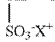Exploring Sodium Percarbonate's Benefits in Drinking Water Systems
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate in Water Treatment: Background and Objectives
Sodium percarbonate has emerged as a promising compound in the field of water treatment, particularly for drinking water systems. This innovative chemical, also known as sodium carbonate peroxyhydrate, has garnered attention due to its unique properties and potential benefits in water purification processes.
The development of sodium percarbonate can be traced back to the early 20th century, with its initial applications primarily in the laundry and cleaning industry. However, recent advancements in water treatment technologies have led to a renewed interest in exploring its potential for improving drinking water quality. The compound's ability to release hydrogen peroxide and sodium carbonate upon dissolution in water has made it an attractive option for addressing various water treatment challenges.
The evolution of sodium percarbonate in water treatment has been driven by the growing global demand for safe and clean drinking water. As populations increase and urbanization accelerates, the need for efficient and cost-effective water treatment solutions has become more pressing. Traditional methods of water treatment, while effective, often involve complex processes and the use of multiple chemicals. Sodium percarbonate offers the potential to simplify these processes while maintaining or even improving water quality.
In recent years, research efforts have focused on understanding the mechanisms by which sodium percarbonate interacts with various contaminants in water. Studies have shown promising results in its ability to oxidize organic compounds, inactivate pathogens, and remove certain heavy metals. These findings have paved the way for exploring sodium percarbonate's application in both centralized water treatment facilities and point-of-use systems.
The primary objectives of investigating sodium percarbonate in drinking water systems are multifaceted. Firstly, researchers aim to establish its efficacy in removing a wide range of contaminants, including microorganisms, organic pollutants, and inorganic compounds. Secondly, there is a focus on optimizing the dosage and application methods to ensure maximum effectiveness while minimizing any potential negative impacts on water quality or human health.
Another key objective is to assess the economic viability of incorporating sodium percarbonate into existing water treatment infrastructures. This includes evaluating its cost-effectiveness compared to traditional treatment methods and exploring potential synergies with other treatment processes. Additionally, researchers are investigating the long-term stability and storage requirements of sodium percarbonate to ensure its practicality in various environmental conditions.
As environmental regulations become more stringent and public awareness of water quality issues grows, the exploration of sodium percarbonate's benefits in drinking water systems aligns with the broader goal of developing sustainable and environmentally friendly water treatment solutions. By leveraging the unique properties of this compound, researchers and water treatment professionals aim to address current challenges in water purification while anticipating future needs in this critical field.
The development of sodium percarbonate can be traced back to the early 20th century, with its initial applications primarily in the laundry and cleaning industry. However, recent advancements in water treatment technologies have led to a renewed interest in exploring its potential for improving drinking water quality. The compound's ability to release hydrogen peroxide and sodium carbonate upon dissolution in water has made it an attractive option for addressing various water treatment challenges.
The evolution of sodium percarbonate in water treatment has been driven by the growing global demand for safe and clean drinking water. As populations increase and urbanization accelerates, the need for efficient and cost-effective water treatment solutions has become more pressing. Traditional methods of water treatment, while effective, often involve complex processes and the use of multiple chemicals. Sodium percarbonate offers the potential to simplify these processes while maintaining or even improving water quality.
In recent years, research efforts have focused on understanding the mechanisms by which sodium percarbonate interacts with various contaminants in water. Studies have shown promising results in its ability to oxidize organic compounds, inactivate pathogens, and remove certain heavy metals. These findings have paved the way for exploring sodium percarbonate's application in both centralized water treatment facilities and point-of-use systems.
The primary objectives of investigating sodium percarbonate in drinking water systems are multifaceted. Firstly, researchers aim to establish its efficacy in removing a wide range of contaminants, including microorganisms, organic pollutants, and inorganic compounds. Secondly, there is a focus on optimizing the dosage and application methods to ensure maximum effectiveness while minimizing any potential negative impacts on water quality or human health.
Another key objective is to assess the economic viability of incorporating sodium percarbonate into existing water treatment infrastructures. This includes evaluating its cost-effectiveness compared to traditional treatment methods and exploring potential synergies with other treatment processes. Additionally, researchers are investigating the long-term stability and storage requirements of sodium percarbonate to ensure its practicality in various environmental conditions.
As environmental regulations become more stringent and public awareness of water quality issues grows, the exploration of sodium percarbonate's benefits in drinking water systems aligns with the broader goal of developing sustainable and environmentally friendly water treatment solutions. By leveraging the unique properties of this compound, researchers and water treatment professionals aim to address current challenges in water purification while anticipating future needs in this critical field.
Market Analysis for Advanced Water Purification Solutions
The global market for advanced water purification solutions has been experiencing significant growth in recent years, driven by increasing concerns over water quality and the need for sustainable water management practices. The introduction of sodium percarbonate as a potential treatment option in drinking water systems represents an exciting development in this rapidly evolving sector.
Market analysis indicates that the demand for innovative water treatment technologies is on the rise, with a particular focus on solutions that offer improved efficiency, cost-effectiveness, and environmental sustainability. Sodium percarbonate, known for its powerful oxidizing properties and eco-friendly nature, aligns well with these market requirements, positioning it as a promising contender in the advanced water purification landscape.
The drinking water treatment market is characterized by a diverse range of stakeholders, including municipal water authorities, private water companies, industrial users, and residential consumers. Each of these segments presents unique opportunities and challenges for the adoption of sodium percarbonate-based solutions. Municipal water treatment facilities, in particular, are showing increased interest in technologies that can effectively address emerging contaminants while minimizing the use of harsh chemicals.
Consumer awareness regarding water quality issues has been growing steadily, leading to a surge in demand for point-of-use and point-of-entry water treatment systems. This trend creates a potential market for sodium percarbonate-based products in the residential sector, especially in regions with inadequate public water infrastructure or persistent water quality concerns.
The industrial sector, including food and beverage manufacturing, pharmaceuticals, and power generation, represents another significant market opportunity for advanced water purification solutions. These industries require high-quality water for their processes and are increasingly looking for treatment options that can meet stringent regulatory standards while also improving operational efficiency.
Geographically, the market for advanced water purification technologies shows varying levels of maturity and growth potential. Developed regions such as North America and Europe are focusing on upgrading existing water treatment infrastructure, while emerging economies in Asia-Pacific and Latin America are investing heavily in new water treatment facilities to address rapid urbanization and industrialization.
The competitive landscape in the advanced water purification market is characterized by a mix of established players and innovative startups. Major water treatment companies are actively exploring new technologies, including sodium percarbonate-based solutions, to maintain their market position and address evolving customer needs. This dynamic environment is fostering partnerships and collaborations between technology developers, equipment manufacturers, and service providers.
Market analysis indicates that the demand for innovative water treatment technologies is on the rise, with a particular focus on solutions that offer improved efficiency, cost-effectiveness, and environmental sustainability. Sodium percarbonate, known for its powerful oxidizing properties and eco-friendly nature, aligns well with these market requirements, positioning it as a promising contender in the advanced water purification landscape.
The drinking water treatment market is characterized by a diverse range of stakeholders, including municipal water authorities, private water companies, industrial users, and residential consumers. Each of these segments presents unique opportunities and challenges for the adoption of sodium percarbonate-based solutions. Municipal water treatment facilities, in particular, are showing increased interest in technologies that can effectively address emerging contaminants while minimizing the use of harsh chemicals.
Consumer awareness regarding water quality issues has been growing steadily, leading to a surge in demand for point-of-use and point-of-entry water treatment systems. This trend creates a potential market for sodium percarbonate-based products in the residential sector, especially in regions with inadequate public water infrastructure or persistent water quality concerns.
The industrial sector, including food and beverage manufacturing, pharmaceuticals, and power generation, represents another significant market opportunity for advanced water purification solutions. These industries require high-quality water for their processes and are increasingly looking for treatment options that can meet stringent regulatory standards while also improving operational efficiency.
Geographically, the market for advanced water purification technologies shows varying levels of maturity and growth potential. Developed regions such as North America and Europe are focusing on upgrading existing water treatment infrastructure, while emerging economies in Asia-Pacific and Latin America are investing heavily in new water treatment facilities to address rapid urbanization and industrialization.
The competitive landscape in the advanced water purification market is characterized by a mix of established players and innovative startups. Major water treatment companies are actively exploring new technologies, including sodium percarbonate-based solutions, to maintain their market position and address evolving customer needs. This dynamic environment is fostering partnerships and collaborations between technology developers, equipment manufacturers, and service providers.
Current Challenges in Drinking Water Disinfection
Drinking water disinfection remains a critical challenge in ensuring public health and safety worldwide. Despite significant advancements in water treatment technologies, several persistent issues continue to plague the industry. One of the primary concerns is the formation of disinfection by-products (DBPs) during traditional chlorination processes. These DBPs, such as trihalomethanes and haloacetic acids, have been linked to potential health risks, including cancer and reproductive problems.
Another significant challenge is the increasing resistance of waterborne pathogens to conventional disinfection methods. Microorganisms like Cryptosporidium and Giardia have shown resilience against chlorine-based treatments, necessitating more robust disinfection strategies. This resistance not only compromises the effectiveness of water treatment but also poses a substantial threat to public health, particularly in areas with limited access to advanced water treatment facilities.
The presence of emerging contaminants, such as pharmaceuticals, personal care products, and microplastics, further complicates the disinfection process. These contaminants are often not effectively removed by traditional treatment methods and can interfere with disinfection efficacy. Additionally, they may react with disinfectants to form new, potentially harmful compounds, exacerbating the DBP issue.
Balancing disinfection efficacy with taste and odor concerns presents another ongoing challenge. While ensuring microbial safety is paramount, excessive use of disinfectants can lead to unpalatable water, potentially driving consumers to less safe alternatives. This balance is particularly crucial in maintaining public trust and compliance with water safety measures.
The variability in source water quality, influenced by factors such as climate change, urbanization, and industrial activities, adds another layer of complexity to water disinfection. Treatment plants must adapt to fluctuating water conditions, which can affect the efficacy of disinfection processes and the formation of DBPs. This variability necessitates more flexible and adaptive treatment strategies.
Lastly, the energy intensity and environmental impact of current disinfection methods pose sustainability challenges. Many disinfection processes, particularly those involving UV irradiation or ozonation, are energy-intensive and may contribute to a significant carbon footprint. Finding more sustainable, eco-friendly disinfection solutions that maintain high efficacy remains a pressing need in the water treatment industry.
Another significant challenge is the increasing resistance of waterborne pathogens to conventional disinfection methods. Microorganisms like Cryptosporidium and Giardia have shown resilience against chlorine-based treatments, necessitating more robust disinfection strategies. This resistance not only compromises the effectiveness of water treatment but also poses a substantial threat to public health, particularly in areas with limited access to advanced water treatment facilities.
The presence of emerging contaminants, such as pharmaceuticals, personal care products, and microplastics, further complicates the disinfection process. These contaminants are often not effectively removed by traditional treatment methods and can interfere with disinfection efficacy. Additionally, they may react with disinfectants to form new, potentially harmful compounds, exacerbating the DBP issue.
Balancing disinfection efficacy with taste and odor concerns presents another ongoing challenge. While ensuring microbial safety is paramount, excessive use of disinfectants can lead to unpalatable water, potentially driving consumers to less safe alternatives. This balance is particularly crucial in maintaining public trust and compliance with water safety measures.
The variability in source water quality, influenced by factors such as climate change, urbanization, and industrial activities, adds another layer of complexity to water disinfection. Treatment plants must adapt to fluctuating water conditions, which can affect the efficacy of disinfection processes and the formation of DBPs. This variability necessitates more flexible and adaptive treatment strategies.
Lastly, the energy intensity and environmental impact of current disinfection methods pose sustainability challenges. Many disinfection processes, particularly those involving UV irradiation or ozonation, are energy-intensive and may contribute to a significant carbon footprint. Finding more sustainable, eco-friendly disinfection solutions that maintain high efficacy remains a pressing need in the water treatment industry.
Existing Sodium Percarbonate Applications in Water Systems
01 Synthesis and production of sodium percarbonate
Various methods for synthesizing and producing sodium percarbonate are described. These methods involve the reaction of sodium carbonate with hydrogen peroxide under specific conditions to form stable sodium percarbonate crystals. The processes may include steps such as crystallization, drying, and stabilization to improve the quality and stability of the final product.- Synthesis and production of sodium percarbonate: Various methods for synthesizing and producing sodium percarbonate are described. These processes typically involve the reaction of sodium carbonate with hydrogen peroxide under controlled conditions. The production methods aim to improve yield, stability, and purity of the final product.
- Stabilization of sodium percarbonate: Techniques for enhancing the stability of sodium percarbonate are discussed. These may include coating the particles, adding stabilizing agents, or modifying the crystal structure. Improved stability helps maintain the product's effectiveness during storage and use.
- Applications in cleaning and bleaching: Sodium percarbonate is widely used in cleaning and bleaching applications. It serves as an effective oxygen-based bleach in laundry detergents and other cleaning products. Its ability to release hydrogen peroxide in aqueous solutions makes it valuable for stain removal and disinfection.
- Formulation in personal care products: Sodium percarbonate is incorporated into various personal care products, such as tooth whitening formulations and hair bleaching agents. Its oxidizing properties are utilized to achieve desired cosmetic effects while maintaining safety for personal use.
- Environmental and safety considerations: Research and development efforts focus on improving the environmental profile and safety of sodium percarbonate. This includes studies on its biodegradability, ecotoxicity, and potential health effects. Efforts are made to optimize its use while minimizing any negative impacts.
02 Stabilization of sodium percarbonate
Techniques for stabilizing sodium percarbonate to improve its shelf life and performance are discussed. These may include coating the particles with stabilizing agents, incorporating additives, or modifying the crystal structure. Stabilization helps prevent decomposition and maintains the active oxygen content of the compound during storage and use.Expand Specific Solutions03 Applications in cleaning and bleaching
Sodium percarbonate is widely used in cleaning and bleaching applications. It serves as an effective oxygen-based bleaching agent in laundry detergents, dishwashing products, and other household cleaners. The compound releases hydrogen peroxide when dissolved in water, providing powerful stain removal and disinfecting properties.Expand Specific Solutions04 Formulation in personal care products
Sodium percarbonate is incorporated into various personal care products, such as tooth whitening formulations and hair bleaching agents. Its oxygen-releasing properties make it effective for removing stains and discoloration in dental and cosmetic applications. Formulations may include additional ingredients to enhance stability and efficacy.Expand Specific Solutions05 Environmental and safety considerations
Research and development efforts focus on improving the environmental profile and safety of sodium percarbonate. This includes developing more eco-friendly production processes, reducing impurities, and enhancing the biodegradability of formulations containing sodium percarbonate. Safety measures for handling and storage are also addressed to prevent accidental decomposition or reactivity.Expand Specific Solutions
Key Players in Water Treatment Industry
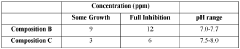
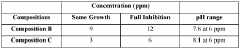
The exploration of sodium percarbonate's benefits in drinking water systems is currently in an emerging phase, with the market showing promising growth potential. The global market for water treatment chemicals, including sodium percarbonate, is expanding due to increasing water quality concerns and stringent regulations. Technologically, the field is advancing rapidly, with companies like Solvay SA, Ecolab USA, Inc., and Kemira Oyj leading innovation. These firms are developing more efficient and environmentally friendly applications of sodium percarbonate in water treatment. The technology's maturity is moderate, with ongoing research focusing on optimizing its effectiveness and sustainability in various water system contexts.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate formulations for drinking water treatment. Their technology involves stabilized sodium percarbonate particles that release controlled amounts of hydrogen peroxide and sodium carbonate when dissolved in water. This controlled release mechanism ensures a sustained disinfection effect while maintaining optimal pH levels. Solvay's sodium percarbonate products are designed to effectively remove organic contaminants, pathogens, and biofilms in water distribution systems[1]. The company has also implemented a proprietary coating process that enhances the stability and shelf-life of sodium percarbonate, making it more suitable for long-term storage and transportation in various climatic conditions[2].
Strengths: Controlled release technology, enhanced stability, and broad-spectrum disinfection. Weaknesses: Potentially higher cost compared to traditional disinfectants, and may require specialized handling and storage.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed a comprehensive water treatment solution incorporating sodium percarbonate for drinking water systems. Their approach combines sodium percarbonate with other synergistic compounds to create a multi-functional treatment system. This system not only disinfects water but also addresses issues such as scale formation and corrosion in distribution networks. Ecolab's technology utilizes a smart dosing system that adjusts the release of sodium percarbonate based on real-time water quality parameters, ensuring optimal treatment efficiency[3]. The company has also developed specialized formulations that enhance the oxidative power of sodium percarbonate, making it effective against a wider range of contaminants, including emerging pollutants[4].
Strengths: Integrated treatment approach, smart dosing technology, and enhanced oxidative power. Weaknesses: May require more complex implementation and monitoring systems, potentially increasing operational costs.
Core Innovations in Sodium Percarbonate-based Water Treatment
Use of medium chain peracids for biofilm inhibition in industrial recirculating water systems
PatentWO2019060814A1
Innovation
- The use of peracid compositions, specifically a combination of short chain and medium chain peracids, is employed to sanitize water systems, effectively reducing both planktonic and sessile microbial populations, thereby preventing biofilm growth without relying on biocidal agents.
Use of peracetic acid/hydrogen peroxide and peroxide-reducing agents for treatment of drilling fluids, FRAC fluids, flowback water and disposal water
PatentWO2013148200A1
Innovation
- The use of percarboxylic acid compositions treated with peroxide-reducing agents like catalase or metals to reduce hydrogen peroxide levels, creating a stable antimicrobial solution that does not interfere with friction reducers and viscosity enhancers, and minimizes microbial-induced corrosion.
Environmental Impact of Sodium Percarbonate Usage
The use of sodium percarbonate in drinking water systems has significant environmental implications that warrant careful consideration. As a powerful oxidizing agent, sodium percarbonate can effectively remove contaminants and improve water quality, but its widespread application may also lead to unintended consequences for aquatic ecosystems and surrounding environments.
One of the primary environmental benefits of sodium percarbonate is its ability to break down into harmless components. When dissolved in water, it decomposes into sodium carbonate and hydrogen peroxide, both of which are environmentally benign substances. This characteristic makes sodium percarbonate an attractive alternative to more persistent chemical treatments, reducing the long-term impact on water bodies and aquatic life.
However, the release of oxygen during the decomposition process can potentially lead to temporary oversaturation in water systems. While this may benefit aerobic organisms in the short term, it can disrupt the natural balance of dissolved gases in aquatic environments. Careful dosing and monitoring are essential to prevent adverse effects on sensitive aquatic species that may be adapted to specific oxygen levels.
The introduction of sodium carbonate as a byproduct of sodium percarbonate decomposition can affect water pH levels. In areas with naturally soft water, this may help to increase alkalinity and buffer against acidification. Conversely, in regions with already high pH levels, additional sodium carbonate could potentially lead to alkaline conditions that may stress certain aquatic organisms or alter ecosystem dynamics.
Another environmental consideration is the impact of sodium percarbonate on microbial communities in water systems. While its antimicrobial properties are beneficial for controlling harmful pathogens, there is a risk of disrupting beneficial microbial populations that play crucial roles in nutrient cycling and ecosystem health. The extent of this impact likely depends on dosage and frequency of application, highlighting the need for balanced and targeted use.
The production and transportation of sodium percarbonate also contribute to its overall environmental footprint. Manufacturing processes require energy and resources, potentially leading to greenhouse gas emissions and other industrial impacts. Additionally, the transportation of the chemical to water treatment facilities adds to carbon emissions. However, when compared to alternative water treatment methods, the environmental cost of sodium percarbonate production and distribution may be offset by its efficiency and effectiveness in water purification.
In conclusion, while sodium percarbonate offers significant benefits for drinking water treatment, its environmental impact is multifaceted. Proper management and application strategies are crucial to maximize its positive effects while minimizing potential ecological disruptions. Ongoing research and monitoring will be essential to fully understand and mitigate any long-term environmental consequences associated with its use in drinking water systems.
One of the primary environmental benefits of sodium percarbonate is its ability to break down into harmless components. When dissolved in water, it decomposes into sodium carbonate and hydrogen peroxide, both of which are environmentally benign substances. This characteristic makes sodium percarbonate an attractive alternative to more persistent chemical treatments, reducing the long-term impact on water bodies and aquatic life.
However, the release of oxygen during the decomposition process can potentially lead to temporary oversaturation in water systems. While this may benefit aerobic organisms in the short term, it can disrupt the natural balance of dissolved gases in aquatic environments. Careful dosing and monitoring are essential to prevent adverse effects on sensitive aquatic species that may be adapted to specific oxygen levels.
The introduction of sodium carbonate as a byproduct of sodium percarbonate decomposition can affect water pH levels. In areas with naturally soft water, this may help to increase alkalinity and buffer against acidification. Conversely, in regions with already high pH levels, additional sodium carbonate could potentially lead to alkaline conditions that may stress certain aquatic organisms or alter ecosystem dynamics.
Another environmental consideration is the impact of sodium percarbonate on microbial communities in water systems. While its antimicrobial properties are beneficial for controlling harmful pathogens, there is a risk of disrupting beneficial microbial populations that play crucial roles in nutrient cycling and ecosystem health. The extent of this impact likely depends on dosage and frequency of application, highlighting the need for balanced and targeted use.
The production and transportation of sodium percarbonate also contribute to its overall environmental footprint. Manufacturing processes require energy and resources, potentially leading to greenhouse gas emissions and other industrial impacts. Additionally, the transportation of the chemical to water treatment facilities adds to carbon emissions. However, when compared to alternative water treatment methods, the environmental cost of sodium percarbonate production and distribution may be offset by its efficiency and effectiveness in water purification.
In conclusion, while sodium percarbonate offers significant benefits for drinking water treatment, its environmental impact is multifaceted. Proper management and application strategies are crucial to maximize its positive effects while minimizing potential ecological disruptions. Ongoing research and monitoring will be essential to fully understand and mitigate any long-term environmental consequences associated with its use in drinking water systems.
Regulatory Framework for Water Treatment Chemicals
The regulatory framework for water treatment chemicals is a critical aspect of ensuring safe drinking water systems. In the context of exploring sodium percarbonate's benefits, it is essential to understand the existing regulations and guidelines that govern its use.
At the federal level in the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating water treatment chemicals. Under the Safe Drinking Water Act (SDWA), the EPA sets standards for drinking water quality and oversees the states, localities, and water suppliers who implement those standards. The agency has established maximum contaminant levels (MCLs) for various substances in drinking water, which water treatment facilities must adhere to.
For sodium percarbonate specifically, its use in drinking water systems falls under the broader category of oxidizing agents. The EPA requires that all chemicals used in water treatment, including sodium percarbonate, must be certified to NSF/ANSI Standard 60: Drinking Water Treatment Chemicals - Health Effects. This standard ensures that chemicals used in water treatment do not contribute contaminants to water at levels that could cause adverse health effects.
At the state level, regulations may vary, with some states imposing additional requirements or stricter standards than those set by the EPA. Water treatment facilities must comply with both federal and state regulations, often requiring them to obtain permits and undergo regular inspections to ensure compliance.
Internationally, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which many countries use as a basis for their national standards. The WHO Guidelines for Drinking-water Quality address chemical safety, including the use of treatment chemicals like sodium percarbonate.
In the European Union, the Drinking Water Directive (98/83/EC) sets standards for drinking water quality, including limits on chemical contaminants. Member states are required to transpose these directives into their national legislation, which may result in variations in regulatory approaches across EU countries.
It is important to note that regulations are not static and are subject to periodic review and updates based on new scientific evidence and technological advancements. As research into sodium percarbonate's benefits in drinking water systems progresses, regulatory bodies may reassess and potentially modify existing guidelines to reflect new findings.
Water treatment facilities and chemical suppliers must stay informed about these regulatory frameworks and any changes that may affect the use of sodium percarbonate in drinking water systems. Compliance with these regulations is crucial not only for legal reasons but also to ensure the safety and quality of drinking water for consumers.
At the federal level in the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating water treatment chemicals. Under the Safe Drinking Water Act (SDWA), the EPA sets standards for drinking water quality and oversees the states, localities, and water suppliers who implement those standards. The agency has established maximum contaminant levels (MCLs) for various substances in drinking water, which water treatment facilities must adhere to.
For sodium percarbonate specifically, its use in drinking water systems falls under the broader category of oxidizing agents. The EPA requires that all chemicals used in water treatment, including sodium percarbonate, must be certified to NSF/ANSI Standard 60: Drinking Water Treatment Chemicals - Health Effects. This standard ensures that chemicals used in water treatment do not contribute contaminants to water at levels that could cause adverse health effects.
At the state level, regulations may vary, with some states imposing additional requirements or stricter standards than those set by the EPA. Water treatment facilities must comply with both federal and state regulations, often requiring them to obtain permits and undergo regular inspections to ensure compliance.
Internationally, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which many countries use as a basis for their national standards. The WHO Guidelines for Drinking-water Quality address chemical safety, including the use of treatment chemicals like sodium percarbonate.
In the European Union, the Drinking Water Directive (98/83/EC) sets standards for drinking water quality, including limits on chemical contaminants. Member states are required to transpose these directives into their national legislation, which may result in variations in regulatory approaches across EU countries.
It is important to note that regulations are not static and are subject to periodic review and updates based on new scientific evidence and technological advancements. As research into sodium percarbonate's benefits in drinking water systems progresses, regulatory bodies may reassess and potentially modify existing guidelines to reflect new findings.
Water treatment facilities and chemical suppliers must stay informed about these regulatory frameworks and any changes that may affect the use of sodium percarbonate in drinking water systems. Compliance with these regulations is crucial not only for legal reasons but also to ensure the safety and quality of drinking water for consumers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!