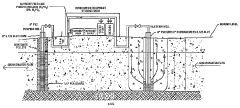
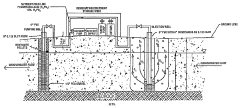
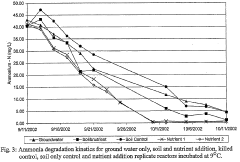
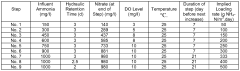
Nitrification In Groundwater Recharge Systems
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrification Technology Background and Objectives
Nitrification in groundwater recharge systems has evolved significantly over the past several decades, transitioning from an observed natural phenomenon to a deliberately engineered process. Initially documented in the 1970s and 1980s, researchers observed that nitrogen compounds underwent transformation during soil aquifer treatment, though the mechanisms were poorly understood. The evolution of this technology has been driven by increasing concerns about nitrogen pollution in water resources and the recognition of groundwater's importance as a strategic resource.
The nitrification process involves the biological oxidation of ammonia (NH₃) to nitrite (NO₂⁻) and subsequently to nitrate (NO₃⁻) through the action of specialized chemolithoautotrophic bacteria. In groundwater recharge contexts, this process occurs within the vadose zone and upper portions of the aquifer, where oxygen availability supports aerobic microbial activity. The efficiency of this natural treatment process has inspired engineered approaches to enhance and control nitrification in managed aquifer recharge (MAR) systems.
Recent technological developments have focused on optimizing conditions for nitrification in recharge systems through careful management of hydraulic loading rates, pre-treatment processes, and enhancement of the soil-aquifer interface. Advanced monitoring techniques, including real-time sensors and molecular biological tools, have enabled better understanding and control of the microbial communities responsible for nitrification.
The primary technical objectives in this field include maximizing nitrification efficiency while minimizing energy inputs and operational costs. This involves developing systems that can maintain appropriate oxygen levels, temperature ranges, and hydraulic retention times to support robust nitrifying bacterial populations. Additionally, there is significant interest in designing systems that can operate effectively under variable influent conditions and recover quickly from perturbations.
Another key objective is integrating nitrification processes with subsequent denitrification to achieve complete nitrogen removal, particularly in areas where nitrate contamination of groundwater is a concern. This requires careful management of redox conditions within the subsurface environment to create zones that support both aerobic nitrification and anoxic denitrification.
The technology trajectory is moving toward more sophisticated, nature-based solutions that leverage and enhance natural microbial processes while incorporating smart monitoring and control systems. Research is increasingly focused on understanding the microbial ecology of nitrifying communities in these systems and how they respond to environmental variables and operational parameters.
Future objectives include developing predictive models that can anticipate system performance under changing conditions, including climate change impacts, and designing more resilient systems that can adapt to these changes while maintaining treatment efficacy.
The nitrification process involves the biological oxidation of ammonia (NH₃) to nitrite (NO₂⁻) and subsequently to nitrate (NO₃⁻) through the action of specialized chemolithoautotrophic bacteria. In groundwater recharge contexts, this process occurs within the vadose zone and upper portions of the aquifer, where oxygen availability supports aerobic microbial activity. The efficiency of this natural treatment process has inspired engineered approaches to enhance and control nitrification in managed aquifer recharge (MAR) systems.
Recent technological developments have focused on optimizing conditions for nitrification in recharge systems through careful management of hydraulic loading rates, pre-treatment processes, and enhancement of the soil-aquifer interface. Advanced monitoring techniques, including real-time sensors and molecular biological tools, have enabled better understanding and control of the microbial communities responsible for nitrification.
The primary technical objectives in this field include maximizing nitrification efficiency while minimizing energy inputs and operational costs. This involves developing systems that can maintain appropriate oxygen levels, temperature ranges, and hydraulic retention times to support robust nitrifying bacterial populations. Additionally, there is significant interest in designing systems that can operate effectively under variable influent conditions and recover quickly from perturbations.
Another key objective is integrating nitrification processes with subsequent denitrification to achieve complete nitrogen removal, particularly in areas where nitrate contamination of groundwater is a concern. This requires careful management of redox conditions within the subsurface environment to create zones that support both aerobic nitrification and anoxic denitrification.
The technology trajectory is moving toward more sophisticated, nature-based solutions that leverage and enhance natural microbial processes while incorporating smart monitoring and control systems. Research is increasingly focused on understanding the microbial ecology of nitrifying communities in these systems and how they respond to environmental variables and operational parameters.
Future objectives include developing predictive models that can anticipate system performance under changing conditions, including climate change impacts, and designing more resilient systems that can adapt to these changes while maintaining treatment efficacy.
Market Analysis for Groundwater Recharge Solutions
The global market for groundwater recharge solutions is experiencing significant growth, driven by increasing water scarcity concerns and the need for sustainable water management practices. Current market valuations indicate that the groundwater management sector reached approximately $14.2 billion in 2022, with projections suggesting growth to $20.5 billion by 2027, representing a compound annual growth rate of 7.6%.
Regionally, North America currently dominates the market with about 35% share, followed by Europe (28%) and Asia-Pacific (22%). However, the fastest growth is anticipated in developing regions, particularly in water-stressed countries across Asia and Africa, where annual growth rates exceed 9%.
The market for nitrification-specific technologies within groundwater recharge systems represents a specialized but expanding segment. This growth is primarily driven by increasing regulatory pressures regarding nitrogen contamination in groundwater, with the EPA and European Water Framework Directive imposing stricter limits on nitrate levels in drinking water sources.
Municipal water utilities constitute the largest customer segment (42%), followed by agricultural operations (27%) and industrial users (18%). The remaining market share is distributed among research institutions, environmental remediation companies, and private water management firms. This distribution reflects the critical importance of nitrification management across multiple sectors.
Key market drivers include escalating concerns about groundwater contamination from agricultural runoff, industrial discharge, and wastewater effluent. Additionally, climate change impacts on water availability are compelling governments and private entities to invest in groundwater recharge infrastructure as a resilience strategy.
Customer demand patterns reveal increasing preference for integrated solutions that address multiple water quality parameters simultaneously, with nitrification control being a critical component. There is also growing interest in nature-based solutions that leverage biological processes for nitrification management, reflecting broader sustainability trends.
Market barriers include high initial capital costs for advanced recharge systems, regulatory complexities across different jurisdictions, and limited public awareness about groundwater management issues. Technical challenges related to site-specific optimization of nitrification processes also constrain market expansion in certain regions.
Emerging opportunities exist in the development of smart monitoring systems for real-time nitrification tracking, modular and scalable solutions for smaller communities, and public-private partnership models that distribute financial risks while accelerating technology deployment.
Regionally, North America currently dominates the market with about 35% share, followed by Europe (28%) and Asia-Pacific (22%). However, the fastest growth is anticipated in developing regions, particularly in water-stressed countries across Asia and Africa, where annual growth rates exceed 9%.
The market for nitrification-specific technologies within groundwater recharge systems represents a specialized but expanding segment. This growth is primarily driven by increasing regulatory pressures regarding nitrogen contamination in groundwater, with the EPA and European Water Framework Directive imposing stricter limits on nitrate levels in drinking water sources.
Municipal water utilities constitute the largest customer segment (42%), followed by agricultural operations (27%) and industrial users (18%). The remaining market share is distributed among research institutions, environmental remediation companies, and private water management firms. This distribution reflects the critical importance of nitrification management across multiple sectors.
Key market drivers include escalating concerns about groundwater contamination from agricultural runoff, industrial discharge, and wastewater effluent. Additionally, climate change impacts on water availability are compelling governments and private entities to invest in groundwater recharge infrastructure as a resilience strategy.
Customer demand patterns reveal increasing preference for integrated solutions that address multiple water quality parameters simultaneously, with nitrification control being a critical component. There is also growing interest in nature-based solutions that leverage biological processes for nitrification management, reflecting broader sustainability trends.
Market barriers include high initial capital costs for advanced recharge systems, regulatory complexities across different jurisdictions, and limited public awareness about groundwater management issues. Technical challenges related to site-specific optimization of nitrification processes also constrain market expansion in certain regions.
Emerging opportunities exist in the development of smart monitoring systems for real-time nitrification tracking, modular and scalable solutions for smaller communities, and public-private partnership models that distribute financial risks while accelerating technology deployment.
Current Nitrification Challenges in Aquifer Systems
Nitrification processes in groundwater recharge systems face significant challenges that impact their efficiency and effectiveness. The primary obstacle is the variability in environmental conditions within aquifer systems, which directly affects nitrifying bacterial communities. Temperature fluctuations, particularly seasonal changes, can dramatically alter nitrification rates, with optimal activity typically occurring between 20-30°C. During colder periods, nitrification efficiency decreases substantially, creating inconsistent treatment outcomes throughout the year.
Oxygen availability represents another critical limitation, as nitrification is fundamentally an aerobic process. In many groundwater recharge systems, especially those utilizing infiltration basins or injection wells, oxygen depletion occurs rapidly with depth. This creates anoxic zones where nitrification cannot proceed, resulting in incomplete ammonia transformation and potential groundwater contamination. Engineered solutions to maintain adequate dissolved oxygen levels often increase operational costs significantly.
pH variability presents additional complications, as nitrifying bacteria operate optimally within a narrow range of 7.5-8.5. Groundwater systems with naturally acidic conditions or those receiving acidic recharge water experience inhibited nitrification rates. Furthermore, the buffering capacity of soil and aquifer materials may be insufficient to maintain optimal pH levels throughout the treatment zone.
Organic carbon interference poses a substantial challenge in many recharge systems. High concentrations of biodegradable organic matter create competition between heterotrophic bacteria and nitrifiers for available oxygen. This competition typically favors the faster-growing heterotrophs, suppressing nitrification processes. Additionally, certain organic compounds can directly inhibit nitrifying bacteria through toxic effects.
Hydraulic loading rates and residence time constraints further complicate nitrification management. Excessive loading rates reduce contact time between nitrifying bacteria and ammonia, while insufficient residence time prevents complete nitrification reactions. Finding the optimal balance between treatment efficiency and system throughput remains challenging for system operators.
Emerging contaminants, including pharmaceuticals, personal care products, and industrial chemicals, have demonstrated inhibitory effects on nitrifying bacterial communities. These compounds can disrupt enzyme functions or damage cellular structures of nitrifying bacteria, even at trace concentrations. The increasing presence of these contaminants in wastewater and surface water sources used for recharge compounds the challenges facing nitrification processes.
Biofilm development and management issues also impact nitrification efficiency. While biofilms provide habitat for nitrifying bacteria, excessive growth can lead to clogging, preferential flow paths, and reduced treatment efficiency. Conversely, insufficient biofilm development limits nitrification capacity, particularly in newly established recharge systems.
Oxygen availability represents another critical limitation, as nitrification is fundamentally an aerobic process. In many groundwater recharge systems, especially those utilizing infiltration basins or injection wells, oxygen depletion occurs rapidly with depth. This creates anoxic zones where nitrification cannot proceed, resulting in incomplete ammonia transformation and potential groundwater contamination. Engineered solutions to maintain adequate dissolved oxygen levels often increase operational costs significantly.
pH variability presents additional complications, as nitrifying bacteria operate optimally within a narrow range of 7.5-8.5. Groundwater systems with naturally acidic conditions or those receiving acidic recharge water experience inhibited nitrification rates. Furthermore, the buffering capacity of soil and aquifer materials may be insufficient to maintain optimal pH levels throughout the treatment zone.
Organic carbon interference poses a substantial challenge in many recharge systems. High concentrations of biodegradable organic matter create competition between heterotrophic bacteria and nitrifiers for available oxygen. This competition typically favors the faster-growing heterotrophs, suppressing nitrification processes. Additionally, certain organic compounds can directly inhibit nitrifying bacteria through toxic effects.
Hydraulic loading rates and residence time constraints further complicate nitrification management. Excessive loading rates reduce contact time between nitrifying bacteria and ammonia, while insufficient residence time prevents complete nitrification reactions. Finding the optimal balance between treatment efficiency and system throughput remains challenging for system operators.
Emerging contaminants, including pharmaceuticals, personal care products, and industrial chemicals, have demonstrated inhibitory effects on nitrifying bacterial communities. These compounds can disrupt enzyme functions or damage cellular structures of nitrifying bacteria, even at trace concentrations. The increasing presence of these contaminants in wastewater and surface water sources used for recharge compounds the challenges facing nitrification processes.
Biofilm development and management issues also impact nitrification efficiency. While biofilms provide habitat for nitrifying bacteria, excessive growth can lead to clogging, preferential flow paths, and reduced treatment efficiency. Conversely, insufficient biofilm development limits nitrification capacity, particularly in newly established recharge systems.
Current Nitrification Process Solutions
01 Biological nitrification processes
Biological nitrification involves the oxidation of ammonia to nitrite by ammonia-oxidizing bacteria (AOB), followed by the oxidation of nitrite to nitrate by nitrite-oxidizing bacteria (NOB). This two-step process is crucial for nitrogen transformation in wastewater treatment systems and natural environments. The efficiency of biological nitrification depends on factors such as dissolved oxygen levels, temperature, pH, and the presence of inhibitory compounds.- Biological nitrification processes: Biological nitrification involves the oxidation of ammonia to nitrite by ammonia-oxidizing bacteria (AOB), followed by the oxidation of nitrite to nitrate by nitrite-oxidizing bacteria (NOB). This two-step process is crucial in wastewater treatment systems and natural nitrogen cycling. The process requires specific environmental conditions including adequate oxygen levels, appropriate pH range, and suitable temperature to maintain microbial activity and efficiency of nitrogen transformation.
- Enhanced nitrification systems for wastewater treatment: Advanced systems for enhancing nitrification in wastewater treatment incorporate specialized reactor designs, biofilm carriers, and controlled environmental parameters. These systems optimize the conversion of ammonia to nitrate while minimizing the production of greenhouse gases. Technologies include moving bed biofilm reactors (MBBR), sequencing batch reactors (SBR), and integrated fixed-film activated sludge processes that provide efficient nitrogen removal while accommodating fluctuating load conditions.
- Agricultural applications of nitrification control: Controlling nitrification processes in agricultural settings involves the use of nitrification inhibitors, specialized fertilizer formulations, and application techniques to regulate the transformation of nitrogen in soil. These approaches help maintain nitrogen in the ammonium form longer, reducing nitrate leaching and improving nitrogen use efficiency by crops. Strategic management of nitrification can significantly reduce environmental impacts while optimizing crop yields and nutrient utilization.
- Novel microbial consortia for nitrogen transformation: Specialized microbial consortia have been developed to enhance nitrification processes under various environmental conditions. These consortia include specific strains of ammonia-oxidizing and nitrite-oxidizing microorganisms that can operate efficiently in challenging environments such as low temperatures, high salinity, or fluctuating pH levels. The application of these microbial communities improves nitrogen removal rates and process stability in both natural and engineered systems.
- Monitoring and control systems for nitrification processes: Advanced monitoring and control systems for nitrification processes utilize sensors, real-time analytics, and automated feedback mechanisms to optimize nitrogen transformation. These systems track key parameters such as dissolved oxygen, pH, temperature, and nitrogen compound concentrations to maintain optimal conditions for nitrifying microorganisms. Implementation of these control strategies improves process stability, reduces energy consumption, and ensures consistent nitrogen removal efficiency in treatment systems.
02 Enhanced nitrification systems for wastewater treatment
Advanced systems for enhancing nitrification in wastewater treatment incorporate specialized reactor designs, biofilm carriers, and controlled environmental conditions. These systems aim to optimize the growth and activity of nitrifying microorganisms, resulting in more efficient nitrogen transformation. Technologies include moving bed biofilm reactors (MBBR), sequencing batch reactors (SBR), and integrated fixed-film activated sludge (IFAS) systems that provide ideal conditions for nitrification processes.Expand Specific Solutions03 Agricultural applications of nitrification control
Controlling nitrification processes in agricultural settings is essential for optimizing nitrogen use efficiency and reducing environmental impacts. This includes the use of nitrification inhibitors that slow the conversion of ammonium to nitrate, reducing nitrogen losses through leaching and denitrification. Various formulations and application methods have been developed to regulate nitrogen transformation in soil, improving nutrient availability to crops while minimizing environmental pollution.Expand Specific Solutions04 Novel microbial communities for nitrogen transformation
Research into specialized microbial communities has led to the discovery and development of novel nitrifying consortia with enhanced capabilities for nitrogen transformation. These include anammox bacteria that can convert ammonium directly to nitrogen gas, and other microorganisms that perform nitrification under extreme or unusual conditions. The cultivation and application of these specialized microbial communities offer new approaches to nitrogen management in various environmental and industrial contexts.Expand Specific Solutions05 Monitoring and control systems for nitrification processes
Advanced monitoring and control systems have been developed to optimize nitrification processes in real-time. These systems utilize sensors for parameters such as dissolved oxygen, ammonia, nitrite, and nitrate concentrations, along with automated control algorithms to maintain optimal conditions for nitrogen transformation. Implementation of these technologies allows for more efficient operation of nitrification processes, reduced energy consumption, and improved nitrogen removal efficiency in treatment systems.Expand Specific Solutions
Key Industry Players in Groundwater Treatment
Nitrification in groundwater recharge systems is currently in a growth phase, with the market expanding due to increasing water scarcity and stricter environmental regulations. The global market for groundwater recharge technologies is projected to grow significantly as water resource management becomes critical worldwide. Technologically, the field shows varying maturity levels, with established players like Kurita Water Industries, Veolia, and Sulzer offering commercial solutions, while academic institutions such as Zhejiang University, Tianjin University, and Nanjing University drive fundamental research. Companies like Hitachi and Mitsubishi Chemical Aqua Solutions are integrating advanced monitoring and control systems, while newer entrants like Nitricity are developing innovative nitrogen management approaches. The competitive landscape features collaboration between industrial water treatment specialists and research institutions to address efficiency and sustainability challenges.
Kurita Water Industries Ltd.
Technical Solution: Kurita Water Industries has developed advanced biological nitrification systems specifically designed for groundwater recharge applications. Their technology employs specialized biofilm carriers with optimized surface area that support the growth of nitrifying bacteria (Nitrosomonas and Nitrobacter). The system incorporates a two-stage process: first converting ammonia to nitrite, then nitrite to nitrate, with precise oxygen control mechanisms to maintain optimal dissolved oxygen levels (5-7 mg/L) for nitrification. Kurita's approach includes automated monitoring systems that continuously track nitrogen compounds, pH, and temperature, with feedback controls that adjust aeration rates and hydraulic retention times. Their technology also features innovative backwashing mechanisms that prevent clogging while preserving the integrity of the microbial communities essential for nitrification processes.
Strengths: Superior biofilm carrier design maximizes nitrification efficiency even at lower temperatures; integrated monitoring systems ensure process stability. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized maintenance expertise for optimal performance.
Sulzer AG
Technical Solution: Sulzer has developed the AquaNOx® system specifically for nitrification in groundwater recharge applications. This technology utilizes a hybrid fixed-film/suspended growth process with proprietary structured packing media that provides optimal surface area (>400 m²/m³) for nitrifying bacteria colonization. The system incorporates fine-bubble aeration technology that achieves oxygen transfer efficiency exceeding 30%, crucial for the energy-intensive nitrification process. Sulzer's approach includes a pre-treatment zone for ammonia equalization followed by multiple nitrification stages with progressively specialized microbial communities. The technology features automated process control that adjusts operational parameters based on influent characteristics and temperature variations, maintaining nitrification efficiency above 95% even during cold weather operations (down to 5°C). Their design also incorporates innovative hydraulic distribution systems that ensure uniform flow across the biological media, preventing short-circuiting and maximizing treatment efficiency.
Strengths: Exceptional resistance to shock loads and temperature fluctuations; modular design allows for easy capacity expansion; low sludge production compared to conventional systems. Weaknesses: Higher initial capital costs; requires specialized media replacement approximately every 7-10 years; more complex operation than basic treatment systems.
Critical Patents in Biological Nitrogen Removal
In-SITU groundwater nitrification and de-nitrification remediation system
PatentInactiveCA2610722C
Innovation
- An in-situ groundwater remediation system that alternates between nitrification and de-nitrification processes by extracting groundwater, adding oxygen or carbon sources along with nutrients, and re-injecting it into the ground to oxidize ammonia to nitrate or reduce nitrate to nitrogen gas, respectively, utilizing naturally occurring bacteria to encourage growth and reproduction.
Nitrification method and system
PatentWO2014172121A1
Innovation
- A nitrification method and system that uses a reactor with a balanced population of Ammonia Oxidizing Bacteria (AOB) and Nitrite Oxidizing Bacteria (NOB) and controlled dissolved oxygen levels to convert at least 40% of ammonia in the centrate stream to nitrates, with the ability to increase conversion rates up to 90% by optimizing bacterial activity and oxygen utilization.
Environmental Impact Assessment
The environmental impacts of nitrification processes in groundwater recharge systems extend across multiple ecological dimensions. These systems, designed to replenish aquifers with treated wastewater or surface water, create unique biogeochemical environments where nitrogen transformation significantly influences surrounding ecosystems. The conversion of ammonia to nitrite and subsequently to nitrate through nitrification alters groundwater chemistry in ways that can persist for decades.
Primary environmental concerns include potential nitrate leaching into adjacent water bodies, which may trigger eutrophication in receiving surface waters. Studies have documented increased algal blooms and decreased dissolved oxygen levels in watersheds downstream from recharge facilities with incomplete nitrification management. The ecological cascade effect can disrupt aquatic food webs and reduce biodiversity in affected water bodies.
Soil structure and composition undergo notable changes in recharge zones where nitrification occurs. The process generates acidity that can mobilize heavy metals and alter soil mineral composition. Long-term monitoring at established recharge facilities has revealed shifts in soil microbial communities, with nitrifying bacteria becoming dominant in previously diverse microbial ecosystems.
Greenhouse gas emissions represent another significant environmental consideration. Incomplete nitrification can lead to nitrous oxide (N₂O) production, a greenhouse gas approximately 300 times more potent than carbon dioxide. Measurements at operational recharge facilities indicate emission rates varying between 0.1-2.5% of nitrogen processed, depending on system design and operational parameters.
Habitat modification occurs as recharge basins create artificial wetland-like environments. While these areas can provide beneficial habitat for certain species, the altered nitrogen cycling may favor invasive plant species adapted to high-nitrate conditions. The resulting vegetation changes can displace native plant communities and modify wildlife usage patterns in surrounding areas.
Groundwater quality impacts extend beyond the immediate recharge zone, with nitrification-derived nitrate plumes potentially affecting drinking water supplies. The World Health Organization has established a 50 mg/L nitrate limit for drinking water, a threshold exceeded in numerous documented cases where recharge system nitrification was inadequately managed.
Climate adaptation considerations are increasingly relevant, as changing precipitation patterns and temperature regimes affect nitrification kinetics in recharge systems. Higher temperatures generally accelerate nitrification rates but may also increase volatilization losses and alter the microbial communities responsible for the process.
Primary environmental concerns include potential nitrate leaching into adjacent water bodies, which may trigger eutrophication in receiving surface waters. Studies have documented increased algal blooms and decreased dissolved oxygen levels in watersheds downstream from recharge facilities with incomplete nitrification management. The ecological cascade effect can disrupt aquatic food webs and reduce biodiversity in affected water bodies.
Soil structure and composition undergo notable changes in recharge zones where nitrification occurs. The process generates acidity that can mobilize heavy metals and alter soil mineral composition. Long-term monitoring at established recharge facilities has revealed shifts in soil microbial communities, with nitrifying bacteria becoming dominant in previously diverse microbial ecosystems.
Greenhouse gas emissions represent another significant environmental consideration. Incomplete nitrification can lead to nitrous oxide (N₂O) production, a greenhouse gas approximately 300 times more potent than carbon dioxide. Measurements at operational recharge facilities indicate emission rates varying between 0.1-2.5% of nitrogen processed, depending on system design and operational parameters.
Habitat modification occurs as recharge basins create artificial wetland-like environments. While these areas can provide beneficial habitat for certain species, the altered nitrogen cycling may favor invasive plant species adapted to high-nitrate conditions. The resulting vegetation changes can displace native plant communities and modify wildlife usage patterns in surrounding areas.
Groundwater quality impacts extend beyond the immediate recharge zone, with nitrification-derived nitrate plumes potentially affecting drinking water supplies. The World Health Organization has established a 50 mg/L nitrate limit for drinking water, a threshold exceeded in numerous documented cases where recharge system nitrification was inadequately managed.
Climate adaptation considerations are increasingly relevant, as changing precipitation patterns and temperature regimes affect nitrification kinetics in recharge systems. Higher temperatures generally accelerate nitrification rates but may also increase volatilization losses and alter the microbial communities responsible for the process.
Regulatory Framework for Groundwater Quality
The regulatory landscape governing groundwater quality has evolved significantly in response to increasing concerns about contamination and the critical role of groundwater in water supply systems. In the context of nitrification processes in groundwater recharge systems, regulatory frameworks have been established at international, national, and local levels to ensure the protection of this vital resource.
At the international level, organizations such as the World Health Organization (WHO) have developed guidelines that establish maximum contaminant levels for nitrates and nitrites in drinking water. These guidelines serve as reference points for many national regulatory bodies, recommending nitrate concentrations below 50 mg/L to protect against methemoglobinemia and other health risks associated with elevated nitrogen compounds.
In the United States, the Environmental Protection Agency (EPA) enforces the Safe Drinking Water Act, which establishes a Maximum Contaminant Level (MCL) of 10 mg/L for nitrate-nitrogen in public water systems. Additionally, the Clean Water Act provides regulatory authority for protecting surface waters that may interact with groundwater systems through recharge zones.
The European Union's Water Framework Directive and Groundwater Directive collectively establish comprehensive protection measures, requiring member states to prevent deterioration of groundwater quality and implement monitoring programs. These directives specifically address nitrate pollution through the Nitrates Directive (91/676/EEC), which requires the designation of Nitrate Vulnerable Zones and implementation of action programs.
Regulatory approaches for managed aquifer recharge (MAR) systems, including those utilizing nitrification processes, typically involve permitting requirements that address source water quality, treatment processes, and monitoring protocols. These regulations often mandate pre-treatment standards, operational controls, and regular assessment of both influent and effluent water quality parameters.
Compliance monitoring represents a critical component of the regulatory framework, with requirements for regular sampling and analysis of nitrogen compounds throughout the recharge system. Many jurisdictions have established trigger levels that, when exceeded, necessitate corrective actions or system modifications to maintain compliance with water quality standards.
Recent regulatory trends reflect an increasing emphasis on integrated water resource management approaches that consider the interconnections between surface water, groundwater, and ecological systems. This holistic perspective has led to the development of watershed-based regulatory frameworks that address nitrogen loading from multiple sources, including agricultural runoff, wastewater discharges, and atmospheric deposition.
As scientific understanding of nitrification processes advances, regulatory frameworks continue to evolve, incorporating new knowledge about transformation pathways, microbial ecology, and treatment technologies to better protect groundwater resources while enabling sustainable water management practices.
At the international level, organizations such as the World Health Organization (WHO) have developed guidelines that establish maximum contaminant levels for nitrates and nitrites in drinking water. These guidelines serve as reference points for many national regulatory bodies, recommending nitrate concentrations below 50 mg/L to protect against methemoglobinemia and other health risks associated with elevated nitrogen compounds.
In the United States, the Environmental Protection Agency (EPA) enforces the Safe Drinking Water Act, which establishes a Maximum Contaminant Level (MCL) of 10 mg/L for nitrate-nitrogen in public water systems. Additionally, the Clean Water Act provides regulatory authority for protecting surface waters that may interact with groundwater systems through recharge zones.
The European Union's Water Framework Directive and Groundwater Directive collectively establish comprehensive protection measures, requiring member states to prevent deterioration of groundwater quality and implement monitoring programs. These directives specifically address nitrate pollution through the Nitrates Directive (91/676/EEC), which requires the designation of Nitrate Vulnerable Zones and implementation of action programs.
Regulatory approaches for managed aquifer recharge (MAR) systems, including those utilizing nitrification processes, typically involve permitting requirements that address source water quality, treatment processes, and monitoring protocols. These regulations often mandate pre-treatment standards, operational controls, and regular assessment of both influent and effluent water quality parameters.
Compliance monitoring represents a critical component of the regulatory framework, with requirements for regular sampling and analysis of nitrogen compounds throughout the recharge system. Many jurisdictions have established trigger levels that, when exceeded, necessitate corrective actions or system modifications to maintain compliance with water quality standards.
Recent regulatory trends reflect an increasing emphasis on integrated water resource management approaches that consider the interconnections between surface water, groundwater, and ecological systems. This holistic perspective has led to the development of watershed-based regulatory frameworks that address nitrogen loading from multiple sources, including agricultural runoff, wastewater discharges, and atmospheric deposition.
As scientific understanding of nitrification processes advances, regulatory frameworks continue to evolve, incorporating new knowledge about transformation pathways, microbial ecology, and treatment technologies to better protect groundwater resources while enabling sustainable water management practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!