Nitrification Monitoring Using Stable Isotope Techniques
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotope Nitrification Monitoring Background and Objectives
Nitrification, the biological oxidation of ammonia to nitrite and subsequently to nitrate, represents a critical process in the global nitrogen cycle. This transformation significantly impacts agricultural productivity, environmental quality, and ecosystem health. The evolution of monitoring techniques for nitrification has progressed substantially over the past decades, from basic chemical assays to sophisticated molecular approaches. Among these, stable isotope techniques have emerged as powerful tools that provide unique insights into nitrification dynamics that conventional methods cannot offer.
The historical development of stable isotope applications in nitrification studies dates back to the 1950s, when researchers first utilized 15N to trace nitrogen transformations in soils. Since then, technological advancements in mass spectrometry and analytical chemistry have dramatically enhanced the sensitivity, precision, and accessibility of isotope-based methods. The integration of these techniques with modern molecular biology approaches has further revolutionized our understanding of nitrification processes across diverse ecosystems.
Current technological trends in this field include the development of compound-specific isotope analysis, high-throughput isotope ratio mass spectrometry, and in-situ measurement capabilities. These innovations are enabling researchers to monitor nitrification at unprecedented spatial and temporal resolutions, providing real-time data on nitrogen transformation rates and pathways in complex environmental matrices.
The primary objectives of isotope-based nitrification monitoring encompass several dimensions. First, to quantify nitrification rates with high precision in various environmental contexts, from agricultural soils to wastewater treatment systems and natural aquatic ecosystems. Second, to differentiate between various nitrogen transformation pathways, including classical nitrification, anaerobic ammonium oxidation (anammox), and heterotrophic nitrification. Third, to identify and characterize the microbial communities responsible for nitrification processes under different environmental conditions.
Additionally, these techniques aim to evaluate the efficiency of nitrification inhibitors in agricultural settings, assess the impact of environmental factors on nitrification rates, and monitor nitrogen losses through various pathways. The ultimate goal is to develop comprehensive models of nitrogen cycling that can inform sustainable management practices across sectors, from agriculture to wastewater treatment and ecosystem conservation.
As climate change and anthropogenic activities continue to alter global nitrogen cycles, the importance of accurate nitrification monitoring grows increasingly critical. Stable isotope techniques stand at the forefront of efforts to understand these complex biogeochemical processes and develop effective mitigation strategies for nitrogen-related environmental challenges.
The historical development of stable isotope applications in nitrification studies dates back to the 1950s, when researchers first utilized 15N to trace nitrogen transformations in soils. Since then, technological advancements in mass spectrometry and analytical chemistry have dramatically enhanced the sensitivity, precision, and accessibility of isotope-based methods. The integration of these techniques with modern molecular biology approaches has further revolutionized our understanding of nitrification processes across diverse ecosystems.
Current technological trends in this field include the development of compound-specific isotope analysis, high-throughput isotope ratio mass spectrometry, and in-situ measurement capabilities. These innovations are enabling researchers to monitor nitrification at unprecedented spatial and temporal resolutions, providing real-time data on nitrogen transformation rates and pathways in complex environmental matrices.
The primary objectives of isotope-based nitrification monitoring encompass several dimensions. First, to quantify nitrification rates with high precision in various environmental contexts, from agricultural soils to wastewater treatment systems and natural aquatic ecosystems. Second, to differentiate between various nitrogen transformation pathways, including classical nitrification, anaerobic ammonium oxidation (anammox), and heterotrophic nitrification. Third, to identify and characterize the microbial communities responsible for nitrification processes under different environmental conditions.
Additionally, these techniques aim to evaluate the efficiency of nitrification inhibitors in agricultural settings, assess the impact of environmental factors on nitrification rates, and monitor nitrogen losses through various pathways. The ultimate goal is to develop comprehensive models of nitrogen cycling that can inform sustainable management practices across sectors, from agriculture to wastewater treatment and ecosystem conservation.
As climate change and anthropogenic activities continue to alter global nitrogen cycles, the importance of accurate nitrification monitoring grows increasingly critical. Stable isotope techniques stand at the forefront of efforts to understand these complex biogeochemical processes and develop effective mitigation strategies for nitrogen-related environmental challenges.
Market Applications for Stable Isotope Nitrification Tracking
Stable isotope techniques for nitrification monitoring have established significant market applications across multiple sectors, with environmental management representing the largest application area. Water quality management agencies utilize these techniques to track nitrogen pollution sources in watersheds, enabling targeted remediation efforts and regulatory compliance. Municipal water treatment facilities increasingly employ stable isotope analysis to optimize nitrification processes, resulting in operational cost reductions of up to 15% through improved chemical dosing and energy efficiency.
The agricultural sector presents a rapidly growing market for stable isotope nitrification tracking. Precision agriculture companies integrate these techniques into soil management systems to optimize fertilizer application, reducing input costs while minimizing environmental impacts. Several major agricultural technology providers have launched commercial services combining stable isotope analysis with digital farming platforms, creating comprehensive nitrogen management solutions for large-scale operations.
Research institutions and environmental consultancies form another substantial market segment, utilizing stable isotope techniques for both fundamental research and commercial environmental assessment services. The academic research market alone generates significant demand for isotope analysis equipment and services, particularly in regions with stringent environmental regulations.
Emerging applications in aquaculture and wastewater management are expanding the market footprint of stable isotope nitrification tracking. Aquaculture operations increasingly monitor nitrogen cycling to optimize feed utilization and minimize environmental impacts, while industrial wastewater treatment facilities adopt these techniques to demonstrate regulatory compliance and improve treatment efficiency.
Geographically, North America and Europe currently dominate market adoption, driven by stringent environmental regulations and established research infrastructure. However, the Asia-Pacific region shows the fastest growth trajectory, particularly in China and India, where water quality management and agricultural optimization have become national priorities.
The market for stable isotope analysis equipment specifically designed for nitrification monitoring has evolved from specialized laboratory instruments to more field-deployable solutions. Recent technological advances have reduced both the cost and complexity of analysis, expanding potential applications beyond traditional research settings into routine monitoring programs.
Market barriers include the relatively high initial investment costs for analytical equipment and the technical expertise required for data interpretation. However, the development of service-based business models, where specialized laboratories provide analysis services to end-users, has significantly lowered market entry barriers for many applications.
The agricultural sector presents a rapidly growing market for stable isotope nitrification tracking. Precision agriculture companies integrate these techniques into soil management systems to optimize fertilizer application, reducing input costs while minimizing environmental impacts. Several major agricultural technology providers have launched commercial services combining stable isotope analysis with digital farming platforms, creating comprehensive nitrogen management solutions for large-scale operations.
Research institutions and environmental consultancies form another substantial market segment, utilizing stable isotope techniques for both fundamental research and commercial environmental assessment services. The academic research market alone generates significant demand for isotope analysis equipment and services, particularly in regions with stringent environmental regulations.
Emerging applications in aquaculture and wastewater management are expanding the market footprint of stable isotope nitrification tracking. Aquaculture operations increasingly monitor nitrogen cycling to optimize feed utilization and minimize environmental impacts, while industrial wastewater treatment facilities adopt these techniques to demonstrate regulatory compliance and improve treatment efficiency.
Geographically, North America and Europe currently dominate market adoption, driven by stringent environmental regulations and established research infrastructure. However, the Asia-Pacific region shows the fastest growth trajectory, particularly in China and India, where water quality management and agricultural optimization have become national priorities.
The market for stable isotope analysis equipment specifically designed for nitrification monitoring has evolved from specialized laboratory instruments to more field-deployable solutions. Recent technological advances have reduced both the cost and complexity of analysis, expanding potential applications beyond traditional research settings into routine monitoring programs.
Market barriers include the relatively high initial investment costs for analytical equipment and the technical expertise required for data interpretation. However, the development of service-based business models, where specialized laboratories provide analysis services to end-users, has significantly lowered market entry barriers for many applications.
Current Challenges in Isotope-Based Nitrification Monitoring
Despite significant advancements in stable isotope techniques for nitrification monitoring, several critical challenges continue to impede widespread implementation and reliability of these methods. The complexity of sample preparation represents a major hurdle, as it requires specialized equipment and expertise to properly extract, purify, and prepare samples for isotopic analysis. This process is particularly challenging when dealing with environmental samples containing multiple nitrogen species and potential contaminants that can interfere with accurate measurements.
Analytical sensitivity limitations pose another significant challenge, especially when monitoring nitrification in environments with low nitrogen concentrations or slow nitrification rates. Current mass spectrometry techniques often struggle to detect the subtle isotopic shifts that occur during nitrification processes in such conditions, potentially missing important biogeochemical signals.
The high cost associated with stable isotope analysis remains prohibitive for many research institutions and environmental monitoring programs. Both the initial investment in analytical equipment and the ongoing operational expenses restrict the frequency and scale of monitoring efforts, limiting the temporal and spatial resolution of nitrification data.
Interpretation complexities further complicate isotope-based monitoring approaches. The nitrogen cycle involves multiple transformation pathways that can simultaneously affect isotopic signatures, making it difficult to isolate and quantify nitrification specifically. Overlapping isotopic effects from processes such as denitrification, dissimilatory nitrate reduction, and nitrogen fixation create ambiguity in data interpretation.
Field application challenges are particularly pronounced, as most isotope techniques were developed and optimized for laboratory settings. Translating these methods to diverse environmental conditions introduces variables that can compromise measurement accuracy and reproducibility. The lack of standardized protocols for field sampling and preservation further exacerbates these issues.
Temporal resolution remains inadequate with current methodologies, which typically provide snapshot measurements rather than continuous monitoring capabilities. This limitation hinders our understanding of nitrification dynamics across diurnal cycles and in response to rapid environmental changes.
Integration with other monitoring parameters presents another challenge, as isotope data alone often provides insufficient context for comprehensive ecosystem assessment. Developing frameworks that effectively combine isotopic information with traditional water quality parameters, microbial community analyses, and environmental variables remains an ongoing challenge in the field.
Analytical sensitivity limitations pose another significant challenge, especially when monitoring nitrification in environments with low nitrogen concentrations or slow nitrification rates. Current mass spectrometry techniques often struggle to detect the subtle isotopic shifts that occur during nitrification processes in such conditions, potentially missing important biogeochemical signals.
The high cost associated with stable isotope analysis remains prohibitive for many research institutions and environmental monitoring programs. Both the initial investment in analytical equipment and the ongoing operational expenses restrict the frequency and scale of monitoring efforts, limiting the temporal and spatial resolution of nitrification data.
Interpretation complexities further complicate isotope-based monitoring approaches. The nitrogen cycle involves multiple transformation pathways that can simultaneously affect isotopic signatures, making it difficult to isolate and quantify nitrification specifically. Overlapping isotopic effects from processes such as denitrification, dissimilatory nitrate reduction, and nitrogen fixation create ambiguity in data interpretation.
Field application challenges are particularly pronounced, as most isotope techniques were developed and optimized for laboratory settings. Translating these methods to diverse environmental conditions introduces variables that can compromise measurement accuracy and reproducibility. The lack of standardized protocols for field sampling and preservation further exacerbates these issues.
Temporal resolution remains inadequate with current methodologies, which typically provide snapshot measurements rather than continuous monitoring capabilities. This limitation hinders our understanding of nitrification dynamics across diurnal cycles and in response to rapid environmental changes.
Integration with other monitoring parameters presents another challenge, as isotope data alone often provides insufficient context for comprehensive ecosystem assessment. Developing frameworks that effectively combine isotopic information with traditional water quality parameters, microbial community analyses, and environmental variables remains an ongoing challenge in the field.
Current Methodologies for Stable Isotope Nitrification Assessment
01 Isotope labeling for nitrification process monitoring
Stable isotope techniques, particularly using nitrogen isotopes (15N), are employed to track and quantify nitrification processes in various environments. These methods allow researchers to monitor the conversion of ammonium to nitrite and nitrate by nitrifying bacteria. By introducing isotopically labeled compounds and tracking their transformation, scientists can measure nitrification rates and efficiencies in wastewater treatment systems, agricultural soils, and natural ecosystems.- Isotope labeling for nitrification process monitoring: Stable isotope techniques, particularly using nitrogen isotopes (15N), are employed to track and quantify nitrification processes in various environments. These techniques allow researchers to monitor the conversion of ammonia to nitrite and nitrate by nitrifying bacteria. By introducing isotopically labeled compounds and tracking their transformation, scientists can measure nitrification rates and efficiencies in wastewater treatment plants, agricultural soils, and natural ecosystems.
- Mass spectrometry methods for isotope analysis in nitrification studies: Advanced mass spectrometry techniques are utilized to detect and quantify stable isotopes in nitrification monitoring. These methods include isotope ratio mass spectrometry (IRMS), gas chromatography-mass spectrometry (GC-MS), and liquid chromatography-mass spectrometry (LC-MS). These analytical approaches enable precise measurement of isotopic compositions and ratios, allowing researchers to track nitrogen transformations during nitrification processes with high sensitivity and accuracy.
- Environmental applications of stable isotope nitrification monitoring: Stable isotope techniques are applied to monitor nitrification in various environmental contexts, including marine ecosystems, freshwater bodies, and soil environments. These methods help assess nitrogen cycling, identify sources of nitrogen pollution, and evaluate the impact of environmental factors on nitrification rates. By tracking isotopically labeled compounds through environmental systems, researchers can better understand nitrogen transformation pathways and develop strategies for managing nitrogen in ecosystems.
- Wastewater treatment optimization using isotope techniques: Stable isotope methods are employed to optimize wastewater treatment processes by monitoring nitrification efficiency. These techniques help operators understand nitrogen removal performance, identify rate-limiting steps, and optimize operational parameters. By tracking isotopically labeled nitrogen compounds through treatment systems, engineers can evaluate the effectiveness of different treatment strategies, troubleshoot process failures, and develop more efficient biological nitrogen removal systems.
- Innovative sampling and preparation methods for isotope-based nitrification monitoring: Novel sampling techniques and sample preparation methods have been developed to enhance the application of stable isotope approaches in nitrification monitoring. These innovations include automated sampling systems, preservation techniques to maintain sample integrity, extraction methods for isolating target compounds, and pretreatment procedures to remove interfering substances. These methodological advances improve the accuracy, precision, and practical applicability of stable isotope techniques for monitoring nitrification processes in complex matrices.
02 Mass spectrometry techniques for isotope analysis
Advanced mass spectrometry methods are crucial for detecting and quantifying stable isotopes in nitrification monitoring. These techniques include isotope ratio mass spectrometry (IRMS), accelerator mass spectrometry, and various forms of coupled chromatography-mass spectrometry systems. These analytical approaches enable high-precision measurements of isotopic ratios and concentrations, allowing researchers to track nitrogen transformations during nitrification with exceptional sensitivity and accuracy.Expand Specific Solutions03 Environmental applications of isotope-based nitrification monitoring
Stable isotope techniques are applied to monitor nitrification in various environmental contexts, including marine ecosystems, groundwater, and soil systems. These methods help assess nitrogen cycling, identify sources of nitrogen pollution, and evaluate the impact of environmental factors on nitrification rates. By tracking isotopically labeled compounds through environmental systems, researchers can better understand nitrogen transformation pathways and develop strategies for managing nitrogen in ecosystems.Expand Specific Solutions04 Wastewater treatment monitoring using stable isotopes
Stable isotope techniques provide valuable tools for monitoring and optimizing nitrification processes in wastewater treatment facilities. These methods allow operators to track nitrogen removal efficiency, identify rate-limiting steps, and optimize treatment conditions. By using isotopically labeled compounds, treatment plant operators can assess the performance of nitrifying bacteria communities and make informed decisions about process adjustments to improve nitrogen removal and reduce environmental impacts.Expand Specific Solutions05 Microbial community analysis in nitrification processes
Stable isotope techniques are combined with molecular biology methods to study the microbial communities responsible for nitrification. These approaches include stable isotope probing (SIP), which identifies active nitrifying microorganisms by tracking their incorporation of labeled substrates. Additionally, isotope techniques help quantify the contribution of different microbial groups to overall nitrification rates and reveal how environmental conditions affect microbial community structure and function in nitrification processes.Expand Specific Solutions
Leading Research Groups and Commercial Entities in Isotope Analysis
Nitrification monitoring using stable isotope techniques is currently in a growth phase, with increasing adoption across environmental science and agriculture sectors. The market size is expanding, driven by growing concerns about nitrogen pollution and sustainable agriculture practices. Technologically, the field is moderately mature with established methodologies, though innovations continue to emerge. Key players demonstrate varying levels of specialization: Agilent Technologies provides advanced analytical instrumentation essential for isotope analysis, while research institutions like Shanghai Research Institute of Chemical Industry and Centre National de la Recherche Scientifique contribute fundamental research advancements. Companies like Shanghai LianHong Isotope Technology specialize directly in isotope applications, while environmental monitoring firms such as Hach Lange GmbH integrate these techniques into broader water quality solutions. Academic-industrial partnerships between universities and commercial entities are accelerating technological refinement and practical applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced 15N isotope dilution techniques for monitoring nitrification processes in various ecosystems. Their approach combines gas chromatography-isotope ratio mass spectrometry (GC-IRMS) with specialized sampling protocols to trace nitrogen transformations in soil and water environments. The methodology involves introducing 15N-labeled compounds into the system and tracking their conversion through nitrification pathways, allowing for precise quantification of nitrification rates. CNRS researchers have refined these techniques to minimize disturbance to natural systems while maximizing sensitivity, enabling detection of nitrification activity even in low-activity environments. Their protocols include specialized extraction methods that preserve isotopic signatures during sample preparation, crucial for accurate measurements.
Strengths: Exceptional sensitivity in detecting nitrification rates in complex environmental matrices; established standardized protocols that have been widely adopted internationally. Weaknesses: Techniques require sophisticated laboratory infrastructure and highly trained personnel, limiting widespread application in resource-limited settings.
Institut National de Recherche Agronomique SA
Technical Solution: INRA has pioneered the development of compound-specific isotope analysis (CSIA) techniques for monitoring nitrification in agricultural systems. Their technical solution integrates 15N and 18O isotope tracing to simultaneously monitor nitrification and denitrification processes in soil. The approach employs specialized microplate-based methods for high-throughput analysis of isotopic signatures in extracted soil samples, allowing for spatial and temporal mapping of nitrification activity across agricultural landscapes. INRA's innovation includes the development of mathematical models that account for isotope fractionation during nitrification, enabling more accurate rate calculations from field measurements. Their system incorporates automated sampling devices that can be deployed in agricultural fields for continuous monitoring of isotopic shifts related to nitrification activity.
Strengths: Highly optimized for agricultural applications with field-deployable components; excellent integration with farm management systems for practical application. Weaknesses: Models require extensive calibration for different soil types and environmental conditions, potentially limiting accuracy in novel environments.
Key Innovations in 15N and 18O Tracer Technologies
Stable isotope enrichment device and stable isotope enrichment method
PatentPendingUS20240082786A1
Innovation
- A stable isotope enrichment device and method incorporating an isotope exchange reactor to regenerate isotope-depleted gases and liquids by increasing their isotope concentration through reactions with substances at natural abundance ratios, reducing raw material usage and environmental impact.
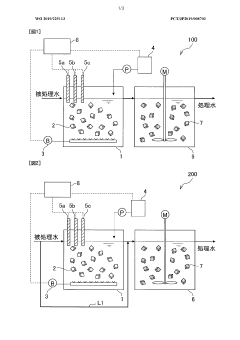
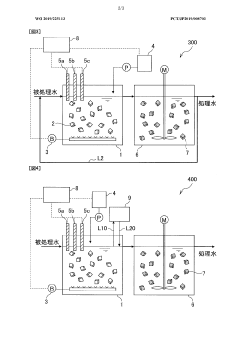
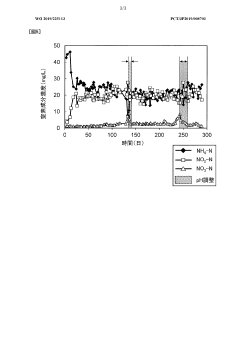
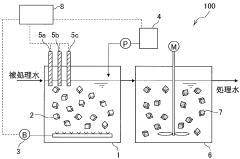
Nitrogen treatment method
PatentWO2019225113A1
Innovation
- A nitrogen treatment method that continuously measures ammonia, nitrite, and nitrate nitrogen concentrations, with pH control increasing the water pH when nitrate nitrogen levels rise, inhibiting nitrite-oxidizing bacteria and stabilizing nitrite nitrogen production.
Environmental Impact Assessment of Nitrification Monitoring Methods
The environmental impact of nitrification monitoring methods varies significantly across different techniques, with stable isotope approaches offering distinct advantages and challenges compared to conventional methods. Traditional monitoring techniques often involve chemical analyses that require substantial reagent use, generating hazardous waste and potentially introducing contaminants into environmental systems. These methods frequently demand extensive field sampling, which can disturb sensitive ecosystems and contribute to habitat disruption.
In contrast, stable isotope techniques for nitrification monitoring generally require smaller sample volumes and cause less environmental disturbance during collection. The 15N isotope approach, in particular, allows for more precise tracking of nitrogen transformation pathways without introducing foreign compounds that might alter ecosystem dynamics. This reduced intervention is especially valuable in pristine environments or protected habitats where minimal disturbance is essential for conservation efforts.
However, stable isotope methods are not without environmental considerations. The production of isotopically labeled compounds requires specialized facilities and energy-intensive processes. The synthesis of 15N-enriched compounds generates industrial waste and contributes to carbon emissions through energy consumption. Additionally, the analytical equipment required for isotope ratio mass spectrometry consumes significant electrical power during operation.
Field application of isotope techniques may involve the controlled release of labeled compounds into environmental systems. While these releases are typically at trace levels, cumulative effects from repeated studies in the same area require careful consideration. Regulatory frameworks in many regions now mandate environmental impact assessments before approval of field studies using isotope tracers, particularly in sensitive aquatic ecosystems or agricultural watersheds.
Laboratory processing of isotope samples generates less chemical waste compared to conventional colorimetric or chromatographic methods, but still produces hazardous materials requiring specialized disposal. The concentrated acids often used in sample preparation pose handling risks and potential environmental contamination if improperly managed.
Long-term monitoring programs using stable isotope techniques must consider the sustainability of their approaches, including energy consumption of analytical equipment, transportation impacts for sample collection, and lifecycle assessment of consumables. Recent innovations in portable isotope analyzers have reduced some of these impacts by enabling in-situ measurements, though these technologies remain limited in sensitivity compared to laboratory-based systems.
In contrast, stable isotope techniques for nitrification monitoring generally require smaller sample volumes and cause less environmental disturbance during collection. The 15N isotope approach, in particular, allows for more precise tracking of nitrogen transformation pathways without introducing foreign compounds that might alter ecosystem dynamics. This reduced intervention is especially valuable in pristine environments or protected habitats where minimal disturbance is essential for conservation efforts.
However, stable isotope methods are not without environmental considerations. The production of isotopically labeled compounds requires specialized facilities and energy-intensive processes. The synthesis of 15N-enriched compounds generates industrial waste and contributes to carbon emissions through energy consumption. Additionally, the analytical equipment required for isotope ratio mass spectrometry consumes significant electrical power during operation.
Field application of isotope techniques may involve the controlled release of labeled compounds into environmental systems. While these releases are typically at trace levels, cumulative effects from repeated studies in the same area require careful consideration. Regulatory frameworks in many regions now mandate environmental impact assessments before approval of field studies using isotope tracers, particularly in sensitive aquatic ecosystems or agricultural watersheds.
Laboratory processing of isotope samples generates less chemical waste compared to conventional colorimetric or chromatographic methods, but still produces hazardous materials requiring specialized disposal. The concentrated acids often used in sample preparation pose handling risks and potential environmental contamination if improperly managed.
Long-term monitoring programs using stable isotope techniques must consider the sustainability of their approaches, including energy consumption of analytical equipment, transportation impacts for sample collection, and lifecycle assessment of consumables. Recent innovations in portable isotope analyzers have reduced some of these impacts by enabling in-situ measurements, though these technologies remain limited in sensitivity compared to laboratory-based systems.
Standardization and Quality Control in Isotope Analysis
Standardization and quality control are paramount in stable isotope analysis for nitrification monitoring, as they directly impact the reliability and comparability of research outcomes. The field has established several international reference materials that serve as calibration standards, including IAEA-N1, IAEA-N2, and USGS34 for nitrogen isotopes. These standards have precisely determined isotopic compositions that allow laboratories worldwide to calibrate their instruments and normalize their results to a common scale, typically the atmospheric N₂ for nitrogen isotopes (δ¹⁵N).
Analytical precision in isotope ratio mass spectrometry (IRMS) requires rigorous quality control protocols. Most laboratories implement a three-tier approach: daily instrument performance checks, routine analysis of laboratory reference materials alongside samples, and periodic participation in inter-laboratory comparison exercises. The acceptable precision for δ¹⁵N measurements typically ranges from ±0.1‰ to ±0.3‰, depending on the specific application and instrumentation used.
Sample preparation represents a critical phase where standardization is essential. Procedures for extracting ammonium and nitrate from environmental samples must be consistent to avoid isotopic fractionation that could bias results. Methods such as diffusion, distillation, and microdiffusion have been standardized with detailed protocols published by organizations like the International Atomic Energy Agency (IAEA) and the United States Geological Survey (USGS).
Data reporting conventions have also been standardized to facilitate comparison across studies. Results are typically expressed in delta notation (δ) relative to international standards, with units of per mil (‰). The scientific community has adopted specific guidelines for reporting uncertainty, requiring both analytical precision and overall measurement uncertainty to be clearly stated in publications.
Recent technological advances have introduced automated systems that integrate sample preparation with isotope analysis, reducing human error and improving throughput. These systems incorporate built-in quality control features, such as automatic drift correction and flagging of anomalous results. However, they require regular validation against established manual methods to ensure consistency.
Inter-laboratory comparison programs, such as those organized by the IAEA, play a crucial role in maintaining global standardization. These programs distribute identical samples to participating laboratories, allowing for the identification of systematic biases and methodological issues. Results from these exercises have led to improved analytical protocols and greater harmonization of isotope data worldwide.
Analytical precision in isotope ratio mass spectrometry (IRMS) requires rigorous quality control protocols. Most laboratories implement a three-tier approach: daily instrument performance checks, routine analysis of laboratory reference materials alongside samples, and periodic participation in inter-laboratory comparison exercises. The acceptable precision for δ¹⁵N measurements typically ranges from ±0.1‰ to ±0.3‰, depending on the specific application and instrumentation used.
Sample preparation represents a critical phase where standardization is essential. Procedures for extracting ammonium and nitrate from environmental samples must be consistent to avoid isotopic fractionation that could bias results. Methods such as diffusion, distillation, and microdiffusion have been standardized with detailed protocols published by organizations like the International Atomic Energy Agency (IAEA) and the United States Geological Survey (USGS).
Data reporting conventions have also been standardized to facilitate comparison across studies. Results are typically expressed in delta notation (δ) relative to international standards, with units of per mil (‰). The scientific community has adopted specific guidelines for reporting uncertainty, requiring both analytical precision and overall measurement uncertainty to be clearly stated in publications.
Recent technological advances have introduced automated systems that integrate sample preparation with isotope analysis, reducing human error and improving throughput. These systems incorporate built-in quality control features, such as automatic drift correction and flagging of anomalous results. However, they require regular validation against established manual methods to ensure consistency.
Inter-laboratory comparison programs, such as those organized by the IAEA, play a crucial role in maintaining global standardization. These programs distribute identical samples to participating laboratories, allowing for the identification of systematic biases and methodological issues. Results from these exercises have led to improved analytical protocols and greater harmonization of isotope data worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!