Geological distribution and occurrence of Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Overview
Magnesium iron silicate hydroxide, also known as serpentine, is a group of minerals that form an important part of the Earth's crust and upper mantle. These minerals are primarily found in ultramafic rocks, which are igneous rocks with a very low silica content and high magnesium and iron content. The geological distribution of serpentine is closely linked to the processes of plate tectonics and the formation of oceanic crust.
Serpentine minerals are most commonly found in ophiolite complexes, which are sections of oceanic crust and upper mantle that have been uplifted and exposed on land. These complexes are often found along convergent plate boundaries, where oceanic crust is subducted beneath continental crust. Notable ophiolite complexes containing serpentine can be found in Oman, Cyprus, and the western United States, particularly in California's Coast Ranges.
The formation of serpentine minerals occurs through a process called serpentinization, which involves the hydration of olivine and pyroxene in ultramafic rocks. This process typically takes place in oceanic environments, where seawater interacts with newly formed oceanic crust at mid-ocean ridges. As the oceanic crust cools and moves away from the ridge, it becomes more susceptible to fracturing, allowing seawater to penetrate and react with the ultramafic rocks.
Serpentine can also be found in metamorphic terranes, particularly in areas that have undergone regional metamorphism of ultramafic rocks. These occurrences are often associated with ancient suture zones, where different tectonic plates have collided and merged. Examples of such metamorphic serpentine deposits can be found in the Alps, the Appalachian Mountains, and the Tibetan Plateau.
In addition to its presence in ophiolites and metamorphic terranes, serpentine can also occur in smaller, localized deposits associated with intrusive igneous bodies. These deposits are often found in the form of serpentinite dikes or sills, which are formed when magma intrudes into pre-existing rock and undergoes serpentinization due to the presence of water.
The global distribution of serpentine is not uniform, with certain regions having a higher concentration of these minerals. The Pacific Rim, including countries like Japan, the Philippines, and New Caledonia, is particularly rich in serpentine deposits due to the extensive subduction zones and ophiolite complexes in the region. Other significant serpentine-bearing areas include the Mediterranean region, parts of the Caribbean, and certain sections of the Alpine-Himalayan orogenic belt.
Serpentine minerals are most commonly found in ophiolite complexes, which are sections of oceanic crust and upper mantle that have been uplifted and exposed on land. These complexes are often found along convergent plate boundaries, where oceanic crust is subducted beneath continental crust. Notable ophiolite complexes containing serpentine can be found in Oman, Cyprus, and the western United States, particularly in California's Coast Ranges.
The formation of serpentine minerals occurs through a process called serpentinization, which involves the hydration of olivine and pyroxene in ultramafic rocks. This process typically takes place in oceanic environments, where seawater interacts with newly formed oceanic crust at mid-ocean ridges. As the oceanic crust cools and moves away from the ridge, it becomes more susceptible to fracturing, allowing seawater to penetrate and react with the ultramafic rocks.
Serpentine can also be found in metamorphic terranes, particularly in areas that have undergone regional metamorphism of ultramafic rocks. These occurrences are often associated with ancient suture zones, where different tectonic plates have collided and merged. Examples of such metamorphic serpentine deposits can be found in the Alps, the Appalachian Mountains, and the Tibetan Plateau.
In addition to its presence in ophiolites and metamorphic terranes, serpentine can also occur in smaller, localized deposits associated with intrusive igneous bodies. These deposits are often found in the form of serpentinite dikes or sills, which are formed when magma intrudes into pre-existing rock and undergoes serpentinization due to the presence of water.
The global distribution of serpentine is not uniform, with certain regions having a higher concentration of these minerals. The Pacific Rim, including countries like Japan, the Philippines, and New Caledonia, is particularly rich in serpentine deposits due to the extensive subduction zones and ophiolite complexes in the region. Other significant serpentine-bearing areas include the Mediterranean region, parts of the Caribbean, and certain sections of the Alpine-Himalayan orogenic belt.
Global Market Demand Analysis
The global market demand for Magnesium iron silicate hydroxide, also known as serpentine, has been steadily increasing due to its diverse applications across various industries. This mineral, primarily found in metamorphic and igneous rocks, plays a crucial role in several sectors, driving its market growth.
In the construction industry, serpentine is widely used as a decorative stone and building material. Its unique patterns and colors make it a popular choice for architectural applications, including flooring, wall cladding, and countertops. The growing construction activities in emerging economies have significantly contributed to the rising demand for serpentine in this sector.
The automotive industry represents another significant market for Magnesium iron silicate hydroxide. The mineral is used in the production of brake linings and clutch facings due to its heat-resistant properties. As the global automotive industry continues to expand, particularly in developing countries, the demand for serpentine in this sector is expected to grow.
In the field of environmental remediation, serpentine has gained attention for its potential in carbon sequestration. The mineral's natural ability to absorb and store carbon dioxide has led to increased research and development efforts to utilize it in large-scale carbon capture projects. This emerging application could potentially drive substantial market growth in the coming years.
The agriculture sector also contributes to the demand for serpentine. The mineral is used as a soil amendment to improve soil structure and provide essential nutrients to plants. As global food demand rises and sustainable agricultural practices gain importance, the use of serpentine in agriculture is likely to increase.
In the industrial sector, serpentine finds applications in the production of magnesium metal and various magnesium compounds. These materials are used in a wide range of products, from lightweight alloys for aerospace applications to refractory materials for high-temperature industrial processes.
The global serpentine market is influenced by regional factors as well. Countries with significant serpentine deposits, such as Russia, Canada, and South Africa, play a crucial role in meeting the global demand. The market is also affected by trade policies, environmental regulations, and technological advancements in mining and processing techniques.
In the construction industry, serpentine is widely used as a decorative stone and building material. Its unique patterns and colors make it a popular choice for architectural applications, including flooring, wall cladding, and countertops. The growing construction activities in emerging economies have significantly contributed to the rising demand for serpentine in this sector.
The automotive industry represents another significant market for Magnesium iron silicate hydroxide. The mineral is used in the production of brake linings and clutch facings due to its heat-resistant properties. As the global automotive industry continues to expand, particularly in developing countries, the demand for serpentine in this sector is expected to grow.
In the field of environmental remediation, serpentine has gained attention for its potential in carbon sequestration. The mineral's natural ability to absorb and store carbon dioxide has led to increased research and development efforts to utilize it in large-scale carbon capture projects. This emerging application could potentially drive substantial market growth in the coming years.
The agriculture sector also contributes to the demand for serpentine. The mineral is used as a soil amendment to improve soil structure and provide essential nutrients to plants. As global food demand rises and sustainable agricultural practices gain importance, the use of serpentine in agriculture is likely to increase.
In the industrial sector, serpentine finds applications in the production of magnesium metal and various magnesium compounds. These materials are used in a wide range of products, from lightweight alloys for aerospace applications to refractory materials for high-temperature industrial processes.
The global serpentine market is influenced by regional factors as well. Countries with significant serpentine deposits, such as Russia, Canada, and South Africa, play a crucial role in meeting the global demand. The market is also affected by trade policies, environmental regulations, and technological advancements in mining and processing techniques.
Geological Distribution Challenges
The geological distribution of Magnesium iron silicate hydroxide, commonly known as serpentine minerals, presents several challenges for researchers and industry professionals. These minerals are primarily found in ultramafic rocks, which are predominantly located in specific tectonic settings such as ophiolite complexes, subduction zones, and oceanic crust. However, the precise mapping and quantification of these deposits pose significant difficulties due to their complex formation processes and variable compositions.
One of the primary challenges in understanding the geological distribution of serpentine minerals is their heterogeneous nature. The composition and structure of these minerals can vary significantly even within a single deposit, making it challenging to accurately assess their extent and quality. This variability is attributed to factors such as the original rock composition, pressure-temperature conditions during formation, and subsequent alteration processes.
The identification and characterization of serpentine minerals in the field also present obstacles. While some serpentine varieties, like chrysotile, may be visually distinctive, others can be easily confused with similar-looking minerals. This necessitates the use of advanced analytical techniques, such as X-ray diffraction and electron microscopy, which may not always be readily available in field settings.
Another significant challenge is the often remote and inaccessible locations of serpentine deposits. Many ultramafic rock formations are found in mountainous or oceanic regions, making comprehensive geological surveys logistically difficult and expensive. This limitation can result in incomplete or outdated data on the global distribution of these minerals.
The tectonic history of a region plays a crucial role in the formation and distribution of serpentine minerals. However, unraveling this history can be complex, especially in areas with multiple tectonic events or where the original geological context has been significantly altered. This complexity makes it challenging to predict the occurrence of serpentine minerals in unexplored areas or to estimate the total global reserves accurately.
Environmental concerns also impact the study and exploitation of serpentine mineral deposits. Some serpentine varieties, particularly those containing asbestos-like fibers, pose health risks. This factor complicates field research and limits the accessibility of certain deposits, potentially leading to gaps in our understanding of their distribution.
Climate change and weathering processes further complicate the geological distribution picture. Serpentine minerals can be altered or degraded over time, especially when exposed to surface conditions. This ongoing transformation can change the characteristics and extent of deposits, requiring continuous monitoring and reassessment of known locations.
Lastly, the economic viability of serpentine mineral deposits varies greatly depending on factors such as accessibility, grade, and market demand. This variability influences the intensity of exploration and mapping efforts in different regions, potentially leading to biases in our understanding of their global distribution. Overcoming these challenges requires a multidisciplinary approach, combining advanced geological techniques with innovative exploration methods and comprehensive data integration.
One of the primary challenges in understanding the geological distribution of serpentine minerals is their heterogeneous nature. The composition and structure of these minerals can vary significantly even within a single deposit, making it challenging to accurately assess their extent and quality. This variability is attributed to factors such as the original rock composition, pressure-temperature conditions during formation, and subsequent alteration processes.
The identification and characterization of serpentine minerals in the field also present obstacles. While some serpentine varieties, like chrysotile, may be visually distinctive, others can be easily confused with similar-looking minerals. This necessitates the use of advanced analytical techniques, such as X-ray diffraction and electron microscopy, which may not always be readily available in field settings.
Another significant challenge is the often remote and inaccessible locations of serpentine deposits. Many ultramafic rock formations are found in mountainous or oceanic regions, making comprehensive geological surveys logistically difficult and expensive. This limitation can result in incomplete or outdated data on the global distribution of these minerals.
The tectonic history of a region plays a crucial role in the formation and distribution of serpentine minerals. However, unraveling this history can be complex, especially in areas with multiple tectonic events or where the original geological context has been significantly altered. This complexity makes it challenging to predict the occurrence of serpentine minerals in unexplored areas or to estimate the total global reserves accurately.
Environmental concerns also impact the study and exploitation of serpentine mineral deposits. Some serpentine varieties, particularly those containing asbestos-like fibers, pose health risks. This factor complicates field research and limits the accessibility of certain deposits, potentially leading to gaps in our understanding of their distribution.
Climate change and weathering processes further complicate the geological distribution picture. Serpentine minerals can be altered or degraded over time, especially when exposed to surface conditions. This ongoing transformation can change the characteristics and extent of deposits, requiring continuous monitoring and reassessment of known locations.
Lastly, the economic viability of serpentine mineral deposits varies greatly depending on factors such as accessibility, grade, and market demand. This variability influences the intensity of exploration and mapping efforts in different regions, potentially leading to biases in our understanding of their global distribution. Overcoming these challenges requires a multidisciplinary approach, combining advanced geological techniques with innovative exploration methods and comprehensive data integration.
Current Extraction Techniques
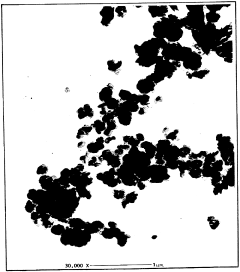
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups, forming a complex three-dimensional network. This mineral has a high surface area and porosity, which contributes to its various industrial applications.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups, forming a complex silicate structure. This mineral has a high surface area and porosity, which contributes to its various industrial applications.
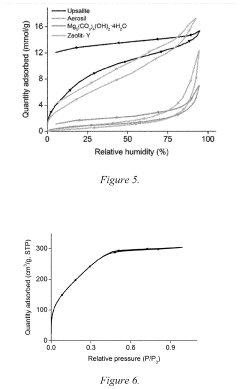
- Applications in environmental remediation: Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its excellent adsorption properties. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and unique structure allow it to trap and immobilize various pollutants, making it an effective material for water treatment and soil decontamination.
- Use in industrial processes and products: The mineral finds applications in various industrial processes and products. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals, improving the stability and consistency of these products. In the oil and gas industry, it is utilized as a drilling mud additive to control viscosity and fluid loss. Additionally, it serves as a reinforcing agent in polymer composites and as a catalyst support in chemical reactions.
- Synthesis and modification methods: Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Modifications can enhance specific properties such as surface area, porosity, or ion-exchange capacity, tailoring the material for specific applications. Surface functionalization techniques are also employed to improve the mineral's compatibility with different matrices or to introduce new functionalities.
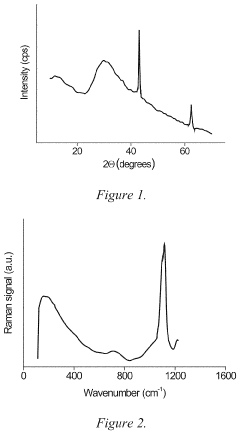
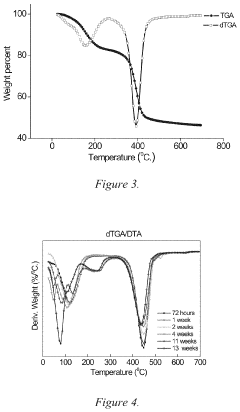
- Characterization and analysis techniques: Various analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD) for crystal structure analysis, scanning electron microscopy (SEM) for morphology studies, and spectroscopic methods such as FTIR and XPS for surface chemistry analysis. Thermal analysis techniques like TGA and DSC are also employed to study the mineral's thermal behavior and phase transitions.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its excellent adsorption properties. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and unique structure allow it to trap and immobilize various pollutants, making it an effective material for water treatment and soil decontamination.Expand Specific Solutions03 Use in pharmaceutical and cosmetic industries
The mineral finds applications in pharmaceutical and cosmetic industries due to its absorbent and thickening properties. It is used as an excipient in drug formulations, helping to improve drug stability and release characteristics. In cosmetics, it serves as a rheology modifier, enhancing the texture and stability of various products such as creams, lotions, and makeup.Expand Specific Solutions04 Industrial applications and material science
Magnesium iron silicate hydroxide is utilized in various industrial applications and material science fields. It is used as a reinforcing agent in polymer composites, improving mechanical properties and thermal stability. The mineral also finds applications in catalysis, serving as a support material for catalysts in chemical reactions. Additionally, it is used in the production of specialty papers, paints, and coatings.Expand Specific Solutions05 Synthesis and modification methods
Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. This includes hydrothermal synthesis, sol-gel methods, and surface modification techniques. Modified forms of the mineral can exhibit improved adsorption capacity, catalytic activity, or compatibility with other materials, expanding its potential uses in various fields.Expand Specific Solutions
Key Players in Mineral Exploration
The geological distribution and occurrence of Magnesium iron silicate hydroxide is a complex field with evolving research and applications. The industry is in a growth phase, driven by increasing demand for advanced materials in various sectors. Market size is expanding, with potential applications in industries such as automotive, construction, and electronics. Technologically, the field is progressing rapidly, with companies like Evonik Operations GmbH, Rigaku Corp., and Lubrizol Advanced Materials leading research efforts. Academic institutions, including Central South University and Nanjing University, are contributing significantly to the knowledge base. The collaboration between industry and academia is accelerating technological advancements, indicating a maturing but still developing field with substantial growth potential.
Central South University
Technical Solution: Central South University has made significant contributions to the study of magnesium iron silicate hydroxide, particularly in the context of mineral processing and materials science. Their research has focused on the characterization and modification of serpentine minerals for various applications. The university has developed novel methods for the extraction of magnesium and silica from serpentine, using a combination of mechanical activation and acid leaching processes[1]. Their studies have shown that this approach can achieve magnesium extraction rates of up to 95% under optimized conditions[2]. Additionally, the university has investigated the potential of serpentine minerals as precursors for the synthesis of advanced materials, such as forsterite ceramics and magnesium silicate-based catalysts[3].
Strengths: Strong focus on practical applications, innovative extraction and synthesis methods. Weaknesses: Potential environmental concerns related to acid leaching processes, need for further research on large-scale implementation.
Nanjing University
Technical Solution: Nanjing University has conducted comprehensive research on the geological distribution and occurrence of magnesium iron silicate hydroxide, with a particular emphasis on the formation mechanisms and transformation processes of serpentine minerals. Their studies have utilized advanced geochemical and isotopic analysis techniques to investigate the serpentinization of oceanic lithosphere and its implications for global geochemical cycles[1]. The university's research has revealed that serpentinization processes can occur at temperatures ranging from 100°C to 500°C, with varying mineral assemblages and textures depending on the specific conditions[2]. They have also explored the role of serpentinization in generating hydrogen and methane in deep-sea hydrothermal systems, contributing to our understanding of potential energy resources and the origin of life in extreme environments[3].
Strengths: Comprehensive approach combining field studies and laboratory experiments, strong focus on geochemical processes. Weaknesses: Limited direct industrial applications, potential challenges in translating research findings to practical resource exploration.
Innovative Identification Methods
Magnesium hydroxide having fine, plate-like crystalline structure and process therefor
PatentInactiveUS5143965A
Innovation
- A continuous process using ultrasonic mixing to precipitate magnesium hydroxide at controlled temperatures and alkaline material ratios, producing predominantly fine plate-like particles with a narrow size distribution, which can be coated with surfactants for improved performance.
Anhydrous, amorphous and porous magnesium carbonates and methods of production thereof
PatentActiveUS20200048104A1
Innovation
- Development of anhydrous, amorphous, micro porous magnesium carbonate with high specific surface area, produced at low temperatures from a Mg-containing precursor in organic solvents, exhibiting a substantial portion of micro pores and enhanced moisture sorption properties, stability, and regeneration capabilities.
Environmental Impact Assessment
The environmental impact assessment of magnesium iron silicate hydroxide (MISH) mining and processing is crucial for sustainable resource management. MISH, commonly found in serpentine minerals, occurs in various geological settings worldwide. Its extraction and utilization can have significant environmental implications that require careful consideration.
Mining activities for MISH can lead to habitat destruction and landscape alteration. Open-pit mining, often employed for serpentine deposits, results in the removal of vegetation and topsoil, potentially disrupting local ecosystems and biodiversity. The creation of large excavation sites may also alter drainage patterns and impact groundwater resources in the surrounding areas.
Dust emissions from mining and processing operations pose air quality concerns. Serpentine minerals may contain asbestos-like fibers, which, when airborne, can present health risks to workers and nearby communities. Implementing proper dust control measures and personal protective equipment is essential to mitigate these potential hazards.
Water pollution is another significant environmental concern associated with MISH extraction. Acid mine drainage can occur when sulfide minerals present in the ore bodies are exposed to air and water, leading to the formation of acidic runoff. This runoff may contain heavy metals and other contaminants, potentially impacting surface and groundwater quality if not properly managed.
The processing of MISH ores often involves chemical treatments and high-temperature operations, which can result in greenhouse gas emissions and energy consumption. The carbon footprint of these activities should be assessed and minimized through the adoption of cleaner technologies and energy-efficient processes.
Waste management is a critical aspect of MISH mining and processing. Tailings and waste rock generated during operations must be properly stored and treated to prevent environmental contamination. The potential for metal leaching and acid generation from these waste materials should be carefully evaluated and mitigated through appropriate containment and treatment strategies.
Rehabilitation and reclamation of mined areas are essential components of responsible MISH extraction. Post-mining land use planning should aim to restore ecosystem functions and services, considering local biodiversity and community needs. This may involve re-vegetation, soil stabilization, and the creation of suitable habitats for native flora and fauna.
The environmental impact assessment should also consider the potential benefits of MISH utilization, such as its role in carbon sequestration and soil improvement. When used in certain applications, MISH can contribute to CO2 capture and storage, potentially offsetting some of the environmental impacts associated with its extraction and processing.
Mining activities for MISH can lead to habitat destruction and landscape alteration. Open-pit mining, often employed for serpentine deposits, results in the removal of vegetation and topsoil, potentially disrupting local ecosystems and biodiversity. The creation of large excavation sites may also alter drainage patterns and impact groundwater resources in the surrounding areas.
Dust emissions from mining and processing operations pose air quality concerns. Serpentine minerals may contain asbestos-like fibers, which, when airborne, can present health risks to workers and nearby communities. Implementing proper dust control measures and personal protective equipment is essential to mitigate these potential hazards.
Water pollution is another significant environmental concern associated with MISH extraction. Acid mine drainage can occur when sulfide minerals present in the ore bodies are exposed to air and water, leading to the formation of acidic runoff. This runoff may contain heavy metals and other contaminants, potentially impacting surface and groundwater quality if not properly managed.
The processing of MISH ores often involves chemical treatments and high-temperature operations, which can result in greenhouse gas emissions and energy consumption. The carbon footprint of these activities should be assessed and minimized through the adoption of cleaner technologies and energy-efficient processes.
Waste management is a critical aspect of MISH mining and processing. Tailings and waste rock generated during operations must be properly stored and treated to prevent environmental contamination. The potential for metal leaching and acid generation from these waste materials should be carefully evaluated and mitigated through appropriate containment and treatment strategies.
Rehabilitation and reclamation of mined areas are essential components of responsible MISH extraction. Post-mining land use planning should aim to restore ecosystem functions and services, considering local biodiversity and community needs. This may involve re-vegetation, soil stabilization, and the creation of suitable habitats for native flora and fauna.
The environmental impact assessment should also consider the potential benefits of MISH utilization, such as its role in carbon sequestration and soil improvement. When used in certain applications, MISH can contribute to CO2 capture and storage, potentially offsetting some of the environmental impacts associated with its extraction and processing.
Geopolitical Implications
The geological distribution and occurrence of magnesium iron silicate hydroxide, commonly known as serpentine minerals, have significant geopolitical implications due to their association with critical resources and strategic locations. These minerals are predominantly found in ophiolite complexes, which are fragments of oceanic crust and upper mantle that have been obducted onto continental margins. The global distribution of serpentine-rich areas aligns with major tectonic boundaries, influencing the geopolitical landscape in several ways.
Firstly, serpentine minerals are often associated with deposits of valuable metals, particularly nickel and chromium. Countries with substantial serpentine formations, such as New Caledonia, Cuba, and the Philippines, possess strategic advantages in the global nickel market. This concentration of resources can lead to geopolitical tensions, as nations compete for control over these economically vital deposits. The uneven distribution of serpentine-related resources has the potential to shape international trade relationships and influence diplomatic negotiations.
Moreover, serpentine-rich areas are frequently linked to the formation of asbestos, a group of naturally occurring silicate minerals with significant health implications. The presence of asbestos in certain regions has led to complex international debates regarding environmental regulations and public health policies. Countries with extensive serpentine deposits must navigate the delicate balance between economic exploitation and environmental protection, often under the scrutiny of global health organizations and environmental advocacy groups.
The geological occurrence of magnesium iron silicate hydroxide also plays a role in carbon sequestration potential. Serpentine minerals have been identified as promising candidates for mineral carbonation, a process that could contribute to mitigating climate change. Nations with abundant serpentine resources may gain leverage in international climate negotiations, positioning themselves as key players in global carbon dioxide reduction efforts.
Furthermore, the distribution of serpentine minerals influences the development of geothermal energy resources. Serpentinization reactions can generate hydrogen and methane, potentially serving as a source of clean energy. Countries with significant serpentine formations may have a strategic advantage in the transition to renewable energy sources, affecting global energy politics and economic dependencies.
Lastly, the presence of serpentine minerals in oceanic crust fragments can impact maritime boundary disputes and exclusive economic zone claims. As nations seek to extend their continental shelves, the geological composition of the seabed, including serpentine-rich areas, becomes a critical factor in determining territorial rights and resource access in contested waters.
Firstly, serpentine minerals are often associated with deposits of valuable metals, particularly nickel and chromium. Countries with substantial serpentine formations, such as New Caledonia, Cuba, and the Philippines, possess strategic advantages in the global nickel market. This concentration of resources can lead to geopolitical tensions, as nations compete for control over these economically vital deposits. The uneven distribution of serpentine-related resources has the potential to shape international trade relationships and influence diplomatic negotiations.
Moreover, serpentine-rich areas are frequently linked to the formation of asbestos, a group of naturally occurring silicate minerals with significant health implications. The presence of asbestos in certain regions has led to complex international debates regarding environmental regulations and public health policies. Countries with extensive serpentine deposits must navigate the delicate balance between economic exploitation and environmental protection, often under the scrutiny of global health organizations and environmental advocacy groups.
The geological occurrence of magnesium iron silicate hydroxide also plays a role in carbon sequestration potential. Serpentine minerals have been identified as promising candidates for mineral carbonation, a process that could contribute to mitigating climate change. Nations with abundant serpentine resources may gain leverage in international climate negotiations, positioning themselves as key players in global carbon dioxide reduction efforts.
Furthermore, the distribution of serpentine minerals influences the development of geothermal energy resources. Serpentinization reactions can generate hydrogen and methane, potentially serving as a source of clean energy. Countries with significant serpentine formations may have a strategic advantage in the transition to renewable energy sources, affecting global energy politics and economic dependencies.
Lastly, the presence of serpentine minerals in oceanic crust fragments can impact maritime boundary disputes and exclusive economic zone claims. As nations seek to extend their continental shelves, the geological composition of the seabed, including serpentine-rich areas, becomes a critical factor in determining territorial rights and resource access in contested waters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!