drive demand for safer materials. The technology is in a developmental phase, with varying levels of maturity across different applications. Key players like Commonwealth Scientific & Industrial Research Organisation, Zhejiang University, and Council of Scientific & Industrial Research are leading research efforts, while companies such as Kyowa Chemical Industry Co. Ltd. and Aspiring Materials Ltd. are exploring commercial applications. As the technology advances, collaboration between academic institutions and industry partners is likely to accelerate progress in addressing environmental safety concerns.
Commonwealth Scientific & Industrial Research Organisation
Technical Solution: CSIRO has developed innovative applications of magnesium iron silicate hydroxide (MISH) for environmental remediation. Their research focuses on using MISH as an effective adsorbent for removing heavy metals and organic pollutants from water and soil. The organization has conducted extensive studies on the synthesis of MISH nanoparticles with enhanced surface area and porosity, optimizing their adsorption capacity[1]. CSIRO's approach involves modifying the MISH structure to improve its selectivity towards specific contaminants, such as arsenic and phosphates. They have also investigated the potential of MISH in soil amendment, demonstrating its ability to immobilize toxic metals and improve soil fertility[2]. Additionally, CSIRO has explored the use of MISH in air purification systems, leveraging its high surface reactivity to capture airborne pollutants[3].
Strengths: Comprehensive research on multiple environmental applications; expertise in nanoparticle synthesis and modification. Weaknesses: Potential scalability issues for large-scale environmental remediation projects; long-term stability of MISH in various environmental conditions needs further investigation.
Council of Scientific & Industrial Research
Technical Solution: CSIR has made significant strides in developing environmentally safe applications of magnesium iron silicate hydroxide (MISH). Their research focuses on utilizing MISH as a sustainable catalyst for various industrial processes, reducing the environmental impact of chemical manufacturing. CSIR has successfully synthesized MISH-based catalysts with high surface area and tunable pore structures, enhancing their catalytic activity and selectivity[1]. These catalysts have shown promising results in organic transformations, such as oxidation and condensation reactions, offering a greener alternative to traditional metal-based catalysts[2]. CSIR has also explored the use of MISH in wastewater treatment, developing novel composite materials that combine MISH with other adsorbents for enhanced pollutant removal. Their studies have demonstrated the effectiveness of these composites in removing heavy metals, dyes, and pharmaceutical contaminants from water[3]. Furthermore, CSIR has investigated the potential of MISH in CO2 capture and conversion, contributing to climate change mitigation efforts[4].
Strengths: Diverse range of environmental applications; focus on sustainable industrial processes; expertise in catalyst design and synthesis. Weaknesses: Limited large-scale implementation data; potential challenges in regeneration and reuse of MISH-based materials in some applications.
Innovative Approaches in Toxicology Studies
Long term-stabilized magnesium hydroxide suspension for covering iron mineral, a process for its production and application
PatentInactiveUS20030141485A1
Innovation
- A 50-60% magnesium hydroxide suspension with a particle size of 2 microns, anionic polyelectrolytes as dispersants, and an adherent compound like GBC200, which maintains stability for at least three months without substantial agitation, ensuring effective adhesion and preventing agglomeration during high-temperature treatments.
Eco-friendly chemical composition for forming metal oxide film having excellent paint adhesion on metal surface, and method for using same
PatentWO2023063492A1
Innovation
- A chemical composition comprising ferric ions, zirconium ions, and nitrate radicals, with a pH range of 1.5 to 5.0, that forms a metal oxide film with excellent paint adhesion on metal surfaces, using industrial water and avoiding harmful ions like zinc, manganese, and phosphorus, and does not require silane coupling agents.
Regulatory Framework for Mineral Applications
The regulatory framework for mineral applications, including magnesium iron silicate hydroxide, is a complex and evolving landscape that varies across different jurisdictions. In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use and environmental impact of mineral-based products. Under the Toxic Substances Control Act (TSCA), the EPA has the authority to evaluate and regulate new and existing chemicals, including mineral-derived substances.
For magnesium iron silicate hydroxide applications, manufacturers must comply with the TSCA's New Chemicals Program if the substance is not already on the TSCA Inventory. This process involves submitting a Pre-Manufacture Notice (PMN) to the EPA, which includes detailed information about the substance's chemical identity, production volume, intended uses, and potential environmental and health impacts.
In the European Union, the regulatory framework is governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers of magnesium iron silicate hydroxide must register the substance with the European Chemicals Agency (ECHA) if they produce or import more than one tonne per year. The registration process requires comprehensive data on the substance's properties, uses, and potential risks.
Additionally, specific regulations may apply depending on the intended application of magnesium iron silicate hydroxide. For instance, if used in cosmetics, it must comply with the EU Cosmetics Regulation (EC) No 1223/2009, which sets safety standards and requires a safety assessment for each product. In the food industry, it may fall under the purview of food additive regulations, such as those set by the European Food Safety Authority (EFSA) or the U.S. Food and Drug Administration (FDA).
Environmental safety considerations are paramount in these regulatory frameworks. Both the EPA and ECHA require thorough environmental risk assessments, including data on biodegradation, bioaccumulation potential, and ecotoxicity. These assessments help determine whether the substance poses any significant risks to aquatic or terrestrial ecosystems.
Furthermore, many countries have implemented specific regulations for nanomaterials, which may apply to certain forms of magnesium iron silicate hydroxide. For example, the EU has introduced nano-specific provisions in REACH and the Cosmetics Regulation, requiring additional safety data and labeling for nanomaterials.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the lifecycle impact of mineral applications. This includes considerations of sustainable sourcing, energy-efficient production methods, and end-of-life disposal or recycling options. Manufacturers of magnesium iron silicate hydroxide products must stay abreast of these evolving regulations to ensure compliance and maintain market access.
For magnesium iron silicate hydroxide applications, manufacturers must comply with the TSCA's New Chemicals Program if the substance is not already on the TSCA Inventory. This process involves submitting a Pre-Manufacture Notice (PMN) to the EPA, which includes detailed information about the substance's chemical identity, production volume, intended uses, and potential environmental and health impacts.
In the European Union, the regulatory framework is governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers of magnesium iron silicate hydroxide must register the substance with the European Chemicals Agency (ECHA) if they produce or import more than one tonne per year. The registration process requires comprehensive data on the substance's properties, uses, and potential risks.
Additionally, specific regulations may apply depending on the intended application of magnesium iron silicate hydroxide. For instance, if used in cosmetics, it must comply with the EU Cosmetics Regulation (EC) No 1223/2009, which sets safety standards and requires a safety assessment for each product. In the food industry, it may fall under the purview of food additive regulations, such as those set by the European Food Safety Authority (EFSA) or the U.S. Food and Drug Administration (FDA).
Environmental safety considerations are paramount in these regulatory frameworks. Both the EPA and ECHA require thorough environmental risk assessments, including data on biodegradation, bioaccumulation potential, and ecotoxicity. These assessments help determine whether the substance poses any significant risks to aquatic or terrestrial ecosystems.
Furthermore, many countries have implemented specific regulations for nanomaterials, which may apply to certain forms of magnesium iron silicate hydroxide. For example, the EU has introduced nano-specific provisions in REACH and the Cosmetics Regulation, requiring additional safety data and labeling for nanomaterials.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the lifecycle impact of mineral applications. This includes considerations of sustainable sourcing, energy-efficient production methods, and end-of-life disposal or recycling options. Manufacturers of magnesium iron silicate hydroxide products must stay abreast of these evolving regulations to ensure compliance and maintain market access.
Life Cycle Assessment of Magnesium Iron Silicate Hydroxide
Life Cycle Assessment (LCA) of Magnesium Iron Silicate Hydroxide (MISH) is a crucial tool for evaluating the environmental impacts associated with this material throughout its entire lifecycle. This comprehensive analysis encompasses raw material extraction, production processes, use phase, and end-of-life disposal or recycling.
The extraction phase of MISH involves mining operations, which can have significant environmental implications. These include land use changes, habitat disruption, and potential water pollution. The energy consumption and emissions associated with extraction machinery also contribute to the overall environmental footprint.
Production processes for MISH typically involve high-temperature reactions and chemical treatments. These steps require substantial energy inputs, often derived from fossil fuels, leading to greenhouse gas emissions. Additionally, the use of chemical reagents in production may result in the generation of hazardous waste streams that require careful management and disposal.
During the use phase, MISH applications demonstrate several environmental benefits. Its fire-retardant properties can enhance the safety of various products, potentially reducing the environmental impact of fire incidents. Moreover, MISH's use in lightweight materials can contribute to improved fuel efficiency in transportation applications, indirectly lowering carbon emissions.
End-of-life considerations for MISH-containing products are critical in the LCA. The recyclability of these materials varies depending on the specific application and product design. In some cases, MISH can be recovered and reused, reducing the demand for virgin material extraction. However, challenges may arise in separating MISH from composite materials, potentially limiting recycling options.
The LCA also examines the potential for MISH to leach into the environment during use or after disposal. This aspect is particularly important for assessing long-term ecological impacts and potential bioaccumulation in ecosystems. Studies have shown that the leaching behavior of MISH is generally low, but it can vary depending on environmental conditions and the specific formulation of the material.
Water consumption throughout the MISH lifecycle is another crucial factor evaluated in the LCA. From mining operations to production processes and potential leaching during use, the impact on water resources must be carefully assessed. This includes both quantitative water use and qualitative aspects such as changes in water chemistry and potential impacts on aquatic ecosystems.
In conclusion, the Life Cycle Assessment of Magnesium Iron Silicate Hydroxide provides a holistic view of its environmental performance. While MISH offers certain environmental benefits in its applications, the LCA highlights areas where improvements in production processes and end-of-life management could further enhance its overall sustainability profile.
The extraction phase of MISH involves mining operations, which can have significant environmental implications. These include land use changes, habitat disruption, and potential water pollution. The energy consumption and emissions associated with extraction machinery also contribute to the overall environmental footprint.
Production processes for MISH typically involve high-temperature reactions and chemical treatments. These steps require substantial energy inputs, often derived from fossil fuels, leading to greenhouse gas emissions. Additionally, the use of chemical reagents in production may result in the generation of hazardous waste streams that require careful management and disposal.
During the use phase, MISH applications demonstrate several environmental benefits. Its fire-retardant properties can enhance the safety of various products, potentially reducing the environmental impact of fire incidents. Moreover, MISH's use in lightweight materials can contribute to improved fuel efficiency in transportation applications, indirectly lowering carbon emissions.
End-of-life considerations for MISH-containing products are critical in the LCA. The recyclability of these materials varies depending on the specific application and product design. In some cases, MISH can be recovered and reused, reducing the demand for virgin material extraction. However, challenges may arise in separating MISH from composite materials, potentially limiting recycling options.
The LCA also examines the potential for MISH to leach into the environment during use or after disposal. This aspect is particularly important for assessing long-term ecological impacts and potential bioaccumulation in ecosystems. Studies have shown that the leaching behavior of MISH is generally low, but it can vary depending on environmental conditions and the specific formulation of the material.
Water consumption throughout the MISH lifecycle is another crucial factor evaluated in the LCA. From mining operations to production processes and potential leaching during use, the impact on water resources must be carefully assessed. This includes both quantitative water use and qualitative aspects such as changes in water chemistry and potential impacts on aquatic ecosystems.
In conclusion, the Life Cycle Assessment of Magnesium Iron Silicate Hydroxide provides a holistic view of its environmental performance. While MISH offers certain environmental benefits in its applications, the LCA highlights areas where improvements in production processes and end-of-life management could further enhance its overall sustainability profile.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!