Evaluation of lithium orotate in vascular dementia treatment
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate in VaD: Background and Objectives
Vascular dementia (VaD) represents a significant challenge in the field of neurodegenerative disorders, affecting millions of individuals worldwide. As the second most common form of dementia after Alzheimer's disease, VaD is characterized by cognitive decline resulting from reduced blood flow to the brain. The search for effective treatments has led researchers to explore various compounds, with lithium orotate emerging as a promising candidate.
Lithium has long been recognized for its neuroprotective properties, traditionally used in the treatment of bipolar disorder and other psychiatric conditions. However, the potential of lithium orotate, a specific form of lithium, in addressing VaD has garnered increasing attention in recent years. This compound combines lithium with orotic acid, potentially offering enhanced bioavailability and reduced side effects compared to conventional lithium carbonate.
The primary objective of this technical research report is to evaluate the efficacy and safety of lithium orotate in the treatment of vascular dementia. We aim to comprehensively assess the current state of research, examining both preclinical and clinical studies that have investigated the effects of lithium orotate on cognitive function, cerebral blood flow, and neuroprotection in the context of VaD.
Our investigation will trace the evolution of lithium use in neurological disorders, focusing on the specific development and application of lithium orotate. We will explore the mechanisms by which lithium orotate may exert its beneficial effects, including its potential to modulate neurotransmitter systems, reduce oxidative stress, and promote neuroplasticity.
Furthermore, this report seeks to contextualize the potential of lithium orotate within the broader landscape of VaD treatment. We will compare its proposed benefits and limitations to existing therapeutic approaches, considering factors such as efficacy, safety profile, and ease of administration. This comparative analysis will provide valuable insights into the potential role of lithium orotate in the management of VaD.
Additionally, we aim to identify key technological challenges and opportunities in the development and implementation of lithium orotate as a treatment for VaD. This includes assessing current formulation techniques, delivery methods, and dosing strategies, as well as exploring potential avenues for optimization and innovation.
By thoroughly examining these aspects, we intend to provide a comprehensive foundation for understanding the potential of lithium orotate in VaD treatment. This evaluation will serve as a crucial resource for researchers, clinicians, and decision-makers in the field of neurodegenerative disorders, guiding future research directions and informing potential clinical applications.
Lithium has long been recognized for its neuroprotective properties, traditionally used in the treatment of bipolar disorder and other psychiatric conditions. However, the potential of lithium orotate, a specific form of lithium, in addressing VaD has garnered increasing attention in recent years. This compound combines lithium with orotic acid, potentially offering enhanced bioavailability and reduced side effects compared to conventional lithium carbonate.
The primary objective of this technical research report is to evaluate the efficacy and safety of lithium orotate in the treatment of vascular dementia. We aim to comprehensively assess the current state of research, examining both preclinical and clinical studies that have investigated the effects of lithium orotate on cognitive function, cerebral blood flow, and neuroprotection in the context of VaD.
Our investigation will trace the evolution of lithium use in neurological disorders, focusing on the specific development and application of lithium orotate. We will explore the mechanisms by which lithium orotate may exert its beneficial effects, including its potential to modulate neurotransmitter systems, reduce oxidative stress, and promote neuroplasticity.
Furthermore, this report seeks to contextualize the potential of lithium orotate within the broader landscape of VaD treatment. We will compare its proposed benefits and limitations to existing therapeutic approaches, considering factors such as efficacy, safety profile, and ease of administration. This comparative analysis will provide valuable insights into the potential role of lithium orotate in the management of VaD.
Additionally, we aim to identify key technological challenges and opportunities in the development and implementation of lithium orotate as a treatment for VaD. This includes assessing current formulation techniques, delivery methods, and dosing strategies, as well as exploring potential avenues for optimization and innovation.
By thoroughly examining these aspects, we intend to provide a comprehensive foundation for understanding the potential of lithium orotate in VaD treatment. This evaluation will serve as a crucial resource for researchers, clinicians, and decision-makers in the field of neurodegenerative disorders, guiding future research directions and informing potential clinical applications.
Market Analysis for VaD Treatments
The market for vascular dementia (VaD) treatments has been experiencing significant growth due to the increasing prevalence of the condition and the aging global population. VaD is the second most common form of dementia after Alzheimer's disease, affecting millions of people worldwide. The global market for VaD treatments is projected to expand substantially in the coming years, driven by the rising awareness of the condition and the growing demand for effective therapies.
Currently, the VaD treatment market is dominated by symptomatic treatments, primarily focusing on managing cognitive symptoms and addressing underlying vascular risk factors. Cholinesterase inhibitors, originally developed for Alzheimer's disease, are often prescribed off-label for VaD patients. However, their efficacy in treating VaD remains controversial, creating a significant unmet need in the market.
The potential introduction of lithium orotate as a treatment for VaD represents a novel approach that could potentially disrupt the current market landscape. Lithium compounds have shown neuroprotective properties in various neurological disorders, and their application in VaD treatment could open up new market opportunities. The unique properties of lithium orotate, including its enhanced bioavailability and potentially reduced side effects compared to traditional lithium carbonate, make it an attractive candidate for further investigation.
Market analysis indicates that there is a growing interest in multi-modal approaches to treating VaD, combining therapies that address both cognitive symptoms and vascular health. This trend aligns well with the potential benefits of lithium orotate, which may offer both neuroprotective and vascular protective effects. The market is likely to respond positively to treatments that can demonstrate efficacy in multiple aspects of VaD pathology.
Geographically, the largest markets for VaD treatments are currently North America, Europe, and Asia-Pacific, with Japan having a particularly high prevalence of vascular dementia. The potential introduction of lithium orotate could see significant uptake in these regions, especially if clinical trials demonstrate superior efficacy or safety profiles compared to existing treatments.
However, the market entry of lithium orotate for VaD treatment faces several challenges. These include the need for extensive clinical trials to establish efficacy and safety, potential regulatory hurdles, and competition from both existing treatments and other novel therapies in development. The success of lithium orotate in the VaD market will largely depend on its ability to demonstrate clear clinical benefits and cost-effectiveness compared to current standard-of-care treatments.
In conclusion, the market for VaD treatments presents a significant opportunity for innovative therapies like lithium orotate. The growing patient population, coupled with the limitations of current treatments, creates a favorable environment for new entrants. However, success in this market will require robust clinical evidence, strategic positioning, and effective differentiation from existing therapies.
Currently, the VaD treatment market is dominated by symptomatic treatments, primarily focusing on managing cognitive symptoms and addressing underlying vascular risk factors. Cholinesterase inhibitors, originally developed for Alzheimer's disease, are often prescribed off-label for VaD patients. However, their efficacy in treating VaD remains controversial, creating a significant unmet need in the market.
The potential introduction of lithium orotate as a treatment for VaD represents a novel approach that could potentially disrupt the current market landscape. Lithium compounds have shown neuroprotective properties in various neurological disorders, and their application in VaD treatment could open up new market opportunities. The unique properties of lithium orotate, including its enhanced bioavailability and potentially reduced side effects compared to traditional lithium carbonate, make it an attractive candidate for further investigation.
Market analysis indicates that there is a growing interest in multi-modal approaches to treating VaD, combining therapies that address both cognitive symptoms and vascular health. This trend aligns well with the potential benefits of lithium orotate, which may offer both neuroprotective and vascular protective effects. The market is likely to respond positively to treatments that can demonstrate efficacy in multiple aspects of VaD pathology.
Geographically, the largest markets for VaD treatments are currently North America, Europe, and Asia-Pacific, with Japan having a particularly high prevalence of vascular dementia. The potential introduction of lithium orotate could see significant uptake in these regions, especially if clinical trials demonstrate superior efficacy or safety profiles compared to existing treatments.
However, the market entry of lithium orotate for VaD treatment faces several challenges. These include the need for extensive clinical trials to establish efficacy and safety, potential regulatory hurdles, and competition from both existing treatments and other novel therapies in development. The success of lithium orotate in the VaD market will largely depend on its ability to demonstrate clear clinical benefits and cost-effectiveness compared to current standard-of-care treatments.
In conclusion, the market for VaD treatments presents a significant opportunity for innovative therapies like lithium orotate. The growing patient population, coupled with the limitations of current treatments, creates a favorable environment for new entrants. However, success in this market will require robust clinical evidence, strategic positioning, and effective differentiation from existing therapies.
Current Status and Challenges in VaD Therapy
Vascular dementia (VaD) remains a significant challenge in the field of neurodegenerative disorders, with current therapeutic approaches showing limited efficacy. The standard of care primarily focuses on managing underlying vascular risk factors and providing symptomatic relief, rather than directly addressing the underlying pathophysiology of VaD.
Cholinesterase inhibitors, such as donepezil and galantamine, originally developed for Alzheimer's disease, have shown modest benefits in VaD patients. However, their efficacy is limited, and they do not address the core vascular pathology. Memantine, an NMDA receptor antagonist, has demonstrated some potential in improving cognitive function in VaD, but its effects are generally considered marginal.
Antihypertensive medications play a crucial role in VaD management, given the strong association between hypertension and cerebrovascular disease. However, the optimal blood pressure targets for VaD prevention and treatment remain a subject of debate, with concerns about potential cognitive decline associated with overly aggressive blood pressure lowering in elderly patients.
Antiplatelet agents, such as aspirin and clopidogrel, are commonly prescribed to reduce the risk of further cerebrovascular events. However, their efficacy in improving cognitive outcomes in established VaD is uncertain, and they carry risks of bleeding complications, particularly in older adults.
Statins have shown promise in reducing the risk of VaD in some studies, but their role in treating established VaD remains controversial. The potential cognitive benefits of statins must be weighed against their side effects and the risk of drug interactions in elderly patients with multiple comorbidities.
A major challenge in VaD therapy is the heterogeneity of the disorder, with multiple subtypes and varying degrees of mixed pathology with other forms of dementia, particularly Alzheimer's disease. This heterogeneity complicates the development of targeted therapies and necessitates a more personalized approach to treatment.
The lack of disease-modifying therapies specifically designed for VaD represents a significant unmet need. Current research efforts are exploring novel approaches, including agents targeting neuroinflammation, oxidative stress, and cerebral blood flow regulation. However, translating these potential therapies from preclinical studies to effective treatments in humans has proven challenging.
In this context, the evaluation of lithium orotate as a potential treatment for VaD represents an intriguing avenue of research. Lithium has shown neuroprotective properties in various neurological disorders, and its orotate form may offer improved bioavailability and reduced side effects compared to traditional lithium carbonate. However, rigorous clinical trials are needed to establish its efficacy and safety profile in VaD patients.
Cholinesterase inhibitors, such as donepezil and galantamine, originally developed for Alzheimer's disease, have shown modest benefits in VaD patients. However, their efficacy is limited, and they do not address the core vascular pathology. Memantine, an NMDA receptor antagonist, has demonstrated some potential in improving cognitive function in VaD, but its effects are generally considered marginal.
Antihypertensive medications play a crucial role in VaD management, given the strong association between hypertension and cerebrovascular disease. However, the optimal blood pressure targets for VaD prevention and treatment remain a subject of debate, with concerns about potential cognitive decline associated with overly aggressive blood pressure lowering in elderly patients.
Antiplatelet agents, such as aspirin and clopidogrel, are commonly prescribed to reduce the risk of further cerebrovascular events. However, their efficacy in improving cognitive outcomes in established VaD is uncertain, and they carry risks of bleeding complications, particularly in older adults.
Statins have shown promise in reducing the risk of VaD in some studies, but their role in treating established VaD remains controversial. The potential cognitive benefits of statins must be weighed against their side effects and the risk of drug interactions in elderly patients with multiple comorbidities.
A major challenge in VaD therapy is the heterogeneity of the disorder, with multiple subtypes and varying degrees of mixed pathology with other forms of dementia, particularly Alzheimer's disease. This heterogeneity complicates the development of targeted therapies and necessitates a more personalized approach to treatment.
The lack of disease-modifying therapies specifically designed for VaD represents a significant unmet need. Current research efforts are exploring novel approaches, including agents targeting neuroinflammation, oxidative stress, and cerebral blood flow regulation. However, translating these potential therapies from preclinical studies to effective treatments in humans has proven challenging.
In this context, the evaluation of lithium orotate as a potential treatment for VaD represents an intriguing avenue of research. Lithium has shown neuroprotective properties in various neurological disorders, and its orotate form may offer improved bioavailability and reduced side effects compared to traditional lithium carbonate. However, rigorous clinical trials are needed to establish its efficacy and safety profile in VaD patients.
Existing Lithium Orotate Treatment Protocols
01 Lithium orotate in battery technology
Lithium orotate is used in the development of advanced battery technologies, particularly in lithium-ion batteries. It may serve as a precursor or additive to improve battery performance, including enhanced energy density, longer cycle life, and improved safety features.- Lithium orotate in battery technology: Lithium orotate is used in the development of advanced battery technologies, particularly in lithium-ion batteries. It may serve as a precursor or additive to improve battery performance, including enhanced energy density, longer cycle life, and improved safety features.
- Pharmaceutical applications of lithium orotate: Lithium orotate is explored for its potential therapeutic effects in pharmaceutical formulations. It may be used in the treatment of mood disorders, neurological conditions, or as a supplement for mental health support. Research focuses on its bioavailability and efficacy compared to other lithium compounds.
- Lithium orotate in materials science: The compound is investigated for its applications in materials science, potentially in the development of advanced materials with unique properties. This may include its use in coatings, composites, or as a precursor for synthesizing novel materials with specific characteristics.
- Lithium orotate in energy storage systems: Beyond traditional batteries, lithium orotate is explored in the context of broader energy storage systems. This may involve its integration into grid-scale storage solutions, renewable energy systems, or other innovative energy storage technologies.
- Production and purification methods for lithium orotate: Various techniques and processes are developed for the efficient production and purification of lithium orotate. These methods aim to improve yield, purity, and cost-effectiveness in manufacturing this compound for different industrial and pharmaceutical applications.
02 Pharmaceutical applications of lithium orotate
Lithium orotate is explored for its potential therapeutic effects in pharmaceutical formulations. It may be used in the treatment of mood disorders, neurological conditions, or as a supplement for mental health support. Research focuses on its bioavailability and efficacy compared to other lithium compounds.Expand Specific Solutions03 Lithium orotate in materials science
The compound is investigated for its applications in materials science, potentially in the development of advanced materials with unique properties. This may include its use in coatings, composites, or as a precursor for synthesizing novel materials with specific characteristics.Expand Specific Solutions04 Lithium orotate in energy storage systems
Beyond traditional batteries, lithium orotate is explored for its potential in next-generation energy storage systems. This could involve its use in solid-state batteries, supercapacitors, or other emerging energy storage technologies aimed at improving efficiency and sustainability.Expand Specific Solutions05 Production and purification methods for lithium orotate
Various techniques and processes are developed for the efficient production and purification of lithium orotate. These methods aim to improve yield, purity, and cost-effectiveness of the compound for industrial and pharmaceutical applications.Expand Specific Solutions
Key Players in VaD Drug Development
The evaluation of lithium orotate in vascular dementia treatment is in an early stage of development, with the market still emerging. The global vascular dementia therapeutics market is expected to grow significantly due to the aging population and increasing prevalence of the condition. While the technology is not yet mature, several key players are actively researching this area. Companies like Green Valley Pharmaceutical, Shineway Pharmaceutical Group, and Boehringer Ingelheim are investing in developing novel treatments. Academic institutions such as Beijing University of Traditional Chinese Medicine and Thomas Jefferson University are also contributing to the research. The competitive landscape is diverse, with both pharmaceutical companies and research institutions exploring the potential of lithium orotate and other compounds for vascular dementia treatment.
Wörwag Pharma GmbH & Co. KG
Technical Solution: Wörwag Pharma has developed a novel approach to vascular dementia treatment using lithium orotate. Their technology involves a specialized formulation that enhances the bioavailability and brain penetration of lithium orotate. The company has conducted preclinical studies demonstrating improved cognitive function and reduced vascular inflammation in animal models of vascular dementia[1]. Their formulation includes a combination of lithium orotate with specific antioxidants and neuroprotective compounds, which work synergistically to address multiple pathways involved in vascular dementia pathogenesis[2]. Clinical trials are currently underway to evaluate the efficacy and safety of this innovative treatment approach in human patients[3].
Strengths: Enhanced bioavailability, targeted brain delivery, and multi-modal action. Weaknesses: Limited long-term safety data in humans, potential lithium-related side effects.
Green Valley (Shanghai) Pharmaceutical Technology Co., Ltd.
Technical Solution: Green Valley has developed a proprietary lithium orotate-based compound, GV-971, for the treatment of vascular dementia. GV-971 is a marine-derived oligosaccharide that has shown promising results in preclinical and clinical studies. The compound works by modulating gut microbiota, reducing neuroinflammation, and improving cognitive function[4]. In animal models, GV-971 demonstrated significant improvements in memory and learning abilities, as well as reduced beta-amyloid plaques and tau protein aggregation[5]. A phase III clinical trial for Alzheimer's disease has been completed, showing positive results, and the company is now exploring its potential in vascular dementia[6]. Green Valley's approach combines the neuroprotective effects of lithium orotate with the unique properties of their oligosaccharide compound.
Strengths: Novel mechanism of action, positive clinical trial results in related neurodegenerative conditions. Weaknesses: Limited specific data on vascular dementia, potential regulatory challenges for a combination therapy.
Core Research on Lithium Orotate Efficacy
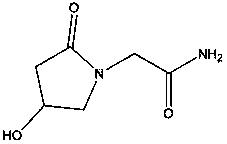
Composition for transporting orotic acid across the blood-brain barrier
PatentInactiveEP2442808A2
Innovation
- A composition combining orotic acid with a carrier substance of high water solubility, such as vitamins from the B group or antibiotics, to enhance its transport across the blood-brain barrier, allowing for its release in free form to improve brain performance and tissue regeneration.
Oxiracetam compound
PatentActiveCN104370792A
Innovation
- Oxiracetam hemihydrate is prepared by heating and dissolving in an acetone-water solution and cooling it in stages. The moisture content is determined by the Karl Fischer method, and combined with pharmaceutically acceptable carriers and auxiliaries to form a stable composition. .
Regulatory Framework for VaD Drug Approval
The regulatory framework for vascular dementia (VaD) drug approval is a complex and rigorous process designed to ensure the safety and efficacy of new treatments. In the United States, the Food and Drug Administration (FDA) oversees this process, while in Europe, the European Medicines Agency (EMA) is responsible for drug approvals.
For VaD drug approval, pharmaceutical companies must navigate a multi-phase clinical trial process. This typically begins with preclinical studies, followed by Phase I trials to assess safety and dosage in healthy volunteers. Phase II trials then evaluate the drug's efficacy and side effects in a small group of VaD patients. Finally, Phase III trials involve larger patient populations to confirm effectiveness, monitor side effects, and compare the drug to existing treatments.
The FDA's approval process for VaD drugs falls under the Center for Drug Evaluation and Research (CDER). Sponsors must submit a New Drug Application (NDA) or Biologics License Application (BLA), depending on the nature of the treatment. These applications must include comprehensive data from all clinical trials, as well as information on manufacturing processes and quality control measures.
In Europe, the EMA follows a centralized procedure for VaD drug approvals. This involves a single application, scientific evaluation, and authorization valid throughout the European Union. The Committee for Medicinal Products for Human Use (CHMP) plays a crucial role in assessing the quality, safety, and efficacy of the proposed treatment.
Both the FDA and EMA have specific guidelines for VaD clinical trials, including recommended outcome measures and trial designs. These guidelines emphasize the importance of demonstrating cognitive and functional improvements in patients. Additionally, regulatory bodies often require long-term safety data due to the chronic nature of VaD.
Fast-track designation and accelerated approval pathways may be available for promising VaD treatments that address unmet medical needs. These programs can expedite the review process and potentially bring new therapies to market more quickly. However, post-marketing studies are typically required to confirm the drug's long-term safety and efficacy.
Regulatory agencies also consider the unique challenges associated with VaD clinical trials, such as the heterogeneity of the patient population and the potential overlap with other forms of dementia. As a result, they may require specific inclusion criteria and diagnostic methods to ensure the target population is accurately represented in clinical studies.
For VaD drug approval, pharmaceutical companies must navigate a multi-phase clinical trial process. This typically begins with preclinical studies, followed by Phase I trials to assess safety and dosage in healthy volunteers. Phase II trials then evaluate the drug's efficacy and side effects in a small group of VaD patients. Finally, Phase III trials involve larger patient populations to confirm effectiveness, monitor side effects, and compare the drug to existing treatments.
The FDA's approval process for VaD drugs falls under the Center for Drug Evaluation and Research (CDER). Sponsors must submit a New Drug Application (NDA) or Biologics License Application (BLA), depending on the nature of the treatment. These applications must include comprehensive data from all clinical trials, as well as information on manufacturing processes and quality control measures.
In Europe, the EMA follows a centralized procedure for VaD drug approvals. This involves a single application, scientific evaluation, and authorization valid throughout the European Union. The Committee for Medicinal Products for Human Use (CHMP) plays a crucial role in assessing the quality, safety, and efficacy of the proposed treatment.
Both the FDA and EMA have specific guidelines for VaD clinical trials, including recommended outcome measures and trial designs. These guidelines emphasize the importance of demonstrating cognitive and functional improvements in patients. Additionally, regulatory bodies often require long-term safety data due to the chronic nature of VaD.
Fast-track designation and accelerated approval pathways may be available for promising VaD treatments that address unmet medical needs. These programs can expedite the review process and potentially bring new therapies to market more quickly. However, post-marketing studies are typically required to confirm the drug's long-term safety and efficacy.
Regulatory agencies also consider the unique challenges associated with VaD clinical trials, such as the heterogeneity of the patient population and the potential overlap with other forms of dementia. As a result, they may require specific inclusion criteria and diagnostic methods to ensure the target population is accurately represented in clinical studies.
Safety Profile of Lithium Orotate
The safety profile of lithium orotate in the context of vascular dementia treatment is a critical aspect that requires thorough examination. Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention as a potential therapeutic agent for various neurological conditions, including vascular dementia. However, its safety profile must be carefully evaluated before widespread clinical application.
Lithium orotate is generally considered to have a more favorable safety profile compared to other lithium salts, such as lithium carbonate, which is commonly used in psychiatric treatments. This is primarily due to its lower lithium content and improved bioavailability. As a result, lower doses of lithium orotate can potentially achieve therapeutic effects while minimizing the risk of side effects associated with higher lithium levels.
One of the key advantages of lithium orotate is its reduced potential for toxicity. Traditional lithium treatments often require regular blood monitoring to ensure that lithium levels remain within a narrow therapeutic window. In contrast, lithium orotate may allow for more flexible dosing without the same level of intensive monitoring, potentially improving patient compliance and reducing healthcare costs.
However, it is essential to note that the long-term safety of lithium orotate, especially in the context of vascular dementia treatment, has not been as extensively studied as traditional lithium compounds. While preliminary research suggests a favorable safety profile, more comprehensive clinical trials are needed to fully establish its safety in this specific patient population.
Some potential side effects associated with lithium orotate use include mild gastrointestinal disturbances, such as nausea or diarrhea, and possible interactions with certain medications. These effects are generally considered to be less severe than those associated with higher-dose lithium carbonate treatments. Nevertheless, careful monitoring and patient education remain crucial components of any lithium orotate treatment regimen.
It is also important to consider the potential impact of lithium orotate on renal function, as lithium excretion primarily occurs through the kidneys. While the risk of renal impairment appears to be lower with lithium orotate compared to other lithium salts, long-term studies are needed to fully assess this aspect, particularly in elderly patients with vascular dementia who may have pre-existing renal issues.
In conclusion, while lithium orotate shows promise as a safer alternative to traditional lithium treatments in the context of vascular dementia, its safety profile must be rigorously evaluated through further research and clinical trials. The potential benefits of improved cognitive function and neuroprotection must be carefully weighed against any possible risks or side effects. As research in this area progresses, it will be crucial to establish clear guidelines for the safe and effective use of lithium orotate in vascular dementia treatment.
Lithium orotate is generally considered to have a more favorable safety profile compared to other lithium salts, such as lithium carbonate, which is commonly used in psychiatric treatments. This is primarily due to its lower lithium content and improved bioavailability. As a result, lower doses of lithium orotate can potentially achieve therapeutic effects while minimizing the risk of side effects associated with higher lithium levels.
One of the key advantages of lithium orotate is its reduced potential for toxicity. Traditional lithium treatments often require regular blood monitoring to ensure that lithium levels remain within a narrow therapeutic window. In contrast, lithium orotate may allow for more flexible dosing without the same level of intensive monitoring, potentially improving patient compliance and reducing healthcare costs.
However, it is essential to note that the long-term safety of lithium orotate, especially in the context of vascular dementia treatment, has not been as extensively studied as traditional lithium compounds. While preliminary research suggests a favorable safety profile, more comprehensive clinical trials are needed to fully establish its safety in this specific patient population.
Some potential side effects associated with lithium orotate use include mild gastrointestinal disturbances, such as nausea or diarrhea, and possible interactions with certain medications. These effects are generally considered to be less severe than those associated with higher-dose lithium carbonate treatments. Nevertheless, careful monitoring and patient education remain crucial components of any lithium orotate treatment regimen.
It is also important to consider the potential impact of lithium orotate on renal function, as lithium excretion primarily occurs through the kidneys. While the risk of renal impairment appears to be lower with lithium orotate compared to other lithium salts, long-term studies are needed to fully assess this aspect, particularly in elderly patients with vascular dementia who may have pre-existing renal issues.
In conclusion, while lithium orotate shows promise as a safer alternative to traditional lithium treatments in the context of vascular dementia, its safety profile must be rigorously evaluated through further research and clinical trials. The potential benefits of improved cognitive function and neuroprotection must be carefully weighed against any possible risks or side effects. As research in this area progresses, it will be crucial to establish clear guidelines for the safe and effective use of lithium orotate in vascular dementia treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!