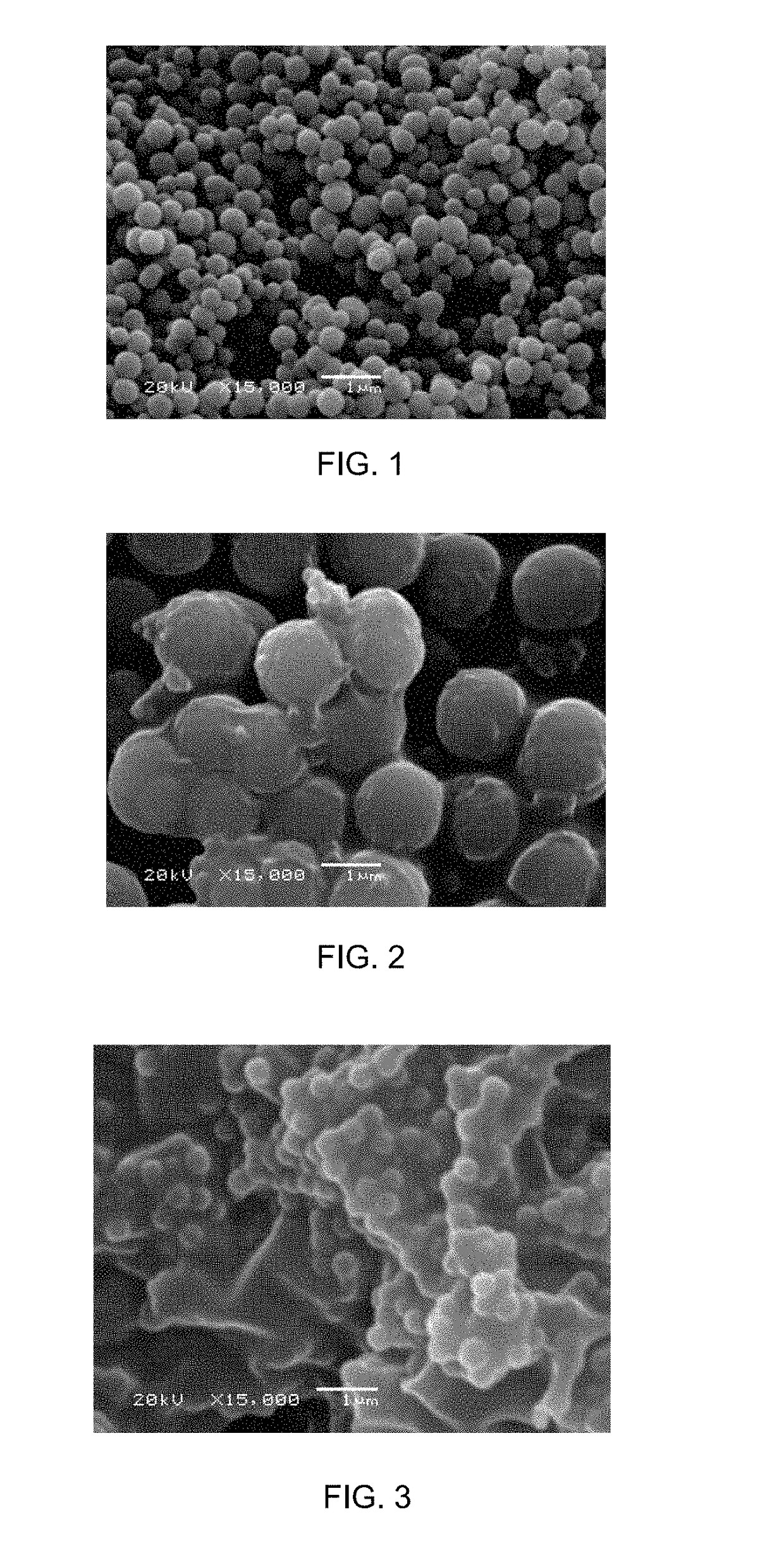
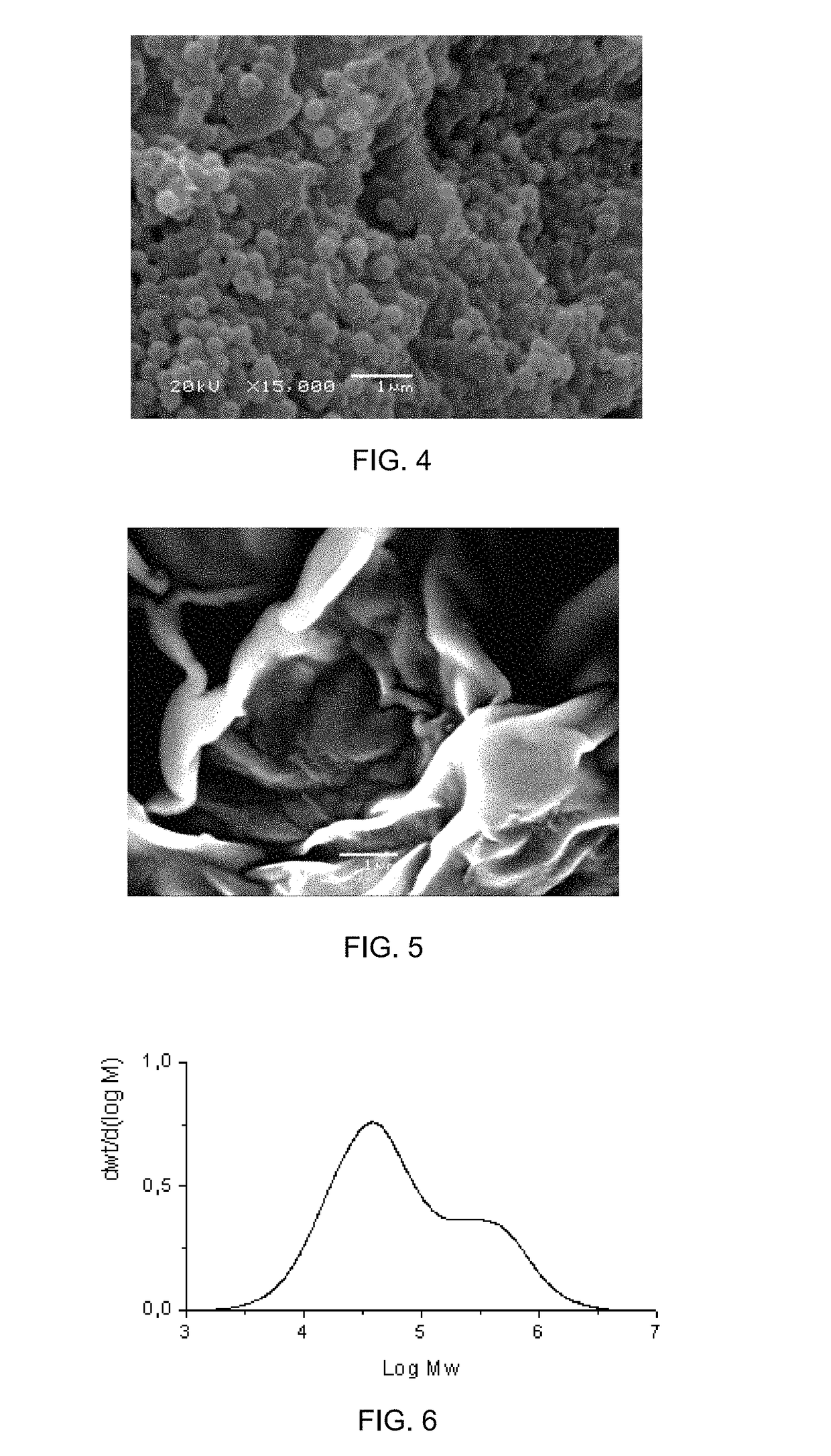
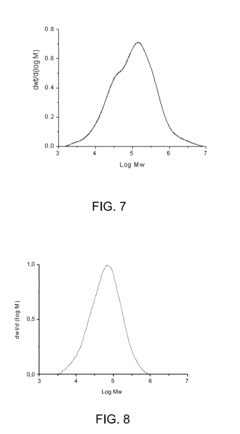
Formation pathways of Magnesium iron silicate hydroxide in basaltic settings.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Fe Silicate Hydroxide Formation Background
Magnesium iron silicate hydroxide, commonly known as serpentine, is a crucial mineral group in the context of basaltic settings. The formation of these minerals is intrinsically linked to the process of serpentinization, which plays a significant role in various geological and geochemical processes on Earth and potentially on other planetary bodies.
The background of Mg-Fe silicate hydroxide formation in basaltic environments is rooted in the interaction between ultramafic rocks and water. This process typically occurs in oceanic crust, particularly at mid-ocean ridges and subduction zones. As seawater penetrates the fractured oceanic crust, it reacts with olivine and pyroxene, the primary minerals in basaltic rocks, leading to the formation of serpentine minerals.
The geological significance of this process extends beyond the mere transformation of minerals. Serpentinization is a key player in the global geochemical cycle, influencing the distribution of elements between the Earth's crust and mantle. It also has implications for the generation of hydrogen and methane, which can support microbial communities in deep-sea environments.
The study of Mg-Fe silicate hydroxide formation has gained increased attention in recent years due to its potential applications in carbon sequestration. The reaction of serpentine with carbon dioxide can result in the formation of carbonate minerals, offering a natural mechanism for long-term carbon storage. This has led to research into engineered serpentinization processes as a means of mitigating climate change.
Furthermore, the presence of serpentine minerals on other planetary bodies, such as Mars, has sparked interest in understanding the formation pathways of these minerals in extraterrestrial basaltic settings. This research has implications for the search for past or present water on Mars and the potential for life beyond Earth.
The historical context of studying Mg-Fe silicate hydroxide formation dates back to the early 20th century, with significant advancements in understanding occurring in the latter half of the century. The development of advanced analytical techniques, such as high-resolution electron microscopy and spectroscopy, has greatly enhanced our ability to investigate the complex mineralogical and chemical processes involved in serpentinization.
The background of Mg-Fe silicate hydroxide formation in basaltic environments is rooted in the interaction between ultramafic rocks and water. This process typically occurs in oceanic crust, particularly at mid-ocean ridges and subduction zones. As seawater penetrates the fractured oceanic crust, it reacts with olivine and pyroxene, the primary minerals in basaltic rocks, leading to the formation of serpentine minerals.
The geological significance of this process extends beyond the mere transformation of minerals. Serpentinization is a key player in the global geochemical cycle, influencing the distribution of elements between the Earth's crust and mantle. It also has implications for the generation of hydrogen and methane, which can support microbial communities in deep-sea environments.
The study of Mg-Fe silicate hydroxide formation has gained increased attention in recent years due to its potential applications in carbon sequestration. The reaction of serpentine with carbon dioxide can result in the formation of carbonate minerals, offering a natural mechanism for long-term carbon storage. This has led to research into engineered serpentinization processes as a means of mitigating climate change.
Furthermore, the presence of serpentine minerals on other planetary bodies, such as Mars, has sparked interest in understanding the formation pathways of these minerals in extraterrestrial basaltic settings. This research has implications for the search for past or present water on Mars and the potential for life beyond Earth.
The historical context of studying Mg-Fe silicate hydroxide formation dates back to the early 20th century, with significant advancements in understanding occurring in the latter half of the century. The development of advanced analytical techniques, such as high-resolution electron microscopy and spectroscopy, has greatly enhanced our ability to investigate the complex mineralogical and chemical processes involved in serpentinization.
Geochemical Demand Analysis
The geochemical demand for magnesium iron silicate hydroxide (MISH) in basaltic settings is driven by both scientific research needs and potential industrial applications. In the scientific realm, understanding the formation pathways of MISH is crucial for unraveling the complex processes of rock alteration and mineral transformation in basaltic environments. This knowledge is essential for geologists, petrologists, and geochemists studying the evolution of basaltic rocks and their interaction with fluids over geological timescales.
The study of MISH formation in basaltic settings has significant implications for planetary science, particularly in the exploration of Mars and other celestial bodies with basaltic surfaces. As MISH minerals are potential indicators of past water activity, their presence and formation mechanisms can provide valuable insights into the geological history and potential habitability of these environments. This has led to increased demand for research in this area, driving funding and resources towards MISH-related studies.
In the realm of environmental science and climate change research, there is growing interest in the role of MISH in carbon sequestration processes. Basaltic rocks are known for their potential in mineral carbonation, and understanding the formation of MISH could lead to improved methods for enhancing natural carbon dioxide capture and storage. This has created a demand for geochemical studies focusing on the interaction between basaltic minerals, including MISH, and atmospheric carbon dioxide.
From an industrial perspective, the formation pathways of MISH in basaltic settings have implications for various sectors. In the field of geothermal energy, understanding MISH formation is crucial for predicting and mitigating scaling and clogging issues in geothermal systems located in basaltic terrains. This knowledge can lead to more efficient and cost-effective geothermal energy production, driving demand for research in this area.
The mining and mineral exploration industry also has a vested interest in MISH formation pathways. As secondary minerals, MISH can be indicators of primary ore deposits or alteration zones associated with mineralization. Improved understanding of their formation can enhance exploration techniques and resource assessment in basaltic terrains, potentially leading to the discovery of new mineral deposits.
In the construction and materials science sectors, there is growing interest in utilizing basaltic materials for various applications. Understanding MISH formation can inform the development of more durable and environmentally friendly construction materials, as well as improve the prediction of long-term material behavior in basaltic aggregate concrete and other basalt-derived products.
The water treatment industry is another sector driving demand for MISH-related geochemical research. The potential use of basaltic materials in water purification systems, particularly for the removal of heavy metals and other contaminants, has sparked interest in understanding the role of MISH in these processes. This knowledge could lead to the development of more effective and sustainable water treatment technologies.
The study of MISH formation in basaltic settings has significant implications for planetary science, particularly in the exploration of Mars and other celestial bodies with basaltic surfaces. As MISH minerals are potential indicators of past water activity, their presence and formation mechanisms can provide valuable insights into the geological history and potential habitability of these environments. This has led to increased demand for research in this area, driving funding and resources towards MISH-related studies.
In the realm of environmental science and climate change research, there is growing interest in the role of MISH in carbon sequestration processes. Basaltic rocks are known for their potential in mineral carbonation, and understanding the formation of MISH could lead to improved methods for enhancing natural carbon dioxide capture and storage. This has created a demand for geochemical studies focusing on the interaction between basaltic minerals, including MISH, and atmospheric carbon dioxide.
From an industrial perspective, the formation pathways of MISH in basaltic settings have implications for various sectors. In the field of geothermal energy, understanding MISH formation is crucial for predicting and mitigating scaling and clogging issues in geothermal systems located in basaltic terrains. This knowledge can lead to more efficient and cost-effective geothermal energy production, driving demand for research in this area.
The mining and mineral exploration industry also has a vested interest in MISH formation pathways. As secondary minerals, MISH can be indicators of primary ore deposits or alteration zones associated with mineralization. Improved understanding of their formation can enhance exploration techniques and resource assessment in basaltic terrains, potentially leading to the discovery of new mineral deposits.
In the construction and materials science sectors, there is growing interest in utilizing basaltic materials for various applications. Understanding MISH formation can inform the development of more durable and environmentally friendly construction materials, as well as improve the prediction of long-term material behavior in basaltic aggregate concrete and other basalt-derived products.
The water treatment industry is another sector driving demand for MISH-related geochemical research. The potential use of basaltic materials in water purification systems, particularly for the removal of heavy metals and other contaminants, has sparked interest in understanding the role of MISH in these processes. This knowledge could lead to the development of more effective and sustainable water treatment technologies.
Current State of Basaltic Alteration Research
The field of basaltic alteration research has seen significant advancements in recent years, with a focus on understanding the formation pathways of Magnesium iron silicate hydroxide in basaltic settings. Current research efforts are primarily concentrated on elucidating the complex interactions between basaltic rocks and various fluids, particularly in submarine and subaerial environments.
One of the key areas of investigation is the role of hydrothermal processes in the formation of Magnesium iron silicate hydroxide minerals. Researchers have made substantial progress in identifying the specific conditions that promote the alteration of basaltic rocks, including temperature, pressure, and fluid composition. These studies have revealed that the formation of these minerals is often associated with low-temperature hydrothermal activity, typically below 150°C.
Recent advancements in analytical techniques have allowed for more precise characterization of the mineralogical and geochemical changes occurring during basaltic alteration. High-resolution electron microscopy and spectroscopic methods have provided valuable insights into the micro-scale processes involved in the formation of Magnesium iron silicate hydroxide phases.
The influence of microbial activity on basaltic alteration has emerged as a significant area of research. Studies have shown that microorganisms can play a crucial role in facilitating the weathering of basaltic rocks and the subsequent formation of secondary minerals, including Magnesium iron silicate hydroxides. This has led to increased interdisciplinary collaboration between geologists, microbiologists, and geochemists.
Field studies in various geological settings, such as mid-ocean ridges, oceanic islands, and continental flood basalt provinces, have contributed to a more comprehensive understanding of the spatial and temporal variations in basaltic alteration processes. These investigations have highlighted the importance of factors such as rock texture, fluid flow patterns, and tectonic setting in controlling the extent and nature of alteration.
Experimental studies have also played a crucial role in advancing our understanding of basaltic alteration. Laboratory simulations of hydrothermal alteration under controlled conditions have provided valuable insights into the kinetics and mechanisms of Magnesium iron silicate hydroxide formation. These experiments have helped to constrain the thermodynamic and kinetic parameters governing the alteration processes.
The application of advanced modeling techniques, including reactive transport models and geochemical simulations, has enhanced our ability to predict and interpret basaltic alteration phenomena. These models integrate field observations, experimental data, and theoretical considerations to provide a more holistic understanding of the complex processes involved in the formation of Magnesium iron silicate hydroxide minerals.
One of the key areas of investigation is the role of hydrothermal processes in the formation of Magnesium iron silicate hydroxide minerals. Researchers have made substantial progress in identifying the specific conditions that promote the alteration of basaltic rocks, including temperature, pressure, and fluid composition. These studies have revealed that the formation of these minerals is often associated with low-temperature hydrothermal activity, typically below 150°C.
Recent advancements in analytical techniques have allowed for more precise characterization of the mineralogical and geochemical changes occurring during basaltic alteration. High-resolution electron microscopy and spectroscopic methods have provided valuable insights into the micro-scale processes involved in the formation of Magnesium iron silicate hydroxide phases.
The influence of microbial activity on basaltic alteration has emerged as a significant area of research. Studies have shown that microorganisms can play a crucial role in facilitating the weathering of basaltic rocks and the subsequent formation of secondary minerals, including Magnesium iron silicate hydroxides. This has led to increased interdisciplinary collaboration between geologists, microbiologists, and geochemists.
Field studies in various geological settings, such as mid-ocean ridges, oceanic islands, and continental flood basalt provinces, have contributed to a more comprehensive understanding of the spatial and temporal variations in basaltic alteration processes. These investigations have highlighted the importance of factors such as rock texture, fluid flow patterns, and tectonic setting in controlling the extent and nature of alteration.
Experimental studies have also played a crucial role in advancing our understanding of basaltic alteration. Laboratory simulations of hydrothermal alteration under controlled conditions have provided valuable insights into the kinetics and mechanisms of Magnesium iron silicate hydroxide formation. These experiments have helped to constrain the thermodynamic and kinetic parameters governing the alteration processes.
The application of advanced modeling techniques, including reactive transport models and geochemical simulations, has enhanced our ability to predict and interpret basaltic alteration phenomena. These models integrate field observations, experimental data, and theoretical considerations to provide a more holistic understanding of the complex processes involved in the formation of Magnesium iron silicate hydroxide minerals.
Existing Models of Mg-Fe Silicate Formation
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups, forming elongated particles with channels running through them. This structure gives the material its distinctive properties, including high surface area and adsorption capacity.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups, forming a complex layered silicate structure. This mineral has a high surface area and strong adsorption properties, making it useful in various applications.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its excellent adsorption capabilities. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and unique structure allow it to trap and immobilize various pollutants, making it an effective material for water treatment and soil decontamination.
- Use in pharmaceutical and cosmetic industries: The mineral finds applications in pharmaceutical and cosmetic industries due to its absorbent and thickening properties. It is used as an excipient in drug formulations, helping to control drug release and improve stability. In cosmetics, it is utilized as a thickening agent, absorbent, and texturizer in various products such as creams, lotions, and powders.
- Industrial applications and material science: Magnesium iron silicate hydroxide has various industrial applications, including its use as a rheological modifier in drilling fluids, as a reinforcing agent in polymer composites, and as a catalyst support in chemical processes. Its unique properties contribute to improved performance in these applications, such as enhanced viscosity control, mechanical strength, and catalytic activity.
- Synthesis and modification methods: Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. This includes techniques for controlling particle size, morphology, and surface chemistry. Modified forms of the mineral can exhibit improved adsorption capacity, selectivity, and stability, expanding its potential uses in various fields.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is widely used in environmental remediation due to its excellent adsorption properties. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The material's high surface area and porous structure allow it to trap and immobilize various pollutants, making it valuable for water treatment and soil decontamination processes.Expand Specific Solutions03 Use in industrial and consumer products
The unique properties of magnesium iron silicate hydroxide make it suitable for various industrial and consumer applications. It is used as a rheological modifier in paints, cosmetics, and personal care products. The material also finds applications in the production of ceramics, paper coatings, and as a reinforcing agent in polymers and rubber compounds. Its ability to absorb liquids and gases makes it useful in cat litter and odor control products.Expand Specific Solutions04 Synthesis and modification methods
Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Researchers have also explored ways to modify the surface properties of the material to enhance its performance in specific applications, such as improving its adsorption capacity or increasing its compatibility with polymer matrices.Expand Specific Solutions05 Emerging applications in advanced materials
Recent research has focused on exploring new applications for magnesium iron silicate hydroxide in advanced materials. These include its use as a component in nanocomposites, as a catalyst support in chemical reactions, and as a precursor for the synthesis of other functional materials. The material's unique structure and properties make it a promising candidate for developing novel materials with enhanced performance in areas such as energy storage, catalysis, and sensing applications.Expand Specific Solutions
Key Players in Geochemical Research
The formation pathways of Magnesium iron silicate hydroxide in basaltic settings represent an emerging field of study in geochemistry. The competitive landscape is characterized by early-stage research, with academic institutions like Southeast University and Central South University leading the charge. The market size is relatively small, primarily driven by scientific research funding. Technologically, the field is in its infancy, with companies like Saudi Arabian Oil Co. and Applied Materials, Inc. potentially interested in applications for mineral exploration and materials science. However, the technology's maturity remains low, with most efforts focused on fundamental research rather than commercial applications.
PetroChina Co., Ltd.
Technical Solution: PetroChina has invested in research on magnesium iron silicate hydroxide formation in basaltic settings, particularly in the context of enhanced oil recovery and carbon sequestration. Their approach involves advanced geochemical modeling coupled with laboratory experiments simulating high-temperature and high-pressure conditions typical of basaltic reservoirs[4]. PetroChina's studies have shown that MISH formation can significantly alter the porosity and permeability of basaltic rocks, impacting fluid flow and storage capacity[5]. They have developed novel tracers to monitor MISH precipitation in real-time during fluid injection experiments, providing crucial data on reaction kinetics and spatial distribution of mineral growth[6].
Strengths: Strong focus on practical applications in energy sector. Extensive field testing capabilities. Weaknesses: Research may be limited to specific geological settings relevant to oil and gas exploration in China.
Nanyang Technological University
Technical Solution: Nanyang Technological University (NTU) has made significant contributions to understanding the formation pathways of magnesium iron silicate hydroxide in basaltic settings through interdisciplinary research. Their approach combines advanced analytical techniques, such as high-resolution transmission electron microscopy and in-situ X-ray diffraction, with geochemical modeling to elucidate the nanoscale processes governing MISH formation[10]. NTU researchers have identified novel intermediate phases in the MISH formation pathway, shedding light on the complex reaction mechanisms involved[11]. They have also investigated the role of organic compounds in modulating MISH precipitation, revealing potential links between biological activity and mineral formation in basaltic environments[12].
Strengths: Strong focus on fundamental science and nanoscale processes. Interdisciplinary approach combining geochemistry, materials science, and biology. Weaknesses: May lack direct industry applications compared to energy sector research.
Core Innovations in Basaltic Alteration Studies
Metallocene catalyst supported by hybrid supporting means, process for producing same, polymerization process for producing an ethylene homopolymer or copolymer with broad or bimodal molar mass distribution, use of the supported metallocene catalyst and ethylene polymer with broad or bimodal molar mass distribution
PatentActiveUS20170267790A1
Innovation
- A metallocene catalyst supported on a hybrid catalytic support with aliphatic organic groups, prepared via a hydrolytic sol-gel pathway, is used to produce ethylene polymers with broad or bimodal molar mass distribution by immobilizing a metallocene complex derived from a compound like [L]2-MQ2, where M is a transition metal from group 4 or 5, and L is a ligand such as cyclopentadienyl or indenyl, on a support with an inorganic component and aliphatic organic groups.
Method for forming a self-aligned silicide of a metal oxide semiconductor
PatentInactiveUS6740570B2
Innovation
- The method involves ionic implantation with ions like fluorine, chlorine, or boron to create a barrier that prevents cobalt from penetrating the silicon substrate, allowing for rapid cobalt silicide growth without increasing leakage current or decreasing breakdown voltage, and uses a two-step annealing process to control the silicidation reaction.
Environmental Implications of Serpentinization
Serpentinization, a process involving the hydration of ultramafic rocks, has significant environmental implications that extend beyond its geological context. This process plays a crucial role in various Earth systems, influencing both the lithosphere and the biosphere in profound ways.
One of the most notable environmental impacts of serpentinization is its contribution to the carbon cycle. As water reacts with olivine and pyroxene in ultramafic rocks, it produces hydrogen and methane. These gases can be released into the atmosphere, potentially affecting climate dynamics. Moreover, the process can lead to the sequestration of carbon dioxide through the formation of carbonate minerals, acting as a natural carbon sink and potentially mitigating the effects of greenhouse gas emissions.
The hydrogen produced during serpentinization has implications for microbial ecosystems. In deep-sea hydrothermal vents and other serpentinite-hosted environments, hydrogen serves as an energy source for chemosynthetic microorganisms. This supports unique ecosystems that thrive in the absence of sunlight, contributing to biodiversity in extreme environments. The discovery of these ecosystems has expanded our understanding of the limits of life and has implications for the search for life on other planets.
Serpentinization also affects water chemistry, particularly in submarine and subterranean environments. The process increases the pH of surrounding waters, creating alkaline conditions that can support distinctive microbial communities. These alkaline fluids, when mixed with seawater, can precipitate carbonate minerals, forming structures like the Lost City hydrothermal field in the Atlantic Ocean. Such formations provide habitats for diverse marine life and may have played a role in the origin of life on Earth.
The alteration of ultramafic rocks through serpentinization can lead to the release of trace elements into the environment. Some of these elements, such as nickel and chromium, can be beneficial in small quantities but toxic at higher concentrations. This process influences the geochemical cycling of these elements in marine and terrestrial ecosystems, potentially affecting the health of organisms across trophic levels.
Furthermore, serpentinization has implications for water resources and soil formation. In regions where serpentinite is exposed at the surface, the process can lead to the development of unique soil types with distinct chemical properties. These soils often support specialized plant communities adapted to high levels of magnesium and low calcium availability, contributing to local biodiversity.
One of the most notable environmental impacts of serpentinization is its contribution to the carbon cycle. As water reacts with olivine and pyroxene in ultramafic rocks, it produces hydrogen and methane. These gases can be released into the atmosphere, potentially affecting climate dynamics. Moreover, the process can lead to the sequestration of carbon dioxide through the formation of carbonate minerals, acting as a natural carbon sink and potentially mitigating the effects of greenhouse gas emissions.
The hydrogen produced during serpentinization has implications for microbial ecosystems. In deep-sea hydrothermal vents and other serpentinite-hosted environments, hydrogen serves as an energy source for chemosynthetic microorganisms. This supports unique ecosystems that thrive in the absence of sunlight, contributing to biodiversity in extreme environments. The discovery of these ecosystems has expanded our understanding of the limits of life and has implications for the search for life on other planets.
Serpentinization also affects water chemistry, particularly in submarine and subterranean environments. The process increases the pH of surrounding waters, creating alkaline conditions that can support distinctive microbial communities. These alkaline fluids, when mixed with seawater, can precipitate carbonate minerals, forming structures like the Lost City hydrothermal field in the Atlantic Ocean. Such formations provide habitats for diverse marine life and may have played a role in the origin of life on Earth.
The alteration of ultramafic rocks through serpentinization can lead to the release of trace elements into the environment. Some of these elements, such as nickel and chromium, can be beneficial in small quantities but toxic at higher concentrations. This process influences the geochemical cycling of these elements in marine and terrestrial ecosystems, potentially affecting the health of organisms across trophic levels.
Furthermore, serpentinization has implications for water resources and soil formation. In regions where serpentinite is exposed at the surface, the process can lead to the development of unique soil types with distinct chemical properties. These soils often support specialized plant communities adapted to high levels of magnesium and low calcium availability, contributing to local biodiversity.
Analytical Techniques for Mineral Identification
Analytical techniques for mineral identification play a crucial role in understanding the formation pathways of Magnesium iron silicate hydroxide in basaltic settings. These techniques encompass a wide range of methods, each offering unique insights into the mineral composition and structure.
X-ray diffraction (XRD) is a fundamental technique used to identify crystalline phases in minerals. It provides information on the atomic and molecular structure of crystals, allowing researchers to determine the presence of Magnesium iron silicate hydroxide and its polymorphs. XRD patterns can reveal the specific crystal structure and help distinguish between different mineral phases that may be present in basaltic samples.
Scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS) is another powerful tool for mineral identification. SEM provides high-resolution images of mineral surfaces, revealing morphological features and textures. When combined with EDS, it allows for elemental analysis, enabling the detection of Magnesium, iron, silicon, and oxygen in the mineral structure.
Transmission electron microscopy (TEM) offers even higher resolution imaging and can be used to study the internal structure of minerals at the atomic scale. This technique is particularly useful for examining the crystal lattice of Magnesium iron silicate hydroxide and identifying any defects or intergrowths that may provide clues about its formation pathways.
Raman spectroscopy is a non-destructive technique that can provide information about the molecular structure and composition of minerals. It is particularly useful for identifying hydrous minerals like Magnesium iron silicate hydroxide, as it can detect the presence of OH groups in the mineral structure.
Fourier-transform infrared spectroscopy (FTIR) is another valuable technique for identifying minerals, especially those containing hydroxyl groups. FTIR can provide information about the bonding environment of atoms within the mineral structure, helping to distinguish between different types of silicate minerals.
Electron probe microanalysis (EPMA) is a technique that allows for precise quantitative analysis of mineral compositions. It can determine the elemental concentrations in Magnesium iron silicate hydroxide, providing insights into its stoichiometry and potential variations in composition.
These analytical techniques, when used in combination, provide a comprehensive approach to mineral identification in basaltic settings. They allow researchers to characterize the physical, chemical, and structural properties of Magnesium iron silicate hydroxide, offering valuable insights into its formation pathways and the conditions under which it crystallizes in basaltic environments.
X-ray diffraction (XRD) is a fundamental technique used to identify crystalline phases in minerals. It provides information on the atomic and molecular structure of crystals, allowing researchers to determine the presence of Magnesium iron silicate hydroxide and its polymorphs. XRD patterns can reveal the specific crystal structure and help distinguish between different mineral phases that may be present in basaltic samples.
Scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS) is another powerful tool for mineral identification. SEM provides high-resolution images of mineral surfaces, revealing morphological features and textures. When combined with EDS, it allows for elemental analysis, enabling the detection of Magnesium, iron, silicon, and oxygen in the mineral structure.
Transmission electron microscopy (TEM) offers even higher resolution imaging and can be used to study the internal structure of minerals at the atomic scale. This technique is particularly useful for examining the crystal lattice of Magnesium iron silicate hydroxide and identifying any defects or intergrowths that may provide clues about its formation pathways.
Raman spectroscopy is a non-destructive technique that can provide information about the molecular structure and composition of minerals. It is particularly useful for identifying hydrous minerals like Magnesium iron silicate hydroxide, as it can detect the presence of OH groups in the mineral structure.
Fourier-transform infrared spectroscopy (FTIR) is another valuable technique for identifying minerals, especially those containing hydroxyl groups. FTIR can provide information about the bonding environment of atoms within the mineral structure, helping to distinguish between different types of silicate minerals.
Electron probe microanalysis (EPMA) is a technique that allows for precise quantitative analysis of mineral compositions. It can determine the elemental concentrations in Magnesium iron silicate hydroxide, providing insights into its stoichiometry and potential variations in composition.
These analytical techniques, when used in combination, provide a comprehensive approach to mineral identification in basaltic settings. They allow researchers to characterize the physical, chemical, and structural properties of Magnesium iron silicate hydroxide, offering valuable insights into its formation pathways and the conditions under which it crystallizes in basaltic environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!