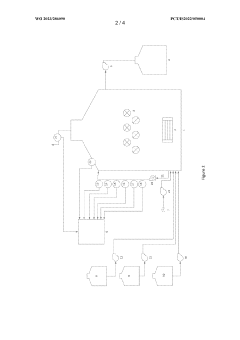
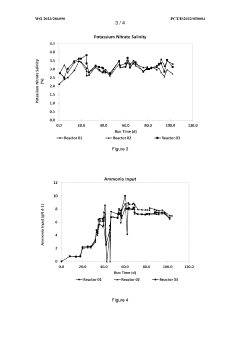
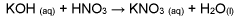How Nitrification Generates Reactive Nitrogen Byproducts?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrification Process Background and Research Objectives
Nitrification represents a fundamental biogeochemical process in the global nitrogen cycle, transforming ammonia (NH3) into nitrate (NO3-) through a series of oxidation reactions. This process, primarily mediated by chemolithoautotrophic microorganisms, has been extensively studied since the late 19th century when Sergei Winogradsky first identified the bacteria responsible. The historical trajectory of nitrification research has evolved from basic understanding of microbial taxonomy to sophisticated molecular analyses of enzymatic pathways and environmental impacts.
The conventional nitrification pathway involves two distinct steps: ammonia oxidation to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB) or archaea (AOA), followed by nitrite oxidation to nitrate by nitrite-oxidizing bacteria (NOB). Recent discoveries have expanded this classical model, revealing complete ammonia oxidation (comammox) organisms capable of performing the entire process independently, representing a significant paradigm shift in our understanding of nitrification ecology.
Current research trends indicate growing interest in the unintended byproducts of nitrification, particularly reactive nitrogen species that contribute to environmental pollution and climate change. These include nitrous oxide (N2O), a potent greenhouse gas with 298 times the global warming potential of carbon dioxide, and various nitrogen oxides (NOx) that contribute to air pollution and acid rain formation. The generation of hydroxylamine (NH2OH), nitric oxide (NO), and other intermediates during nitrification represents critical points where reactive nitrogen can escape controlled metabolic pathways.
The technological significance of understanding nitrification byproducts extends across multiple sectors, including wastewater treatment, agriculture, and environmental management. In wastewater treatment facilities, nitrification is deliberately harnessed to remove ammonia but can inadvertently produce harmful byproducts if not properly controlled. In agricultural systems, nitrification of fertilizers can lead to substantial nitrogen losses and environmental contamination through these reactive intermediates.
This technical research report aims to comprehensively examine how nitrification generates reactive nitrogen byproducts, with specific objectives to: (1) elucidate the biochemical mechanisms and enzymatic pathways leading to byproduct formation; (2) quantify the environmental factors that influence byproduct generation rates; (3) evaluate current mitigation strategies in engineered and natural systems; and (4) identify promising research directions for developing more efficient nitrification processes with minimized byproduct formation.
By addressing these objectives, we seek to bridge fundamental biochemical understanding with practical applications, ultimately contributing to more sustainable nitrogen management practices across multiple sectors and reducing the environmental footprint of essential nitrification processes.
The conventional nitrification pathway involves two distinct steps: ammonia oxidation to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB) or archaea (AOA), followed by nitrite oxidation to nitrate by nitrite-oxidizing bacteria (NOB). Recent discoveries have expanded this classical model, revealing complete ammonia oxidation (comammox) organisms capable of performing the entire process independently, representing a significant paradigm shift in our understanding of nitrification ecology.
Current research trends indicate growing interest in the unintended byproducts of nitrification, particularly reactive nitrogen species that contribute to environmental pollution and climate change. These include nitrous oxide (N2O), a potent greenhouse gas with 298 times the global warming potential of carbon dioxide, and various nitrogen oxides (NOx) that contribute to air pollution and acid rain formation. The generation of hydroxylamine (NH2OH), nitric oxide (NO), and other intermediates during nitrification represents critical points where reactive nitrogen can escape controlled metabolic pathways.
The technological significance of understanding nitrification byproducts extends across multiple sectors, including wastewater treatment, agriculture, and environmental management. In wastewater treatment facilities, nitrification is deliberately harnessed to remove ammonia but can inadvertently produce harmful byproducts if not properly controlled. In agricultural systems, nitrification of fertilizers can lead to substantial nitrogen losses and environmental contamination through these reactive intermediates.
This technical research report aims to comprehensively examine how nitrification generates reactive nitrogen byproducts, with specific objectives to: (1) elucidate the biochemical mechanisms and enzymatic pathways leading to byproduct formation; (2) quantify the environmental factors that influence byproduct generation rates; (3) evaluate current mitigation strategies in engineered and natural systems; and (4) identify promising research directions for developing more efficient nitrification processes with minimized byproduct formation.
By addressing these objectives, we seek to bridge fundamental biochemical understanding with practical applications, ultimately contributing to more sustainable nitrogen management practices across multiple sectors and reducing the environmental footprint of essential nitrification processes.
Market Applications and Environmental Impact Analysis
The market applications of nitrification processes and their reactive nitrogen byproducts span multiple industries, with significant economic implications and environmental considerations. The agricultural sector represents the largest market application, where controlled nitrification processes are essential for optimizing nitrogen fertilizer efficiency. Advanced nitrification inhibitors that specifically target the formation of harmful byproducts have emerged as a growing market segment, projected to reach substantial market value as regulatory pressures increase.
In wastewater treatment, engineered nitrification systems represent another major market application. Municipal and industrial facilities increasingly implement specialized bioreactors designed to optimize ammonia oxidation while minimizing the production of nitrous oxide and other reactive nitrogen intermediates. The global water treatment chemicals market heavily relies on understanding nitrification mechanisms to develop more efficient and environmentally responsible solutions.
The environmental impact of reactive nitrogen byproducts from nitrification processes is profound and multifaceted. Nitrous oxide (N₂O), a potent greenhouse gas with approximately 300 times the warming potential of carbon dioxide, contributes significantly to climate change when released during incomplete nitrification. Recent atmospheric measurements indicate increasing N₂O concentrations globally, with agricultural soils being the primary source.
Water quality degradation represents another critical environmental concern. Nitrite accumulation in aquatic ecosystems can lead to toxic conditions for aquatic organisms, while nitrate leaching into groundwater poses human health risks, particularly methemoglobinemia in infants. The economic burden of treating nitrate-contaminated drinking water supplies is substantial for many municipalities worldwide.
Ecosystem disruption through eutrophication occurs when excess reactive nitrogen compounds enter water bodies, triggering algal blooms that deplete oxygen and create dead zones. The annual economic impact of these eutrophication events includes significant losses in fisheries, tourism, and property values in affected coastal regions.
Regulatory frameworks addressing reactive nitrogen pollution continue to evolve globally. The European Union's Nitrates Directive and Water Framework Directive have established strict limits on nitrogen applications and water quality standards. Similarly, the United States Environmental Protection Agency has implemented increasingly stringent regulations on nitrogen discharge from point sources and is developing new approaches to address non-point source pollution from agricultural operations.
In wastewater treatment, engineered nitrification systems represent another major market application. Municipal and industrial facilities increasingly implement specialized bioreactors designed to optimize ammonia oxidation while minimizing the production of nitrous oxide and other reactive nitrogen intermediates. The global water treatment chemicals market heavily relies on understanding nitrification mechanisms to develop more efficient and environmentally responsible solutions.
The environmental impact of reactive nitrogen byproducts from nitrification processes is profound and multifaceted. Nitrous oxide (N₂O), a potent greenhouse gas with approximately 300 times the warming potential of carbon dioxide, contributes significantly to climate change when released during incomplete nitrification. Recent atmospheric measurements indicate increasing N₂O concentrations globally, with agricultural soils being the primary source.
Water quality degradation represents another critical environmental concern. Nitrite accumulation in aquatic ecosystems can lead to toxic conditions for aquatic organisms, while nitrate leaching into groundwater poses human health risks, particularly methemoglobinemia in infants. The economic burden of treating nitrate-contaminated drinking water supplies is substantial for many municipalities worldwide.
Ecosystem disruption through eutrophication occurs when excess reactive nitrogen compounds enter water bodies, triggering algal blooms that deplete oxygen and create dead zones. The annual economic impact of these eutrophication events includes significant losses in fisheries, tourism, and property values in affected coastal regions.
Regulatory frameworks addressing reactive nitrogen pollution continue to evolve globally. The European Union's Nitrates Directive and Water Framework Directive have established strict limits on nitrogen applications and water quality standards. Similarly, the United States Environmental Protection Agency has implemented increasingly stringent regulations on nitrogen discharge from point sources and is developing new approaches to address non-point source pollution from agricultural operations.
Current Challenges in Reactive Nitrogen Byproduct Formation
The nitrification process, while essential for nitrogen cycling in ecosystems, faces significant challenges related to the generation of reactive nitrogen byproducts. These byproducts, including nitric oxide (NO), nitrous oxide (N2O), and hydroxylamine (NH2OH), pose substantial environmental and health concerns that demand urgent attention from researchers and industry professionals.
One of the primary challenges is the incomplete understanding of the biochemical pathways that lead to reactive nitrogen byproduct formation during nitrification. The complex interactions between ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and nitrite-oxidizing bacteria (NOB) create variable conditions that influence byproduct generation rates. Recent research indicates that enzyme kinetics, particularly those of ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO), play crucial roles in determining byproduct formation, yet the precise mechanisms remain incompletely characterized.
Environmental factors significantly complicate nitrification byproduct management. Fluctuations in oxygen availability create microaerobic zones where incomplete oxidation pathways are favored, leading to increased N2O production. Similarly, pH variations affect enzyme activity and substrate availability, with studies showing that acidic conditions can increase N2O emissions by up to 70% compared to neutral pH environments. Temperature variations further influence microbial community composition and metabolic rates, creating seasonal patterns in byproduct generation that challenge consistent mitigation strategies.
Technological limitations present another significant hurdle. Current monitoring systems lack the sensitivity and specificity needed to detect reactive nitrogen species at environmentally relevant concentrations in real-time. This creates gaps in our understanding of temporal dynamics and spatial distribution of byproduct formation. Additionally, existing treatment technologies struggle to simultaneously address multiple reactive nitrogen species, often targeting either ammonia removal or nitrate reduction without comprehensively managing intermediate byproducts.
Regulatory frameworks worldwide have not kept pace with scientific understanding of reactive nitrogen impacts. Most regulations focus on end-point nitrogen concentrations rather than process-based approaches that would address byproduct formation. This regulatory gap provides insufficient incentives for technology development and implementation of advanced nitrification management strategies.
Economic considerations further complicate solutions, as technologies capable of minimizing reactive nitrogen byproducts typically require higher capital investment and operational costs. The absence of market mechanisms to value environmental benefits of reduced reactive nitrogen emissions creates barriers to widespread adoption of improved nitrification processes, particularly in resource-constrained settings.
One of the primary challenges is the incomplete understanding of the biochemical pathways that lead to reactive nitrogen byproduct formation during nitrification. The complex interactions between ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and nitrite-oxidizing bacteria (NOB) create variable conditions that influence byproduct generation rates. Recent research indicates that enzyme kinetics, particularly those of ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO), play crucial roles in determining byproduct formation, yet the precise mechanisms remain incompletely characterized.
Environmental factors significantly complicate nitrification byproduct management. Fluctuations in oxygen availability create microaerobic zones where incomplete oxidation pathways are favored, leading to increased N2O production. Similarly, pH variations affect enzyme activity and substrate availability, with studies showing that acidic conditions can increase N2O emissions by up to 70% compared to neutral pH environments. Temperature variations further influence microbial community composition and metabolic rates, creating seasonal patterns in byproduct generation that challenge consistent mitigation strategies.
Technological limitations present another significant hurdle. Current monitoring systems lack the sensitivity and specificity needed to detect reactive nitrogen species at environmentally relevant concentrations in real-time. This creates gaps in our understanding of temporal dynamics and spatial distribution of byproduct formation. Additionally, existing treatment technologies struggle to simultaneously address multiple reactive nitrogen species, often targeting either ammonia removal or nitrate reduction without comprehensively managing intermediate byproducts.
Regulatory frameworks worldwide have not kept pace with scientific understanding of reactive nitrogen impacts. Most regulations focus on end-point nitrogen concentrations rather than process-based approaches that would address byproduct formation. This regulatory gap provides insufficient incentives for technology development and implementation of advanced nitrification management strategies.
Economic considerations further complicate solutions, as technologies capable of minimizing reactive nitrogen byproducts typically require higher capital investment and operational costs. The absence of market mechanisms to value environmental benefits of reduced reactive nitrogen emissions creates barriers to widespread adoption of improved nitrification processes, particularly in resource-constrained settings.
Established Mechanisms of Reactive Nitrogen Generation
01 Biological nitrification processes and byproduct management
Biological nitrification processes involve the oxidation of ammonia to nitrite and then to nitrate by microorganisms. These processes can generate reactive nitrogen byproducts that need to be managed. Various techniques are employed to optimize the biological nitrification process while minimizing harmful byproducts, including the use of specific bacterial strains, controlled aeration, and temperature regulation.- Biological nitrification processes and byproducts: Biological nitrification processes involve the oxidation of ammonia to nitrite and then to nitrate by specific microorganisms. During this process, reactive nitrogen byproducts such as nitrous oxide (N2O) can be formed. These processes are essential in wastewater treatment and soil management but can contribute to greenhouse gas emissions when not properly controlled. The biological approach often uses specialized bacteria to facilitate the conversion of ammonia to less harmful nitrogen compounds.
- Chemical nitrification methods and reactive nitrogen management: Chemical nitrification methods involve the use of catalysts and chemical reactions to convert ammonia or other nitrogen compounds into nitrates. These processes can generate various reactive nitrogen byproducts that need to be managed carefully. Chemical approaches often provide faster conversion rates compared to biological methods but may require more precise control to minimize unwanted byproducts such as nitrogen oxides (NOx) and nitrous acid derivatives.
- Monitoring and control systems for nitrification byproducts: Advanced monitoring and control systems are essential for managing reactive nitrogen byproducts during nitrification processes. These systems typically include sensors for detecting ammonia, nitrite, nitrate, and other nitrogen compounds in real-time. By continuously monitoring these parameters, operators can adjust process conditions to minimize the formation of harmful byproducts such as nitrous oxide and nitrogen dioxide. These control systems often incorporate automated feedback mechanisms to maintain optimal conditions.
- Wastewater treatment nitrification technologies: Specialized nitrification technologies for wastewater treatment focus on efficiently converting ammonia to nitrate while minimizing reactive nitrogen byproducts. These technologies often employ sequential batch reactors, membrane bioreactors, or moving bed biofilm reactors to create optimal conditions for nitrifying bacteria. The processes are designed to control oxygen levels, pH, and temperature to reduce the formation of nitrous oxide and other harmful nitrogen compounds during treatment.
- Agricultural applications and fertilizer production: Nitrification processes are crucial in agricultural applications and fertilizer production, where managing reactive nitrogen byproducts is essential for environmental protection. These applications involve controlled release of nitrogen compounds to maximize plant uptake while minimizing leaching and gaseous losses. Nitrification inhibitors are often used to slow the conversion of ammonium to nitrate, reducing the formation of reactive nitrogen byproducts and improving nitrogen use efficiency in agricultural systems.
02 Chemical catalysts for nitrogen conversion and byproduct reduction
Chemical catalysts play a crucial role in nitrogen conversion processes, helping to reduce unwanted reactive nitrogen byproducts. These catalysts facilitate the transformation of nitrogen compounds into less harmful forms. Advanced catalyst technologies can improve reaction efficiency, selectivity, and reduce the formation of toxic intermediates during nitrification processes.Expand Specific Solutions03 Wastewater treatment systems for nitrogen removal
Specialized wastewater treatment systems are designed to remove nitrogen compounds and control reactive nitrogen byproducts. These systems often combine nitrification and denitrification processes to convert ammonia to nitrogen gas. Various reactor configurations, membrane technologies, and sequential treatment approaches are employed to enhance nitrogen removal efficiency while minimizing the release of harmful byproducts.Expand Specific Solutions04 Monitoring and control systems for nitrification processes
Advanced monitoring and control systems are essential for managing nitrification processes and their byproducts. These systems utilize sensors, analyzers, and automated controls to maintain optimal conditions for nitrification while preventing the accumulation of reactive nitrogen intermediates. Real-time monitoring allows for immediate adjustments to process parameters, reducing the risk of harmful byproduct formation.Expand Specific Solutions05 Agricultural applications and fertilizer production
Nitrification processes are widely used in agricultural applications and fertilizer production, where managing reactive nitrogen byproducts is crucial for environmental protection. Controlled-release fertilizers, nitrification inhibitors, and precision application techniques help minimize nitrogen losses and reduce the formation of harmful byproducts such as nitrous oxide. These approaches improve nitrogen use efficiency while reducing environmental impacts.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
The nitrification reactive nitrogen byproducts market is in a growth phase, with increasing environmental regulations driving innovation. The global market size is expanding due to growing concerns about nitrogen pollution in water and air. Technologically, the field shows varying maturity levels across applications, with companies demonstrating different specialization areas. China Petroleum & Chemical Corp. leads in industrial-scale solutions, while Koch Agronomic Services focuses on agricultural applications. Research institutions like Beijing University of Technology and specialized firms such as ToxSorb and Atmonia are advancing novel treatment technologies. CECEP Guozhen and Bayer AG are developing commercial applications, while startups like Complexa are exploring innovative approaches to nitrogen management, indicating a dynamic competitive landscape with opportunities for technological differentiation.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic technologies for controlling reactive nitrogen species during petroleum refining processes. Their approach involves selective catalytic reduction (SCR) systems that convert harmful NOx emissions into nitrogen and water. Sinopec's technology utilizes copper zeolite and vanadium-based catalysts that operate at lower temperatures (180-450°C) compared to conventional systems. Their research has demonstrated that these catalysts can achieve over 90% NOx reduction efficiency while minimizing ammonia slip to less than 5ppm. Additionally, Sinopec has implemented advanced monitoring systems that track the formation of N2O and other reactive nitrogen intermediates during the nitrification process, allowing for real-time adjustments to minimize harmful byproduct generation.
Strengths: Extensive industrial-scale implementation experience; integrated monitoring systems for real-time process optimization; high NOx reduction efficiency. Weaknesses: Technology primarily focused on emissions control rather than fundamental nitrification mechanisms; potential catalyst deactivation issues in presence of sulfur compounds.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has pioneered innovative approaches to understand and control reactive nitrogen species formation during wastewater treatment processes. Their proprietary METEOR™ technology employs specialized microorganisms and biofilm carriers that optimize the nitrification pathway while minimizing the production of nitrous oxide (N2O) and other harmful nitrogen intermediates. The system incorporates a two-stage biological process with carefully controlled dissolved oxygen gradients (1.5-3.0 mg/L in the first stage, 0.3-0.8 mg/L in the second) that favor complete nitrification while suppressing side reactions. Dow's research has identified specific enzyme inhibitors that can selectively block ammonia monooxygenase pathways responsible for generating hydroxylamine and other reactive nitrogen intermediates. Their technology has demonstrated up to 40% reduction in N2O emissions compared to conventional nitrification processes while maintaining treatment efficiency above 95%.
Strengths: Comprehensive biological approach addressing both process efficiency and environmental impact; proven technology with industrial applications; significant reduction in greenhouse gas emissions. Weaknesses: Requires precise control of operating conditions; potentially higher operational complexity compared to conventional systems; performance sensitivity to temperature fluctuations.
Key Scientific Breakthroughs in Nitrification Biochemistry
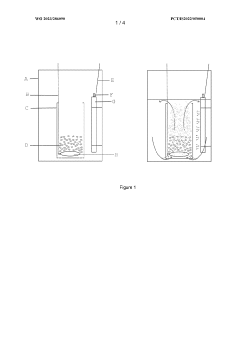
Bioreactor system and process for nitrate production
PatentWO2023286090A1
Innovation
- A bioreactor system and process that uses halophilic nitrifier microorganisms to continuously convert ammonia to nitrate, producing high-concentration nitrate solutions with reduced greenhouse emissions, suitable for both small-scale and large-scale operations, including on-site use at individual farms, utilizing a well-defined aqueous reactor medium and immobilized nitrifier organisms in various reactor types.
Nitrated hydrocarbons, derivatives, and processes for their manufacture
PatentActiveUS20190315768A1
Innovation
- A process utilizing a downflow configured reactor with high pressure and controlled temperature to minimize oxidation byproducts, increase conversion rates, and recover nitric acid, involving the reaction of hydrocarbons with aqueous nitric acid to produce nitrated compounds with reduced waste and improved selectivity.
Regulatory Framework for Nitrogen Pollution Management
The regulatory landscape for nitrogen pollution management has evolved significantly in response to growing scientific understanding of nitrification byproducts and their environmental impacts. At the international level, the United Nations Environment Programme (UNEP) has established the Global Partnership on Nutrient Management, which provides guidelines for countries to address nitrogen pollution through coordinated policy approaches. Similarly, the Convention on Long-range Transboundary Air Pollution includes protocols specifically targeting nitrogen oxide emissions and their atmospheric transport.
In the United States, the Clean Water Act (CWA) and Clean Air Act (CAA) form the backbone of nitrogen pollution regulation. The Environmental Protection Agency (EPA) has established National Pollutant Discharge Elimination System (NPDES) permits that set specific limits on nitrogen compounds in wastewater discharges. Additionally, the EPA's Nutrient Criteria Technical Guidance provides science-based approaches for developing water quality standards that address reactive nitrogen compounds resulting from nitrification processes.
The European Union has implemented the Nitrates Directive (91/676/EEC) and the Water Framework Directive (2000/60/EC), which require member states to identify nitrate vulnerable zones and implement action programs to reduce nitrogen pollution. These directives specifically acknowledge the role of nitrification in generating reactive nitrogen byproducts and mandate monitoring of compounds such as nitrite, nitrate, and nitrous oxide.
Emerging regulatory frameworks are increasingly adopting a more holistic approach to nitrogen management. For instance, New Zealand's National Policy Statement for Freshwater Management incorporates the concept of "nitrogen budgets" that account for all nitrogen transformations, including nitrification pathways and their byproducts. This represents a shift from end-of-pipe regulation to comprehensive nitrogen cycle management.
Compliance mechanisms vary widely across jurisdictions but typically include monitoring requirements, reporting obligations, and penalties for exceedances. Advanced monitoring technologies, such as real-time sensors for nitrous oxide and other reactive nitrogen species, are increasingly being incorporated into regulatory frameworks to improve enforcement capabilities and data collection.
Future regulatory trends point toward more integrated approaches that address multiple environmental compartments simultaneously. The concept of "critical loads" for nitrogen deposition is gaining traction, establishing ecosystem-specific thresholds below which significant harmful effects are not expected to occur according to current scientific knowledge. This approach acknowledges the complex pathways through which nitrification-generated compounds move through environmental systems.
In the United States, the Clean Water Act (CWA) and Clean Air Act (CAA) form the backbone of nitrogen pollution regulation. The Environmental Protection Agency (EPA) has established National Pollutant Discharge Elimination System (NPDES) permits that set specific limits on nitrogen compounds in wastewater discharges. Additionally, the EPA's Nutrient Criteria Technical Guidance provides science-based approaches for developing water quality standards that address reactive nitrogen compounds resulting from nitrification processes.
The European Union has implemented the Nitrates Directive (91/676/EEC) and the Water Framework Directive (2000/60/EC), which require member states to identify nitrate vulnerable zones and implement action programs to reduce nitrogen pollution. These directives specifically acknowledge the role of nitrification in generating reactive nitrogen byproducts and mandate monitoring of compounds such as nitrite, nitrate, and nitrous oxide.
Emerging regulatory frameworks are increasingly adopting a more holistic approach to nitrogen management. For instance, New Zealand's National Policy Statement for Freshwater Management incorporates the concept of "nitrogen budgets" that account for all nitrogen transformations, including nitrification pathways and their byproducts. This represents a shift from end-of-pipe regulation to comprehensive nitrogen cycle management.
Compliance mechanisms vary widely across jurisdictions but typically include monitoring requirements, reporting obligations, and penalties for exceedances. Advanced monitoring technologies, such as real-time sensors for nitrous oxide and other reactive nitrogen species, are increasingly being incorporated into regulatory frameworks to improve enforcement capabilities and data collection.
Future regulatory trends point toward more integrated approaches that address multiple environmental compartments simultaneously. The concept of "critical loads" for nitrogen deposition is gaining traction, establishing ecosystem-specific thresholds below which significant harmful effects are not expected to occur according to current scientific knowledge. This approach acknowledges the complex pathways through which nitrification-generated compounds move through environmental systems.
Mitigation Technologies and Sustainable Solutions
Addressing the environmental and health impacts of reactive nitrogen byproducts from nitrification processes requires comprehensive mitigation strategies and sustainable solutions. Advanced biological treatment systems represent a significant advancement in this field, particularly the implementation of anammox (anaerobic ammonium oxidation) processes that convert ammonium directly to nitrogen gas, bypassing the formation of nitrous oxide and other harmful intermediates.
Engineering controls in wastewater treatment facilities have shown promising results in reducing reactive nitrogen emissions. These include optimized aeration strategies that maintain dissolved oxygen at levels sufficient for complete nitrification while minimizing conditions favorable for N2O production. Intermittent aeration regimes have demonstrated up to 50% reduction in N2O emissions compared to continuous aeration systems in pilot-scale studies.
Chemical inhibitors targeting specific enzymatic pathways offer another approach to mitigate byproduct formation. Compounds such as 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) can selectively inhibit ammonia monooxygenase activity, thereby reducing the generation of hydroxylamine and subsequent reactive nitrogen species. These inhibitors have shown efficacy in both agricultural and wastewater treatment applications.
Precision agriculture techniques represent a sustainable approach to managing nitrogen in agricultural systems. These include variable-rate fertilizer application technologies, enhanced-efficiency fertilizers with controlled-release mechanisms, and nitrification inhibitors that can reduce reactive nitrogen losses by 30-40% compared to conventional practices. Remote sensing and soil monitoring technologies enable real-time adjustment of nitrogen application rates to match crop requirements precisely.
Ecological engineering solutions, such as constructed wetlands and riparian buffer zones, provide natural mitigation systems for reactive nitrogen. These systems leverage plant uptake, microbial denitrification, and soil adsorption processes to capture and transform reactive nitrogen before it reaches water bodies. Studies indicate that well-designed constructed wetlands can remove 60-90% of incoming reactive nitrogen loads.
Policy frameworks and economic instruments are increasingly recognized as essential components of comprehensive mitigation strategies. Carbon pricing mechanisms that incorporate nitrogen pollution externalities, nutrient trading programs, and regulatory standards for nitrogen discharge have demonstrated effectiveness in driving technological innovation and adoption of best management practices across industries.
Emerging technologies such as microbial fuel cells and bioelectrochemical systems offer promising approaches that can simultaneously treat nitrogen-rich wastewaters while generating energy, potentially transforming nitrogen pollution management from a cost center to a value-added process in industrial and municipal applications.
Engineering controls in wastewater treatment facilities have shown promising results in reducing reactive nitrogen emissions. These include optimized aeration strategies that maintain dissolved oxygen at levels sufficient for complete nitrification while minimizing conditions favorable for N2O production. Intermittent aeration regimes have demonstrated up to 50% reduction in N2O emissions compared to continuous aeration systems in pilot-scale studies.
Chemical inhibitors targeting specific enzymatic pathways offer another approach to mitigate byproduct formation. Compounds such as 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) can selectively inhibit ammonia monooxygenase activity, thereby reducing the generation of hydroxylamine and subsequent reactive nitrogen species. These inhibitors have shown efficacy in both agricultural and wastewater treatment applications.
Precision agriculture techniques represent a sustainable approach to managing nitrogen in agricultural systems. These include variable-rate fertilizer application technologies, enhanced-efficiency fertilizers with controlled-release mechanisms, and nitrification inhibitors that can reduce reactive nitrogen losses by 30-40% compared to conventional practices. Remote sensing and soil monitoring technologies enable real-time adjustment of nitrogen application rates to match crop requirements precisely.
Ecological engineering solutions, such as constructed wetlands and riparian buffer zones, provide natural mitigation systems for reactive nitrogen. These systems leverage plant uptake, microbial denitrification, and soil adsorption processes to capture and transform reactive nitrogen before it reaches water bodies. Studies indicate that well-designed constructed wetlands can remove 60-90% of incoming reactive nitrogen loads.
Policy frameworks and economic instruments are increasingly recognized as essential components of comprehensive mitigation strategies. Carbon pricing mechanisms that incorporate nitrogen pollution externalities, nutrient trading programs, and regulatory standards for nitrogen discharge have demonstrated effectiveness in driving technological innovation and adoption of best management practices across industries.
Emerging technologies such as microbial fuel cells and bioelectrochemical systems offer promising approaches that can simultaneously treat nitrogen-rich wastewaters while generating energy, potentially transforming nitrogen pollution management from a cost center to a value-added process in industrial and municipal applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!