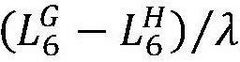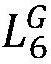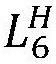Pouch Cell Validation and Scaling of Hard Carbon for Sodium Ion Batteries
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Ion Battery Technology Evolution and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) due to the abundance and widespread distribution of sodium resources. The evolution of SIB technology can be traced back to the 1970s and 1980s when initial research was conducted alongside lithium-ion systems. However, the rapid commercialization of LIBs in the early 1990s shifted focus away from sodium-based systems for nearly two decades.
The resurgence of interest in SIBs began around 2010, driven by concerns about lithium resource limitations and increasing demand for energy storage solutions. This renewed attention has led to significant advancements in electrode materials, electrolytes, and cell designs specifically tailored for sodium-ion chemistry. Hard carbon, in particular, has emerged as a promising anode material due to its ability to accommodate sodium ions effectively.
The technological evolution of SIBs has progressed through several distinct phases. The initial discovery phase focused on fundamental electrochemical principles and material compatibility. This was followed by a materials optimization phase, where researchers explored various carbon structures, including hard carbon derived from different precursors such as biomass, polymers, and petroleum products.
Current development is centered on the engineering and scaling phase, where laboratory-scale discoveries are being translated into commercially viable products. This includes pouch cell validation and scaling of hard carbon production processes, which represents a critical step toward industrial implementation. The optimization of hard carbon's microstructure, porosity, and surface characteristics has been instrumental in improving capacity, cycling stability, and rate capability.
The primary objectives of current research in pouch cell validation and hard carbon scaling include achieving energy densities approaching 160 Wh/kg at the cell level, extending cycle life beyond 2,000 cycles, and reducing production costs to below $100/kWh. Additionally, researchers aim to develop sustainable and scalable synthesis methods for hard carbon that minimize environmental impact while maintaining consistent performance characteristics.
Another key objective is to understand and mitigate the first-cycle irreversible capacity loss in hard carbon anodes, which typically ranges from 20-30%. This phenomenon significantly impacts the overall energy density of sodium-ion cells and represents a major hurdle for commercialization. Strategies being explored include surface pre-sodiation, electrolyte additives, and structural modifications to the hard carbon precursors.
The technology roadmap for SIBs envisions their initial deployment in stationary energy storage applications by 2025, followed by integration into electric vehicles and portable electronics by 2030. This progressive approach allows for continuous improvement of the technology while establishing market presence in less demanding applications first.
The resurgence of interest in SIBs began around 2010, driven by concerns about lithium resource limitations and increasing demand for energy storage solutions. This renewed attention has led to significant advancements in electrode materials, electrolytes, and cell designs specifically tailored for sodium-ion chemistry. Hard carbon, in particular, has emerged as a promising anode material due to its ability to accommodate sodium ions effectively.
The technological evolution of SIBs has progressed through several distinct phases. The initial discovery phase focused on fundamental electrochemical principles and material compatibility. This was followed by a materials optimization phase, where researchers explored various carbon structures, including hard carbon derived from different precursors such as biomass, polymers, and petroleum products.
Current development is centered on the engineering and scaling phase, where laboratory-scale discoveries are being translated into commercially viable products. This includes pouch cell validation and scaling of hard carbon production processes, which represents a critical step toward industrial implementation. The optimization of hard carbon's microstructure, porosity, and surface characteristics has been instrumental in improving capacity, cycling stability, and rate capability.
The primary objectives of current research in pouch cell validation and hard carbon scaling include achieving energy densities approaching 160 Wh/kg at the cell level, extending cycle life beyond 2,000 cycles, and reducing production costs to below $100/kWh. Additionally, researchers aim to develop sustainable and scalable synthesis methods for hard carbon that minimize environmental impact while maintaining consistent performance characteristics.
Another key objective is to understand and mitigate the first-cycle irreversible capacity loss in hard carbon anodes, which typically ranges from 20-30%. This phenomenon significantly impacts the overall energy density of sodium-ion cells and represents a major hurdle for commercialization. Strategies being explored include surface pre-sodiation, electrolyte additives, and structural modifications to the hard carbon precursors.
The technology roadmap for SIBs envisions their initial deployment in stationary energy storage applications by 2025, followed by integration into electric vehicles and portable electronics by 2030. This progressive approach allows for continuous improvement of the technology while establishing market presence in less demanding applications first.
Market Analysis for Sodium Ion Battery Applications
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries. Current market projections indicate that the global sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate exceeding 20% through 2030. This growth is primarily driven by increasing demand for sustainable energy storage solutions and concerns about lithium supply chain vulnerabilities.
Key market segments for sodium-ion batteries include stationary energy storage systems, which represent approximately 45% of potential applications. These systems are particularly valuable for grid stabilization, renewable energy integration, and backup power solutions where energy density is less critical than cost and sustainability. The electric mobility sector, particularly for two and three-wheelers in emerging markets, constitutes about 30% of potential applications, with consumer electronics and low-cost electric vehicles representing another 15%.
Geographically, Asia-Pacific dominates the market landscape with China leading research, development, and commercialization efforts. European markets are rapidly expanding due to stringent environmental regulations and substantial investments in green technology. North America follows with growing interest primarily in the utility-scale storage sector.
The cost advantage of sodium-ion technology is compelling, with raw material costs approximately 30-40% lower than lithium-ion equivalents. Hard carbon, the primary anode material for sodium-ion batteries, is derived from abundant organic precursors, offering significant cost benefits over graphite used in lithium-ion batteries. This economic advantage is particularly attractive for price-sensitive applications where battery cost represents a significant portion of the total system expense.
Market barriers include the technology's lower energy density compared to advanced lithium-ion chemistries, limiting applications in premium electric vehicles and high-end portable electronics. However, recent advancements in hard carbon optimization have narrowed this gap, with energy densities now reaching 160 Wh/kg at the cell level, making them viable for numerous applications.
Customer adoption trends indicate growing acceptance in markets where cost sensitivity outweighs energy density requirements. Utility companies are increasingly incorporating sodium-ion batteries into their renewable energy integration strategies, while manufacturers of low-speed electric vehicles in emerging markets are exploring sodium-ion batteries as cost-effective alternatives to lead-acid batteries.
The competitive landscape is evolving rapidly with traditional battery manufacturers expanding their portfolios to include sodium-ion technology, while specialized startups focus exclusively on advancing sodium-ion chemistry and scaling production capabilities.
Key market segments for sodium-ion batteries include stationary energy storage systems, which represent approximately 45% of potential applications. These systems are particularly valuable for grid stabilization, renewable energy integration, and backup power solutions where energy density is less critical than cost and sustainability. The electric mobility sector, particularly for two and three-wheelers in emerging markets, constitutes about 30% of potential applications, with consumer electronics and low-cost electric vehicles representing another 15%.
Geographically, Asia-Pacific dominates the market landscape with China leading research, development, and commercialization efforts. European markets are rapidly expanding due to stringent environmental regulations and substantial investments in green technology. North America follows with growing interest primarily in the utility-scale storage sector.
The cost advantage of sodium-ion technology is compelling, with raw material costs approximately 30-40% lower than lithium-ion equivalents. Hard carbon, the primary anode material for sodium-ion batteries, is derived from abundant organic precursors, offering significant cost benefits over graphite used in lithium-ion batteries. This economic advantage is particularly attractive for price-sensitive applications where battery cost represents a significant portion of the total system expense.
Market barriers include the technology's lower energy density compared to advanced lithium-ion chemistries, limiting applications in premium electric vehicles and high-end portable electronics. However, recent advancements in hard carbon optimization have narrowed this gap, with energy densities now reaching 160 Wh/kg at the cell level, making them viable for numerous applications.
Customer adoption trends indicate growing acceptance in markets where cost sensitivity outweighs energy density requirements. Utility companies are increasingly incorporating sodium-ion batteries into their renewable energy integration strategies, while manufacturers of low-speed electric vehicles in emerging markets are exploring sodium-ion batteries as cost-effective alternatives to lead-acid batteries.
The competitive landscape is evolving rapidly with traditional battery manufacturers expanding their portfolios to include sodium-ion technology, while specialized startups focus exclusively on advancing sodium-ion chemistry and scaling production capabilities.
Hard Carbon Development Status and Technical Barriers
Hard carbon has emerged as a promising anode material for sodium-ion batteries (SIBs) due to its unique structure and sodium storage capabilities. Currently, hard carbon materials derived from various precursors such as biomass, polymers, and petroleum pitch have demonstrated sodium storage capacities ranging from 250 to 350 mAh/g in laboratory settings. However, commercial-scale production typically yields lower capacities of 220-300 mAh/g, indicating a gap between research achievements and industrial applications.
The synthesis of hard carbon primarily involves pyrolysis of carbon-rich precursors at temperatures between 1000-1500°C under inert atmospheres. While this process is well-established, significant challenges remain in controlling the microstructure, particularly the formation of turbostratic domains and nanopores that are critical for sodium ion storage. The relationship between precursor selection, processing conditions, and final electrochemical performance remains incompletely understood, hindering systematic optimization.
A major technical barrier in hard carbon development is the trade-off between capacity and initial coulombic efficiency (ICE). Materials with higher capacity often suffer from lower ICE (typically 70-85%), which is problematic for full cell applications. This inefficiency is primarily attributed to irreversible sodium consumption during solid electrolyte interphase (SEI) formation and trapping within the carbon structure.
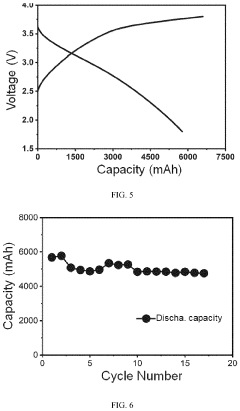
Scaling production from laboratory to industrial levels presents significant challenges in maintaining consistent quality and performance. Batch-to-batch variations in precursor composition, especially for biomass-derived carbons, lead to inconsistent electrochemical properties. Additionally, the high-temperature processing required for hard carbon synthesis is energy-intensive and costly, with current production costs estimated at 15-20 USD/kg, significantly higher than graphite anodes for lithium-ion batteries.
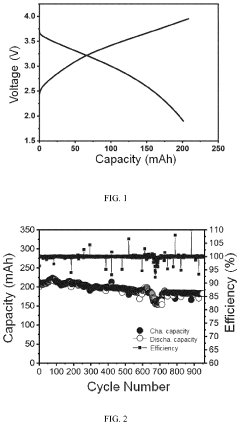
The pouch cell validation of hard carbon anodes reveals additional challenges related to electrode thickness and loading. While thin electrodes (≤100 μm) show promising performance in coin cells, thicker electrodes necessary for practical energy density in pouch cells (150-200 μm) often exhibit increased polarization and reduced rate capability. This thickness-dependent performance degradation is attributed to the limited electronic conductivity of hard carbon (typically 10-100 S/cm) compared to graphite (>1000 S/cm).
Furthermore, the long-term cycling stability of hard carbon remains inferior to graphite anodes, with capacity retention typically limited to 80-90% after 500 cycles. This degradation is associated with continuous SEI evolution and structural changes during repeated sodium insertion/extraction. Recent research indicates that surface modification and electrolyte optimization can mitigate these issues, but comprehensive solutions applicable to industrial-scale production are still under development.
The synthesis of hard carbon primarily involves pyrolysis of carbon-rich precursors at temperatures between 1000-1500°C under inert atmospheres. While this process is well-established, significant challenges remain in controlling the microstructure, particularly the formation of turbostratic domains and nanopores that are critical for sodium ion storage. The relationship between precursor selection, processing conditions, and final electrochemical performance remains incompletely understood, hindering systematic optimization.
A major technical barrier in hard carbon development is the trade-off between capacity and initial coulombic efficiency (ICE). Materials with higher capacity often suffer from lower ICE (typically 70-85%), which is problematic for full cell applications. This inefficiency is primarily attributed to irreversible sodium consumption during solid electrolyte interphase (SEI) formation and trapping within the carbon structure.
Scaling production from laboratory to industrial levels presents significant challenges in maintaining consistent quality and performance. Batch-to-batch variations in precursor composition, especially for biomass-derived carbons, lead to inconsistent electrochemical properties. Additionally, the high-temperature processing required for hard carbon synthesis is energy-intensive and costly, with current production costs estimated at 15-20 USD/kg, significantly higher than graphite anodes for lithium-ion batteries.
The pouch cell validation of hard carbon anodes reveals additional challenges related to electrode thickness and loading. While thin electrodes (≤100 μm) show promising performance in coin cells, thicker electrodes necessary for practical energy density in pouch cells (150-200 μm) often exhibit increased polarization and reduced rate capability. This thickness-dependent performance degradation is attributed to the limited electronic conductivity of hard carbon (typically 10-100 S/cm) compared to graphite (>1000 S/cm).
Furthermore, the long-term cycling stability of hard carbon remains inferior to graphite anodes, with capacity retention typically limited to 80-90% after 500 cycles. This degradation is associated with continuous SEI evolution and structural changes during repeated sodium insertion/extraction. Recent research indicates that surface modification and electrolyte optimization can mitigate these issues, but comprehensive solutions applicable to industrial-scale production are still under development.
Current Hard Carbon Pouch Cell Validation Methods
01 Hard carbon synthesis methods for sodium ion batteries
Various methods can be used to synthesize hard carbon materials for sodium ion batteries, including pyrolysis of organic precursors, hydrothermal carbonization, and template-assisted synthesis. These methods control the microstructure and porosity of hard carbon, which directly affects its sodium storage capacity and cycling stability. Optimized synthesis parameters such as temperature, time, and atmosphere play crucial roles in determining the final properties of hard carbon materials.- Hard carbon synthesis methods for sodium ion batteries: Various methods for synthesizing hard carbon materials suitable for sodium ion batteries are described. These methods include pyrolysis of biomass or organic precursors under controlled conditions, hydrothermal carbonization followed by high-temperature treatment, and chemical activation processes. The synthesis parameters such as temperature, time, and atmosphere significantly influence the microstructure and electrochemical performance of the resulting hard carbon materials.
- Structural optimization of hard carbon for improved sodium storage: Structural optimization of hard carbon materials focuses on creating optimal pore structures, defect sites, and interlayer spacing to enhance sodium ion storage capacity and diffusion kinetics. Techniques include controlled introduction of heteroatoms, creation of hierarchical pore structures, and surface modification. These structural modifications aim to increase the number of active sites for sodium ion adsorption and improve the overall electrochemical performance.
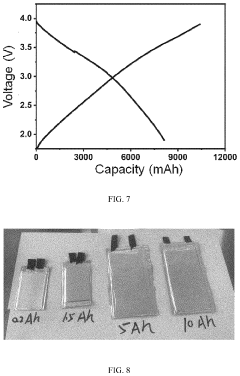
- Scaling up production of hard carbon materials: Scaling up the production of hard carbon materials for commercial sodium ion batteries involves developing continuous manufacturing processes, optimizing reactor designs, and establishing quality control protocols. Key challenges addressed include maintaining consistent material properties during scale-up, reducing production costs, and minimizing environmental impact. Various approaches to large-scale production of hard carbon from sustainable precursors are presented.
- Validation techniques for hard carbon performance: Comprehensive validation techniques for evaluating hard carbon performance in sodium ion batteries include electrochemical characterization methods, structural analysis, and long-term cycling tests. Advanced analytical techniques such as in-situ XRD, TEM, and spectroscopic methods are employed to understand sodium storage mechanisms and degradation pathways. Standardized testing protocols are established to ensure reliable comparison between different hard carbon materials.
- Composite hard carbon materials for enhanced performance: Development of composite hard carbon materials involves combining hard carbon with other components such as metal oxides, conductive additives, or heteroatom-doped carbons to enhance electrochemical performance. These composite materials demonstrate improved capacity, rate capability, and cycling stability compared to conventional hard carbon. The synergistic effects between different components in the composite structure contribute to enhanced sodium storage properties.
02 Precursor selection and optimization for hard carbon production
The choice of precursor materials significantly impacts the performance of hard carbon anodes in sodium ion batteries. Biomass-derived precursors like cellulose, lignin, and various agricultural wastes offer sustainable and cost-effective options. Synthetic polymers and petroleum-based precursors can also be used to produce hard carbon with controlled properties. The pretreatment of precursors, including chemical activation and doping with heteroatoms, can enhance the electrochemical performance of the resulting hard carbon materials.Expand Specific Solutions03 Structural characterization and validation techniques
Comprehensive characterization techniques are essential for validating the structure and properties of hard carbon materials for sodium ion batteries. These include X-ray diffraction (XRD) for crystallinity analysis, Raman spectroscopy for disorder degree evaluation, scanning and transmission electron microscopy (SEM/TEM) for morphological studies, and X-ray photoelectron spectroscopy (XPS) for surface chemistry analysis. Electrochemical techniques such as galvanostatic charge-discharge, cyclic voltammetry, and impedance spectroscopy are used to evaluate battery performance parameters.Expand Specific Solutions04 Scaling up production and industrial manufacturing
Scaling up hard carbon production from laboratory to industrial scale involves several challenges including maintaining consistent quality, optimizing process parameters, and reducing production costs. Continuous production methods, such as spray pyrolysis and rotary kiln carbonization, enable large-scale manufacturing of hard carbon materials. Process monitoring and quality control systems are implemented to ensure batch-to-batch consistency. Economic and environmental assessments are conducted to evaluate the feasibility of commercial production.Expand Specific Solutions05 Performance enhancement strategies for hard carbon anodes
Various strategies can be employed to enhance the performance of hard carbon anodes in sodium ion batteries. These include heteroatom doping (N, S, P, B) to create active sites for sodium storage, surface modification to improve the solid-electrolyte interface stability, composite formation with other materials like graphene or metal oxides, and hierarchical pore structure design to facilitate sodium ion transport. Electrolyte optimization and electrode engineering also play crucial roles in maximizing the performance of hard carbon anodes.Expand Specific Solutions
Leading Companies in Sodium Ion Battery Industry
The sodium-ion battery market is experiencing rapid growth, with the hard carbon pouch cell technology representing a critical advancement in this emerging field. Currently in the early commercialization phase, this technology is gaining momentum as companies seek alternatives to lithium-ion batteries. Key players include established battery manufacturers like CATL and EVE Energy, alongside specialized sodium-ion innovators such as Faradion Ltd. Research institutions including CNRS, College de France, and Tongji University are driving fundamental advancements in hard carbon optimization. Major automotive companies like Toyota and GM are exploring integration possibilities, while materials specialists Haycarb and Ingevity contribute expertise in carbon technologies. The technology is approaching commercial readiness, with scaling challenges being addressed through collaborative efforts between academic institutions and industry partners to improve energy density and cycle life performance.
Faradion Ltd.
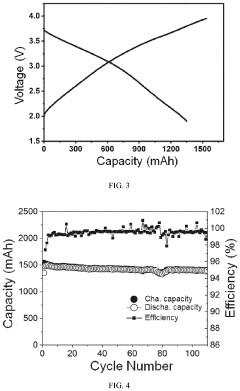
Technical Solution: Faradion has pioneered a proprietary hard carbon synthesis process specifically optimized for sodium-ion pouch cells. Their technology involves a controlled pyrolysis of sustainable organic precursors at temperatures between 1100-1300°C, resulting in hard carbon materials with optimized pore structures and d-spacing. For pouch cell validation, Faradion employs a multi-stage quality control process including electrochemical impedance spectroscopy and long-term cycling tests at various C-rates. Their scaling approach incorporates a continuous flow reactor system that can produce up to 100kg of hard carbon per day while maintaining consistent structural properties. The company has successfully demonstrated pouch cells with energy densities approaching 160 Wh/kg, with capacity retention of >80% after 1000 cycles at 1C charge/discharge rates. Faradion's hard carbon materials exhibit first cycle efficiencies of approximately 78-82%, which they've improved through surface modification techniques.
Strengths: Industry-leading first cycle efficiency for hard carbon anodes; established manufacturing scale-up pathway with proven quality control metrics; materials derived from sustainable precursors reducing environmental impact. Weaknesses: Energy density still lags behind lithium-ion alternatives; higher production costs compared to graphite anodes; temperature performance limitations at extreme cold conditions.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced hard carbon production and validation system for sodium-ion battery pouch cells. Their approach utilizes a two-step carbonization process with precise temperature control between 1200-1400°C to optimize the microstructure of hard carbon derived from biomass precursors. CATL's pouch cell validation methodology incorporates automated high-throughput testing systems capable of simultaneously evaluating hundreds of cells under various operating conditions. Their scaling strategy involves modular production lines that can be rapidly expanded to meet market demand. The company has achieved sodium-ion pouch cells with energy densities of approximately 160 Wh/kg and power densities up to 500 W/kg. CATL's hard carbon materials feature a hierarchical pore structure that facilitates sodium ion diffusion while maintaining structural stability during repeated cycling. Their production process includes proprietary surface treatment technologies that improve the solid electrolyte interphase formation, resulting in first cycle efficiencies exceeding 80% and capacity retention of >90% after 1000 cycles at moderate rates.
Strengths: Massive manufacturing infrastructure allowing rapid scale-up; integrated supply chain from raw materials to finished cells; advanced quality control systems with AI-powered defect detection. Weaknesses: Higher initial investment costs compared to competitors; relatively new to sodium-ion technology compared to their established lithium-ion expertise; current energy density limitations compared to their premium lithium-ion offerings.
Critical Patents in Hard Carbon Scaling Technologies
Ampere-hour-scale sodium-ion pouch cell
PatentPendingUS20240154113A1
Innovation
- The use of O3 layered oxide as a cathode material combined with a novel chemical presodiation process for hard carbon anodes, along with an optimized assembly process, results in a higher compacted density and improved capacity ratios, enabling the production of stable ampere-hour-scale sodium-ion pouch cells.
Hard carbon material and preparation method therefor, negative electrode material, and sodium ion battery
PatentWO2025153073A1
Innovation
- By controlling the lattice curvature of the hard carbon material to be 0.03-0.15 and the closed pore volume is 0.04-0.5cm3·g-1, the microstructure of the hard carbon material is optimized by using atomic pair distribution function technology, combining biomass derivative carbon sources or polymer organics as raw materials, and medium-temperature carbonization treatment is used to prepare hard carbon materials with high closed pore ratio and good pore strength.
Supply Chain Analysis for Hard Carbon Production
The hard carbon supply chain for sodium-ion batteries represents a critical component in the commercialization pathway for this emerging energy storage technology. Current production capacity for battery-grade hard carbon remains limited, with global output estimated at less than 5,000 tons annually, primarily concentrated in Asia. This production volume falls significantly short of the projected demand that would accompany large-scale sodium-ion battery deployment.
Raw material sourcing presents both challenges and opportunities. Hard carbon can be derived from various precursors including petroleum coke, coal tar pitch, and biomass sources such as cellulose, lignin, and agricultural waste. The diversity of potential feedstocks offers flexibility but also creates variability in final product characteristics. Biomass-derived hard carbon has gained particular attention due to sustainability advantages, though standardization of these renewable sources remains challenging.
Processing technologies for hard carbon production typically involve carbonization at temperatures between 1000-1500°C under controlled atmospheres, followed by activation treatments. Current manufacturing methods face scalability issues, with batch processing dominating over continuous production systems. Energy consumption during high-temperature treatment represents a significant cost factor, estimated at 30-40% of total production expenses. Process optimization to reduce energy requirements while maintaining structural properties remains a key technical challenge.
Geographic distribution of hard carbon production capabilities shows significant concentration in China, Japan, and South Korea, with limited capacity in Europe and North America. This geographic imbalance creates potential supply vulnerabilities for Western manufacturers seeking to develop sodium-ion battery production capabilities. Several specialty carbon manufacturers have announced expansion plans specifically targeting sodium-ion battery applications, though most remain in pilot or small-scale production phases.
Cost structures for hard carbon currently range between $15-30/kg, significantly higher than graphite for lithium-ion batteries ($5-15/kg). However, economic modeling suggests potential for cost reduction to $8-12/kg at industrial scale, which would enhance the overall economic competitiveness of sodium-ion technology. Achieving these cost targets requires both process optimization and economies of scale.
Quality control represents another critical supply chain consideration, as structural and electrochemical properties of hard carbon significantly impact battery performance. Current industry lacks standardized specifications for sodium-ion battery-grade hard carbon, creating challenges for consistent material qualification across different suppliers and manufacturing processes.
Raw material sourcing presents both challenges and opportunities. Hard carbon can be derived from various precursors including petroleum coke, coal tar pitch, and biomass sources such as cellulose, lignin, and agricultural waste. The diversity of potential feedstocks offers flexibility but also creates variability in final product characteristics. Biomass-derived hard carbon has gained particular attention due to sustainability advantages, though standardization of these renewable sources remains challenging.
Processing technologies for hard carbon production typically involve carbonization at temperatures between 1000-1500°C under controlled atmospheres, followed by activation treatments. Current manufacturing methods face scalability issues, with batch processing dominating over continuous production systems. Energy consumption during high-temperature treatment represents a significant cost factor, estimated at 30-40% of total production expenses. Process optimization to reduce energy requirements while maintaining structural properties remains a key technical challenge.
Geographic distribution of hard carbon production capabilities shows significant concentration in China, Japan, and South Korea, with limited capacity in Europe and North America. This geographic imbalance creates potential supply vulnerabilities for Western manufacturers seeking to develop sodium-ion battery production capabilities. Several specialty carbon manufacturers have announced expansion plans specifically targeting sodium-ion battery applications, though most remain in pilot or small-scale production phases.
Cost structures for hard carbon currently range between $15-30/kg, significantly higher than graphite for lithium-ion batteries ($5-15/kg). However, economic modeling suggests potential for cost reduction to $8-12/kg at industrial scale, which would enhance the overall economic competitiveness of sodium-ion technology. Achieving these cost targets requires both process optimization and economies of scale.
Quality control represents another critical supply chain consideration, as structural and electrochemical properties of hard carbon significantly impact battery performance. Current industry lacks standardized specifications for sodium-ion battery-grade hard carbon, creating challenges for consistent material qualification across different suppliers and manufacturing processes.
Sustainability Impact of Sodium Ion Battery Technology
The adoption of sodium-ion battery technology represents a significant step towards more sustainable energy storage solutions compared to conventional lithium-ion batteries. The environmental benefits begin with raw material sourcing, as sodium is the sixth most abundant element in the Earth's crust, approximately 1,000 times more plentiful than lithium. This abundance translates to reduced mining impacts, lower habitat destruction, and decreased water consumption associated with extraction processes.
Carbon footprint analyses indicate that sodium-ion battery production can generate up to 30% less CO2 emissions compared to lithium-ion counterparts, primarily due to the reduced energy requirements for sodium processing and the elimination of cobalt and nickel from cathode materials. The use of hard carbon derived from biomass waste as anode material further enhances sustainability by repurposing agricultural residues that would otherwise contribute to waste streams.
Water conservation represents another critical sustainability advantage, with sodium extraction requiring approximately 60% less water than lithium brine operations. This is particularly significant in water-stressed regions where lithium mining has faced increasing scrutiny for depleting local water resources and disrupting ecosystems.
From a circular economy perspective, sodium-ion batteries offer promising end-of-life management opportunities. The absence of critical raw materials like cobalt facilitates more straightforward recycling processes. Preliminary studies suggest recovery rates for sodium compounds could exceed 90% using hydrometallurgical methods, creating closed-loop material flows that minimize waste generation.
Social sustainability dimensions must also be considered, as the shift to sodium-based technologies reduces dependence on materials associated with ethical supply chain concerns. Unlike cobalt mining, which has been linked to human rights violations in regions like the Democratic Republic of Congo, sodium extraction presents fewer social risks and can support more equitable distribution of economic benefits across global supply chains.
The scaling of hard carbon production for sodium-ion batteries presents an opportunity to establish more localized manufacturing ecosystems, reducing transportation emissions and fostering regional economic development. This aligns with broader sustainability goals of creating resilient supply chains that minimize environmental impacts while maximizing socioeconomic benefits.
Carbon footprint analyses indicate that sodium-ion battery production can generate up to 30% less CO2 emissions compared to lithium-ion counterparts, primarily due to the reduced energy requirements for sodium processing and the elimination of cobalt and nickel from cathode materials. The use of hard carbon derived from biomass waste as anode material further enhances sustainability by repurposing agricultural residues that would otherwise contribute to waste streams.
Water conservation represents another critical sustainability advantage, with sodium extraction requiring approximately 60% less water than lithium brine operations. This is particularly significant in water-stressed regions where lithium mining has faced increasing scrutiny for depleting local water resources and disrupting ecosystems.
From a circular economy perspective, sodium-ion batteries offer promising end-of-life management opportunities. The absence of critical raw materials like cobalt facilitates more straightforward recycling processes. Preliminary studies suggest recovery rates for sodium compounds could exceed 90% using hydrometallurgical methods, creating closed-loop material flows that minimize waste generation.
Social sustainability dimensions must also be considered, as the shift to sodium-based technologies reduces dependence on materials associated with ethical supply chain concerns. Unlike cobalt mining, which has been linked to human rights violations in regions like the Democratic Republic of Congo, sodium extraction presents fewer social risks and can support more equitable distribution of economic benefits across global supply chains.
The scaling of hard carbon production for sodium-ion batteries presents an opportunity to establish more localized manufacturing ecosystems, reducing transportation emissions and fostering regional economic development. This aligns with broader sustainability goals of creating resilient supply chains that minimize environmental impacts while maximizing socioeconomic benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!