Full Cell Matching of Hard Carbon Anodes with Prussian White Cathodes
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hard Carbon-Prussian White Battery Technology Background
The development of sustainable energy storage systems has become increasingly critical in the face of growing environmental concerns and the global shift towards renewable energy sources. Within this context, sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. Among various SIB configurations, the combination of hard carbon anodes with Prussian White cathodes represents a particularly promising technological approach.
Hard carbon, derived from various organic precursors through pyrolysis, has gained significant attention as an anode material for SIBs due to its unique structural characteristics. Unlike graphite, which is the standard anode material for lithium-ion batteries, hard carbon possesses a disordered structure with larger interlayer spacing, making it more suitable for accommodating the larger sodium ions. The development of hard carbon materials for SIBs can be traced back to the early 1990s, but significant advancements have been made in the past decade.
On the cathode side, Prussian White compounds, with the general formula Na2M[Fe(CN)6] (where M represents transition metals such as Fe, Mn, Ni, etc.), have demonstrated excellent electrochemical performance. These materials feature an open framework structure that facilitates rapid sodium-ion insertion/extraction, resulting in high rate capability and good cycling stability. The research on Prussian White cathodes for SIBs gained momentum in the early 2010s, with notable improvements in synthesis methods and electrochemical properties.
The integration of hard carbon anodes with Prussian White cathodes in full cells represents a convergence of two independently developed technologies. This combination leverages the complementary properties of both materials: the high capacity and good rate performance of hard carbon anodes, and the stable framework and fast ion transport capabilities of Prussian White cathodes. Early attempts at this integration faced challenges related to capacity matching, electrolyte compatibility, and interface stability.
Recent technological evolution has focused on optimizing the electrochemical performance of full cells through precise control of the anode-to-cathode capacity ratio, electrolyte formulation, and cell design. Researchers have explored various approaches to enhance the initial Coulombic efficiency of hard carbon anodes and improve the structural stability of Prussian White cathodes during long-term cycling.
The development trajectory of Hard Carbon-Prussian White battery technology reflects a broader trend in energy storage research: the pursuit of sustainable, cost-effective alternatives to conventional lithium-ion batteries. This technology aims to address the growing demand for grid-scale energy storage solutions and electric vehicles, where cost considerations are paramount.
Hard carbon, derived from various organic precursors through pyrolysis, has gained significant attention as an anode material for SIBs due to its unique structural characteristics. Unlike graphite, which is the standard anode material for lithium-ion batteries, hard carbon possesses a disordered structure with larger interlayer spacing, making it more suitable for accommodating the larger sodium ions. The development of hard carbon materials for SIBs can be traced back to the early 1990s, but significant advancements have been made in the past decade.
On the cathode side, Prussian White compounds, with the general formula Na2M[Fe(CN)6] (where M represents transition metals such as Fe, Mn, Ni, etc.), have demonstrated excellent electrochemical performance. These materials feature an open framework structure that facilitates rapid sodium-ion insertion/extraction, resulting in high rate capability and good cycling stability. The research on Prussian White cathodes for SIBs gained momentum in the early 2010s, with notable improvements in synthesis methods and electrochemical properties.
The integration of hard carbon anodes with Prussian White cathodes in full cells represents a convergence of two independently developed technologies. This combination leverages the complementary properties of both materials: the high capacity and good rate performance of hard carbon anodes, and the stable framework and fast ion transport capabilities of Prussian White cathodes. Early attempts at this integration faced challenges related to capacity matching, electrolyte compatibility, and interface stability.
Recent technological evolution has focused on optimizing the electrochemical performance of full cells through precise control of the anode-to-cathode capacity ratio, electrolyte formulation, and cell design. Researchers have explored various approaches to enhance the initial Coulombic efficiency of hard carbon anodes and improve the structural stability of Prussian White cathodes during long-term cycling.
The development trajectory of Hard Carbon-Prussian White battery technology reflects a broader trend in energy storage research: the pursuit of sustainable, cost-effective alternatives to conventional lithium-ion batteries. This technology aims to address the growing demand for grid-scale energy storage solutions and electric vehicles, where cost considerations are paramount.
Market Analysis for Sodium-ion Battery Systems
The global sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate a compound annual growth rate of 18-20% between 2023 and 2030, with projections suggesting the market could reach $1.2 billion by 2030. This growth is primarily fueled by the inherent advantages of sodium-ion technology, particularly in grid storage applications where cost considerations often outweigh energy density requirements.
The specific segment focusing on hard carbon anode and Prussian white cathode configurations represents a promising niche within this expanding market. These materials offer a compelling value proposition due to their abundance, lower cost compared to lithium-ion battery materials, and reduced geopolitical supply chain risks. Hard carbon, derived from sustainable biomass sources, provides a cost-effective alternative to graphite anodes used in lithium-ion batteries.
Market segmentation analysis reveals that stationary energy storage currently represents the largest application sector for sodium-ion batteries utilizing hard carbon/Prussian white chemistry, accounting for approximately 65% of current deployments. This is followed by low-speed electric vehicles (18%), consumer electronics (12%), and other applications (5%). The stationary storage segment is expected to maintain dominance through 2028, though electric mobility applications are projected to grow at a faster rate.
Geographically, China leads the market development with over 60% of current sodium-ion battery production capacity, followed by Europe (20%) and North America (15%). This distribution reflects China's strategic investment in sodium-ion technology as a complement to its lithium-ion battery manufacturing ecosystem. European markets show particular interest in sodium-ion technology due to raw material availability and sustainability considerations.
Key market drivers for hard carbon/Prussian white sodium-ion batteries include cost advantages (30-40% lower than comparable lithium-ion systems), material abundance, sustainability credentials, and safety benefits. Market barriers include lower energy density compared to lithium-ion alternatives, limited manufacturing scale, and the need for further cycle life improvements.
Customer adoption analysis indicates that utility companies and renewable energy developers represent the primary early adopters, valuing the technology's cost-effectiveness for grid-level applications. Consumer electronics manufacturers are increasingly exploring sodium-ion batteries for low-cost devices, while electric vehicle manufacturers remain cautious, primarily focusing on specific use cases where weight is less critical.
The specific segment focusing on hard carbon anode and Prussian white cathode configurations represents a promising niche within this expanding market. These materials offer a compelling value proposition due to their abundance, lower cost compared to lithium-ion battery materials, and reduced geopolitical supply chain risks. Hard carbon, derived from sustainable biomass sources, provides a cost-effective alternative to graphite anodes used in lithium-ion batteries.
Market segmentation analysis reveals that stationary energy storage currently represents the largest application sector for sodium-ion batteries utilizing hard carbon/Prussian white chemistry, accounting for approximately 65% of current deployments. This is followed by low-speed electric vehicles (18%), consumer electronics (12%), and other applications (5%). The stationary storage segment is expected to maintain dominance through 2028, though electric mobility applications are projected to grow at a faster rate.
Geographically, China leads the market development with over 60% of current sodium-ion battery production capacity, followed by Europe (20%) and North America (15%). This distribution reflects China's strategic investment in sodium-ion technology as a complement to its lithium-ion battery manufacturing ecosystem. European markets show particular interest in sodium-ion technology due to raw material availability and sustainability considerations.
Key market drivers for hard carbon/Prussian white sodium-ion batteries include cost advantages (30-40% lower than comparable lithium-ion systems), material abundance, sustainability credentials, and safety benefits. Market barriers include lower energy density compared to lithium-ion alternatives, limited manufacturing scale, and the need for further cycle life improvements.
Customer adoption analysis indicates that utility companies and renewable energy developers represent the primary early adopters, valuing the technology's cost-effectiveness for grid-level applications. Consumer electronics manufacturers are increasingly exploring sodium-ion batteries for low-cost devices, while electric vehicle manufacturers remain cautious, primarily focusing on specific use cases where weight is less critical.
Technical Challenges in Full Cell Matching
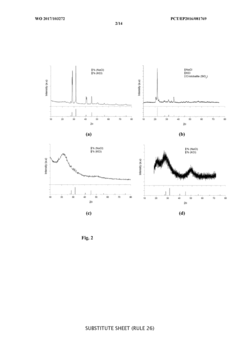
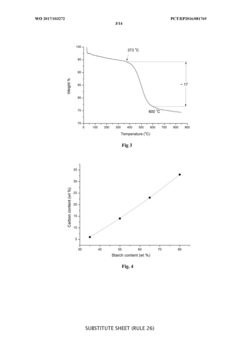
The integration of hard carbon anodes with Prussian White cathodes in full cell configurations presents several significant technical challenges that must be addressed for optimal performance. One of the primary difficulties lies in the capacity matching between these two electrodes. Hard carbon typically delivers specific capacities of 250-350 mAh/g, while Prussian White cathodes generally exhibit lower capacities around 120-170 mAh/g. This substantial capacity mismatch necessitates careful balancing of active material loading ratios to prevent over-discharge or under-utilization of either electrode.
Electrolyte compatibility represents another major challenge in these full cell systems. The optimal electrolyte composition for hard carbon anodes often differs from that ideal for Prussian White cathodes. Hard carbon performs best with electrolytes containing fluorinated salts and certain additives that form stable SEI layers, whereas Prussian White cathodes may require different salt concentrations and additives to prevent iron dissolution and structural degradation.
The differing voltage windows of operation between these materials creates additional complications. Hard carbon operates effectively in the 0-1.5V range (vs. Na/Na+), while Prussian White cathodes typically function in the 2.5-4.2V window. Establishing an appropriate full cell voltage range that maximizes energy density while preventing degradation mechanisms at both electrodes requires extensive optimization.
Initial formation cycles present particular difficulties in these full cell systems. The irreversible capacity loss during the first few cycles is significantly higher in hard carbon (typically 20-30%) compared to Prussian White cathodes (5-15%). This discrepancy leads to capacity fading and efficiency losses if not properly managed through pre-cycling protocols or excess anode capacity provisions.
Interface stability issues emerge during long-term cycling. The open framework structure of Prussian White compounds can release coordinated water molecules or undergo phase transitions during cycling, generating reaction products that may migrate to and react with the hard carbon anode. Similarly, decomposition products from the anode side can affect cathode performance through shuttle mechanisms.
Rate capability matching poses another significant challenge. Hard carbon and Prussian White materials often exhibit different rate performance characteristics, with Prussian White generally allowing faster charge/discharge rates due to its open framework structure. This kinetic mismatch can lead to underutilization of the faster electrode or degradation of the slower one when operating at intermediate rates.
Temperature sensitivity differences between these materials further complicate full cell design. Hard carbon performance is typically more temperature-dependent than Prussian White cathodes, creating additional challenges for maintaining balanced performance across various operating conditions.
Electrolyte compatibility represents another major challenge in these full cell systems. The optimal electrolyte composition for hard carbon anodes often differs from that ideal for Prussian White cathodes. Hard carbon performs best with electrolytes containing fluorinated salts and certain additives that form stable SEI layers, whereas Prussian White cathodes may require different salt concentrations and additives to prevent iron dissolution and structural degradation.
The differing voltage windows of operation between these materials creates additional complications. Hard carbon operates effectively in the 0-1.5V range (vs. Na/Na+), while Prussian White cathodes typically function in the 2.5-4.2V window. Establishing an appropriate full cell voltage range that maximizes energy density while preventing degradation mechanisms at both electrodes requires extensive optimization.
Initial formation cycles present particular difficulties in these full cell systems. The irreversible capacity loss during the first few cycles is significantly higher in hard carbon (typically 20-30%) compared to Prussian White cathodes (5-15%). This discrepancy leads to capacity fading and efficiency losses if not properly managed through pre-cycling protocols or excess anode capacity provisions.
Interface stability issues emerge during long-term cycling. The open framework structure of Prussian White compounds can release coordinated water molecules or undergo phase transitions during cycling, generating reaction products that may migrate to and react with the hard carbon anode. Similarly, decomposition products from the anode side can affect cathode performance through shuttle mechanisms.
Rate capability matching poses another significant challenge. Hard carbon and Prussian White materials often exhibit different rate performance characteristics, with Prussian White generally allowing faster charge/discharge rates due to its open framework structure. This kinetic mismatch can lead to underutilization of the faster electrode or degradation of the slower one when operating at intermediate rates.
Temperature sensitivity differences between these materials further complicate full cell design. Hard carbon performance is typically more temperature-dependent than Prussian White cathodes, creating additional challenges for maintaining balanced performance across various operating conditions.
Current Full Cell Matching Solutions
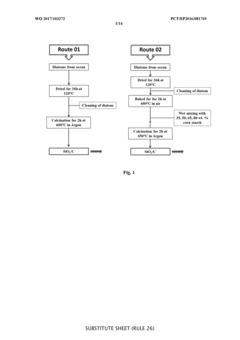
01 Hard carbon anode materials for sodium-ion batteries
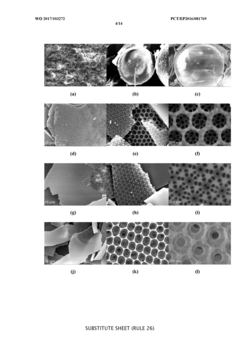
Hard carbon materials are widely used as anode materials in sodium-ion batteries due to their high capacity, good cycling stability, and low cost. These materials have a disordered structure with micropores that can accommodate sodium ions. When paired with Prussian white cathodes, hard carbon anodes can provide a balanced full cell with good energy density and cycle life. The optimization of hard carbon structure, including porosity and surface area, is crucial for improving the electrochemical performance of the full cell.- Hard carbon anode materials for sodium-ion batteries: Hard carbon materials are widely used as anode materials in sodium-ion batteries due to their high capacity, good cycling stability, and low cost. These materials have a disordered structure with micropores that can accommodate sodium ions. When paired with Prussian white cathodes, the hard carbon anodes need to be optimized for capacity matching to ensure efficient full cell performance and cycle life.
- Prussian white cathode composition and structure: Prussian white compounds, typically sodium-rich iron hexacyanoferrates, serve as cathode materials in sodium-ion batteries. These materials feature an open framework structure that allows for rapid sodium ion insertion/extraction. The composition can be modified to improve electrochemical performance, stability, and energy density when matched with hard carbon anodes in full cells.
- Full cell matching and electrolyte optimization: Proper matching of hard carbon anodes with Prussian white cathodes requires balancing the capacity ratio, typically using excess anode capacity. Electrolyte composition plays a crucial role in full cell performance, affecting the solid electrolyte interphase formation on the hard carbon anode and stability of the Prussian white cathode. Optimized electrolytes can mitigate side reactions and improve cycling efficiency.
- Carbon coating and surface modification techniques: Surface modification of hard carbon anodes and Prussian white cathodes can significantly improve their electrochemical performance in full cells. Carbon coating techniques enhance electrical conductivity and structural stability. Various methods including hydrothermal carbonization, pyrolysis of organic precursors, and chemical vapor deposition can be employed to create optimized carbon coatings that improve the interface between active materials and electrolyte.
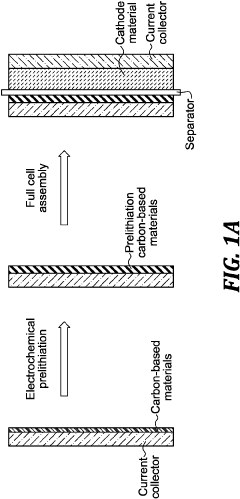
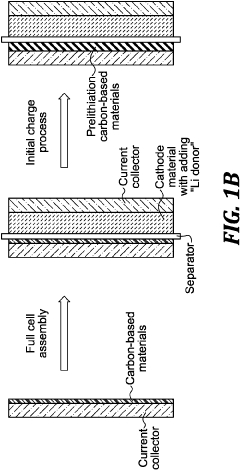
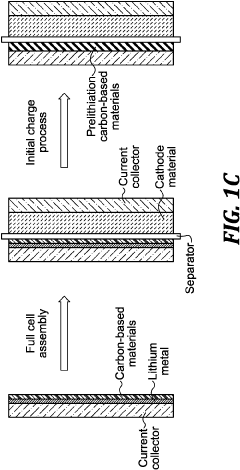
- Pre-lithiation/pre-sodiation strategies for capacity balancing: Pre-sodiation of hard carbon anodes or pre-lithiation techniques can be employed to compensate for irreversible capacity loss during the initial cycles. These strategies help achieve better capacity matching between hard carbon anodes and Prussian white cathodes in full cells. Various electrochemical and chemical methods can be used to introduce sodium or lithium ions into the anode structure before cell assembly, improving first cycle efficiency and overall energy density.
02 Prussian white cathode composition and synthesis
Prussian white compounds, typically sodium-rich iron hexacyanoferrates, serve as cathode materials in sodium-ion batteries. These materials feature an open framework structure that allows for rapid sodium-ion insertion and extraction. The synthesis methods, including co-precipitation and hydrothermal techniques, significantly affect the crystallinity, particle size, and electrochemical performance of Prussian white cathodes. Controlling the sodium content and reducing vacancies in the crystal structure are essential for achieving high capacity and good cycling stability when matched with hard carbon anodes.Expand Specific Solutions03 Electrolyte formulations for hard carbon/Prussian white full cells
The electrolyte composition plays a crucial role in the performance of hard carbon/Prussian white full cells. Optimized electrolytes typically contain sodium salts such as NaPF6 or NaClO4 dissolved in carbonate-based solvents. Additives can be incorporated to form stable solid electrolyte interphase (SEI) layers on the hard carbon anode, preventing continuous electrolyte decomposition and improving cycling stability. The concentration of sodium salts and the ratio of different solvents need to be carefully balanced to ensure good ionic conductivity while maintaining compatibility with both electrode materials.Expand Specific Solutions04 Capacity balancing and electrode design for full cells
Achieving optimal performance in hard carbon/Prussian white full cells requires careful balancing of the capacity ratio between anode and cathode materials. The negative-to-positive capacity ratio typically needs to be greater than 1 to compensate for irreversible capacity loss during the first cycle. Electrode design parameters such as active material loading, thickness, and porosity significantly impact the rate capability and energy density of the full cell. Advanced electrode architectures incorporating conductive additives and binders can enhance the electronic conductivity and mechanical stability of both electrodes.Expand Specific Solutions05 Full cell assembly and performance optimization
The assembly process of hard carbon/Prussian white full cells involves multiple critical steps that affect the final performance. Pre-lithiation or pre-sodiation techniques can be applied to compensate for initial irreversible capacity loss. Proper cell formation protocols, including controlled charge-discharge cycles at low rates, help establish stable interfaces between electrodes and electrolyte. Operating voltage windows need to be optimized to prevent side reactions while maximizing energy density. Advanced characterization techniques can be used to monitor structural changes in both electrodes during cycling, providing insights for further optimization of the full cell system.Expand Specific Solutions
Key Industry Players in Sodium-ion Battery Development
The full cell matching of hard carbon anodes with Prussian White cathodes represents an emerging frontier in sodium-ion battery technology, currently in early commercial development phase. The market is experiencing rapid growth, projected to reach significant scale as industries seek alternatives to lithium-ion batteries. Technologically, companies demonstrate varying levels of maturity: Faradion and LG Energy Solution lead with commercial-ready solutions, while Samsung SDI, NEC, and Sharp are advancing research capabilities. Toyota and CSIR are exploring automotive applications, with universities like IIT Bombay and Jilin University contributing fundamental research. The technology benefits from hard carbon's abundant raw materials and Prussian White's stable structure, positioning it as a promising energy storage solution despite remaining challenges in energy density and cycle life.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced sodium-ion battery systems utilizing hard carbon anodes paired with Prussian white cathodes. Their technology focuses on optimizing the electrochemical interface between these components to maximize energy density and cycle life. The company has engineered a proprietary electrolyte formulation that enhances sodium-ion transport while minimizing side reactions at the hard carbon surface. Their full cell design incorporates pre-sodiation techniques to compensate for initial capacity loss and specialized carbon coatings on the Prussian white cathodes to improve structural stability during cycling. LG's approach includes precise control of the anode-to-cathode capacity ratio (typically 1.1-1.2:1) to account for irreversible capacity losses while maintaining high energy density. Their manufacturing process includes tailored formation protocols that establish stable solid electrolyte interphase layers on the hard carbon anodes.
Strengths: Superior cell balancing expertise from extensive lithium-ion manufacturing experience; advanced electrolyte formulations that enhance compatibility between hard carbon and Prussian white components; established global supply chain and manufacturing infrastructure. Weaknesses: Higher production costs compared to some competitors; relatively new entrant to sodium-ion technology compared to specialized research institutions.
Faradion Ltd.
Technical Solution: Faradion has developed a proprietary full cell sodium-ion technology centered on the pairing of hard carbon anodes with Prussian white cathodes. Their approach features a patented hard carbon synthesis method that creates optimized pore structures specifically designed for sodium-ion intercalation, achieving reversible capacities exceeding 300 mAh/g. On the cathode side, Faradion has engineered Prussian white materials with controlled vacancy concentrations and reduced impurities, resulting in higher initial coulombic efficiency (>80%) and improved cycle stability. Their full cell design incorporates tailored electrolyte formulations containing fluoroethylene carbonate and other additives that form stable solid electrolyte interphase layers on the hard carbon surface. Faradion's manufacturing process includes specialized formation protocols that establish optimal electrode-electrolyte interfaces while minimizing irreversible capacity losses. The company has demonstrated full cells with energy densities approaching 140 Wh/kg at the cell level, with cycle life exceeding 1,000 cycles at 80% depth of discharge.
Strengths: Specialized focus on sodium-ion technology with extensive intellectual property portfolio; advanced hard carbon synthesis techniques yielding superior performance; demonstrated commercial-scale manufacturing capability. Weaknesses: Limited production capacity compared to larger battery manufacturers; challenges in competing with the established lithium-ion ecosystem.
Critical Patents and Research in Electrode Pairing
High energy li batteries with lean lithium metal anodes and methods for prelithiation
PatentPendingUS20230317916A1
Innovation
- A lithium full cell design featuring a prelithiated anode with hard carbon materials and a controlled lithium metal amount, where the anode active material is prelithiated before the initial charge, forming a stable solid-electrolyte interface and suppressing dendrite growth, and is combined with a high-capacity cathode and lean electrolyte to achieve balanced N/P capacity ratios.
Anode containing diatom frustules
PatentWO2017103272A1
Innovation
- The use of calcined diatoms coated in carbon with an electrically conducting filler and a water-soluble binder, such as alginate, creates a porous silicon dioxide network that mitigates expansion issues and enhances electrochemical performance.
Material Supply Chain Analysis
The supply chain for materials used in full cell matching of hard carbon anodes with Prussian White cathodes presents a complex network of sourcing, processing, and distribution challenges. Raw material availability for hard carbon production relies heavily on biomass sources such as coconut shells, wood, and agricultural waste products. These precursors require specialized carbonization and activation processes to achieve the desired porosity and surface characteristics for optimal battery performance.
For Prussian White cathodes, the supply chain begins with the sourcing of transition metals, particularly iron, which benefits from relatively abundant global reserves compared to lithium and cobalt used in conventional lithium-ion batteries. However, the production of high-purity hexacyanoferrate compounds demands specialized chemical processing capabilities that are currently concentrated in a limited number of facilities worldwide.
The geographical distribution of material suppliers reveals significant concentration risks. China dominates the processing of both hard carbon precursors and the chemical compounds required for Prussian White synthesis, controlling approximately 65% of the global production capacity. This concentration creates potential vulnerabilities in the supply chain, particularly as demand for sodium-ion battery materials increases.
Pricing volatility represents another critical factor in the supply chain analysis. Unlike lithium-ion battery materials that have experienced dramatic price fluctuations, the raw materials for hard carbon and Prussian White cathodes have demonstrated relatively stable cost structures. This stability provides manufacturers with more predictable input costs, potentially enabling more competitive pricing strategies for end products.
Sustainability considerations are increasingly influencing supply chain development for these materials. Hard carbon derived from biomass waste streams offers circular economy benefits, while Prussian White cathodes avoid the ethical sourcing concerns associated with cobalt and nickel. However, the environmental impact of chemical processing for Prussian White compounds requires further assessment and optimization.
Scaling production presents significant challenges for both materials. Current manufacturing capacity for battery-grade hard carbon and Prussian White compounds remains limited, with few suppliers capable of meeting the quality specifications required for high-performance sodium-ion batteries. Industry forecasts suggest that supply constraints could emerge as early as 2025 if demand growth follows projected adoption curves for sodium-ion battery technologies.
For Prussian White cathodes, the supply chain begins with the sourcing of transition metals, particularly iron, which benefits from relatively abundant global reserves compared to lithium and cobalt used in conventional lithium-ion batteries. However, the production of high-purity hexacyanoferrate compounds demands specialized chemical processing capabilities that are currently concentrated in a limited number of facilities worldwide.
The geographical distribution of material suppliers reveals significant concentration risks. China dominates the processing of both hard carbon precursors and the chemical compounds required for Prussian White synthesis, controlling approximately 65% of the global production capacity. This concentration creates potential vulnerabilities in the supply chain, particularly as demand for sodium-ion battery materials increases.
Pricing volatility represents another critical factor in the supply chain analysis. Unlike lithium-ion battery materials that have experienced dramatic price fluctuations, the raw materials for hard carbon and Prussian White cathodes have demonstrated relatively stable cost structures. This stability provides manufacturers with more predictable input costs, potentially enabling more competitive pricing strategies for end products.
Sustainability considerations are increasingly influencing supply chain development for these materials. Hard carbon derived from biomass waste streams offers circular economy benefits, while Prussian White cathodes avoid the ethical sourcing concerns associated with cobalt and nickel. However, the environmental impact of chemical processing for Prussian White compounds requires further assessment and optimization.
Scaling production presents significant challenges for both materials. Current manufacturing capacity for battery-grade hard carbon and Prussian White compounds remains limited, with few suppliers capable of meeting the quality specifications required for high-performance sodium-ion batteries. Industry forecasts suggest that supply constraints could emerge as early as 2025 if demand growth follows projected adoption curves for sodium-ion battery technologies.
Environmental Impact and Sustainability Assessment
The environmental impact of sodium-ion battery systems utilizing hard carbon anodes and Prussian White cathodes represents a critical consideration in their development and deployment. These battery systems offer significant sustainability advantages compared to conventional lithium-ion technologies, primarily due to the abundance of sodium resources. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, substantially reducing resource depletion concerns and extraction-related environmental impacts.
The production of hard carbon anodes from biomass waste materials presents a particularly promising sustainability pathway. When derived from agricultural residues, wood processing byproducts, or food industry waste, these carbon materials contribute to circular economy principles by valorizing waste streams. Life cycle assessment studies indicate that biomass-derived hard carbon can reduce the carbon footprint of anode production by 35-45% compared to petroleum-based carbon sources.
Prussian White cathode materials also demonstrate favorable environmental characteristics. Their synthesis typically involves lower temperature processes compared to conventional lithium-ion cathode materials, resulting in reduced energy consumption during manufacturing. Additionally, these materials contain primarily iron, carbon, and nitrogen—elements with relatively low toxicity and environmental impact compared to cobalt, nickel, and manganese used in many lithium-ion cathodes.
Water consumption represents another important environmental consideration. The production processes for both hard carbon anodes and Prussian White cathodes generally require less water than conventional battery materials. However, careful management of process chemicals, particularly cyanide compounds used in Prussian White synthesis, is essential to prevent water contamination risks.
End-of-life management for these sodium-ion batteries shows promising recyclability characteristics. The absence of critical raw materials like cobalt and lithium simplifies recycling processes. Research indicates recovery rates exceeding 90% for iron and sodium components are achievable through hydrometallurgical recycling approaches, though commercial-scale recycling infrastructure remains underdeveloped.
Carbon footprint analyses across the full life cycle reveal that sodium-ion batteries with hard carbon/Prussian White chemistry can achieve 15-30% lower greenhouse gas emissions compared to equivalent lithium-ion systems. This advantage stems primarily from reduced mining impacts, less energy-intensive material processing, and potential for renewable carbon sourcing in anode production.
The production of hard carbon anodes from biomass waste materials presents a particularly promising sustainability pathway. When derived from agricultural residues, wood processing byproducts, or food industry waste, these carbon materials contribute to circular economy principles by valorizing waste streams. Life cycle assessment studies indicate that biomass-derived hard carbon can reduce the carbon footprint of anode production by 35-45% compared to petroleum-based carbon sources.
Prussian White cathode materials also demonstrate favorable environmental characteristics. Their synthesis typically involves lower temperature processes compared to conventional lithium-ion cathode materials, resulting in reduced energy consumption during manufacturing. Additionally, these materials contain primarily iron, carbon, and nitrogen—elements with relatively low toxicity and environmental impact compared to cobalt, nickel, and manganese used in many lithium-ion cathodes.
Water consumption represents another important environmental consideration. The production processes for both hard carbon anodes and Prussian White cathodes generally require less water than conventional battery materials. However, careful management of process chemicals, particularly cyanide compounds used in Prussian White synthesis, is essential to prevent water contamination risks.
End-of-life management for these sodium-ion batteries shows promising recyclability characteristics. The absence of critical raw materials like cobalt and lithium simplifies recycling processes. Research indicates recovery rates exceeding 90% for iron and sodium components are achievable through hydrometallurgical recycling approaches, though commercial-scale recycling infrastructure remains underdeveloped.
Carbon footprint analyses across the full life cycle reveal that sodium-ion batteries with hard carbon/Prussian White chemistry can achieve 15-30% lower greenhouse gas emissions compared to equivalent lithium-ion systems. This advantage stems primarily from reduced mining impacts, less energy-intensive material processing, and potential for renewable carbon sourcing in anode production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!