How PCM Extends Cold-Chain Holdover Time for Biologics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PCM Cold-Chain Technology Background and Objectives
Phase Change Materials (PCM) have emerged as a transformative technology in cold chain management, particularly for temperature-sensitive biologics. The evolution of PCM technology dates back to the 1970s when initial research explored materials with high latent heat properties. However, it wasn't until the early 2000s that PCM applications in pharmaceutical cold chains gained significant traction, driven by the expanding biopharmaceutical market and stricter regulatory requirements for temperature-controlled logistics.
The fundamental principle behind PCM technology lies in its ability to absorb and release thermal energy during phase transitions. When PCM changes from solid to liquid or vice versa, it absorbs or releases large amounts of energy at a nearly constant temperature, creating an ideal buffer against external temperature fluctuations. This property makes PCM particularly valuable for maintaining precise temperature ranges required for biologics, which can lose efficacy or become unsafe when exposed to temperature excursions.
Current technological objectives for PCM in cold chain applications focus on extending holdover times while maintaining temperature stability within narrow therapeutic ranges. The industry aims to develop PCM solutions that can reliably maintain temperatures between 2-8°C (for refrigerated biologics) or -20°C and below (for frozen biologics) for extended periods, even in challenging environmental conditions or during transportation disruptions.
Another critical objective is enhancing the sustainability profile of PCM solutions. Traditional cold chain technologies often rely on mechanical refrigeration or dry ice, both associated with high carbon footprints. PCM technology aims to reduce environmental impact through reusable components and improved energy efficiency, aligning with global sustainability initiatives in healthcare logistics.
Miniaturization and customization represent additional technological goals, as the industry moves toward personalized medicine requiring smaller, more precise temperature-controlled packages. Researchers are exploring nano-enhanced PCMs and composite materials to improve thermal conductivity and energy density, potentially revolutionizing last-mile delivery of temperature-sensitive biologics.
Integration with IoT (Internet of Things) and real-time monitoring systems constitutes another frontier in PCM technology development. The objective is to create smart cold chain solutions that not only maintain temperature but also provide continuous data on storage conditions, enabling proactive interventions and comprehensive documentation for regulatory compliance.
As biologics continue to dominate pharmaceutical pipelines, PCM technology aims to address the increasingly complex temperature requirements of next-generation therapeutics, including cell and gene therapies, which may require ultra-precise temperature control throughout their lifecycle from manufacturing to patient administration.
The fundamental principle behind PCM technology lies in its ability to absorb and release thermal energy during phase transitions. When PCM changes from solid to liquid or vice versa, it absorbs or releases large amounts of energy at a nearly constant temperature, creating an ideal buffer against external temperature fluctuations. This property makes PCM particularly valuable for maintaining precise temperature ranges required for biologics, which can lose efficacy or become unsafe when exposed to temperature excursions.
Current technological objectives for PCM in cold chain applications focus on extending holdover times while maintaining temperature stability within narrow therapeutic ranges. The industry aims to develop PCM solutions that can reliably maintain temperatures between 2-8°C (for refrigerated biologics) or -20°C and below (for frozen biologics) for extended periods, even in challenging environmental conditions or during transportation disruptions.
Another critical objective is enhancing the sustainability profile of PCM solutions. Traditional cold chain technologies often rely on mechanical refrigeration or dry ice, both associated with high carbon footprints. PCM technology aims to reduce environmental impact through reusable components and improved energy efficiency, aligning with global sustainability initiatives in healthcare logistics.
Miniaturization and customization represent additional technological goals, as the industry moves toward personalized medicine requiring smaller, more precise temperature-controlled packages. Researchers are exploring nano-enhanced PCMs and composite materials to improve thermal conductivity and energy density, potentially revolutionizing last-mile delivery of temperature-sensitive biologics.
Integration with IoT (Internet of Things) and real-time monitoring systems constitutes another frontier in PCM technology development. The objective is to create smart cold chain solutions that not only maintain temperature but also provide continuous data on storage conditions, enabling proactive interventions and comprehensive documentation for regulatory compliance.
As biologics continue to dominate pharmaceutical pipelines, PCM technology aims to address the increasingly complex temperature requirements of next-generation therapeutics, including cell and gene therapies, which may require ultra-precise temperature control throughout their lifecycle from manufacturing to patient administration.
Market Demand Analysis for Biologics Cold-Chain Solutions
The global biologics market has witnessed substantial growth, with its value reaching $382 billion in 2020 and projected to exceed $750 billion by 2028, growing at a CAGR of approximately 9.5%. This remarkable expansion is driving unprecedented demand for reliable cold-chain solutions, particularly those that can extend holdover times during transportation and storage of temperature-sensitive biological products.
Temperature excursions remain the primary cause of biologics degradation during distribution, with industry reports indicating that nearly 20% of temperature-sensitive healthcare products are damaged during transport due to cold chain breakdowns. This translates to annual losses exceeding $35 billion globally, creating an urgent market need for advanced thermal management solutions like Phase Change Materials (PCM).
The COVID-19 pandemic has dramatically accelerated this demand, with vaccine distribution requirements highlighting critical gaps in existing cold-chain infrastructure. The ultra-cold storage requirements for mRNA vaccines (-70°C to -80°C) exposed limitations in traditional cold-chain systems, particularly in regions with underdeveloped logistics networks where power interruptions are common.
Healthcare providers and pharmaceutical companies are increasingly seeking solutions that offer extended holdover times without increasing package dimensions or weight. Market research indicates that 78% of biologics manufacturers consider improved temperature stability during transit as their top priority for cold-chain innovation, with 65% specifically interested in PCM-based solutions.
Geographically, North America dominates the biologics cold-chain market with a 38% share, followed by Europe (31%) and Asia-Pacific (22%). However, the fastest growth is occurring in emerging markets, where biologics adoption is accelerating despite infrastructure challenges. These regions show particular interest in PCM solutions that can maintain temperature stability during "last mile" delivery in areas with unreliable power supplies.
The personalized medicine trend is creating demand for smaller-batch, higher-value biologics shipments, increasing the economic impact of each potential temperature excursion. This has shifted market preferences toward premium cold-chain solutions that prioritize reliability over cost, with surveys showing 83% of logistics decision-makers willing to pay premium prices for solutions that demonstrably extend holdover times.
Regulatory pressures are further driving market demand, with agencies worldwide implementing stricter temperature control requirements for biologics. The EU GDP guidelines and FDA regulations now mandate continuous temperature monitoring and validation of thermal protection systems, creating additional market pull for advanced PCM solutions that can provide documented temperature stability and extended holdover capabilities.
Temperature excursions remain the primary cause of biologics degradation during distribution, with industry reports indicating that nearly 20% of temperature-sensitive healthcare products are damaged during transport due to cold chain breakdowns. This translates to annual losses exceeding $35 billion globally, creating an urgent market need for advanced thermal management solutions like Phase Change Materials (PCM).
The COVID-19 pandemic has dramatically accelerated this demand, with vaccine distribution requirements highlighting critical gaps in existing cold-chain infrastructure. The ultra-cold storage requirements for mRNA vaccines (-70°C to -80°C) exposed limitations in traditional cold-chain systems, particularly in regions with underdeveloped logistics networks where power interruptions are common.
Healthcare providers and pharmaceutical companies are increasingly seeking solutions that offer extended holdover times without increasing package dimensions or weight. Market research indicates that 78% of biologics manufacturers consider improved temperature stability during transit as their top priority for cold-chain innovation, with 65% specifically interested in PCM-based solutions.
Geographically, North America dominates the biologics cold-chain market with a 38% share, followed by Europe (31%) and Asia-Pacific (22%). However, the fastest growth is occurring in emerging markets, where biologics adoption is accelerating despite infrastructure challenges. These regions show particular interest in PCM solutions that can maintain temperature stability during "last mile" delivery in areas with unreliable power supplies.
The personalized medicine trend is creating demand for smaller-batch, higher-value biologics shipments, increasing the economic impact of each potential temperature excursion. This has shifted market preferences toward premium cold-chain solutions that prioritize reliability over cost, with surveys showing 83% of logistics decision-makers willing to pay premium prices for solutions that demonstrably extend holdover times.
Regulatory pressures are further driving market demand, with agencies worldwide implementing stricter temperature control requirements for biologics. The EU GDP guidelines and FDA regulations now mandate continuous temperature monitoring and validation of thermal protection systems, creating additional market pull for advanced PCM solutions that can provide documented temperature stability and extended holdover capabilities.
Current PCM Technology Status and Challenges
Phase Change Materials (PCM) technology for cold-chain applications has evolved significantly over the past decade, yet still faces substantial challenges in meeting the stringent requirements for biologics transportation and storage. Current PCM solutions utilize various organic compounds (paraffins, fatty acids), inorganic substances (salt hydrates), and eutectic mixtures to achieve temperature stabilization through latent heat absorption and release during phase transitions.
The global market has witnessed a proliferation of PCM products specifically designed for pharmaceutical cold chains, with solutions available in temperature ranges from -40°C to 8°C to accommodate different biological products. However, performance inconsistencies remain a significant challenge, particularly in maintaining precise temperature control within the narrow therapeutic ranges required for sensitive biologics like mRNA vaccines (±0.5°C in some cases).
Thermal conductivity limitations represent another major technical hurdle. Most commercially available PCMs exhibit relatively low thermal conductivity (0.2-0.5 W/m·K), resulting in slow heat transfer rates that can compromise temperature uniformity throughout storage containers. This creates potential "hot spots" or "cold spots" that may damage biological materials, especially during temperature fluctuations in the external environment.
Supercooling effects present additional complications, as many PCMs do not crystallize at their theoretical freezing point, leading to unpredictable performance in real-world conditions. This phenomenon can reduce effective holdover time by as much as 15-30% compared to laboratory specifications, creating significant reliability concerns for critical biological shipments.
Encapsulation technologies have advanced to address leakage and material compatibility issues, with multi-layer polymer systems and metal-organic frameworks showing promise. However, these solutions often increase cost and weight while potentially reducing overall thermal efficiency. The trade-off between containment security and thermal performance remains a key challenge for manufacturers.
Geographical distribution of PCM technology development shows concentration in North America, Western Europe, and increasingly in Asia-Pacific regions, particularly Japan and China. Research institutions in these regions are actively pursuing next-generation PCM formulations with enhanced properties, though commercialization pathways remain lengthy due to regulatory requirements for pharmaceutical applications.
Sustainability concerns are emerging as another challenge, with traditional petroleum-based PCMs facing scrutiny for their environmental impact. Bio-based alternatives derived from vegetable oils and sugar alcohols show promise but currently demonstrate lower performance metrics and higher production costs, limiting their widespread adoption in critical biological applications where performance reliability cannot be compromised.
The global market has witnessed a proliferation of PCM products specifically designed for pharmaceutical cold chains, with solutions available in temperature ranges from -40°C to 8°C to accommodate different biological products. However, performance inconsistencies remain a significant challenge, particularly in maintaining precise temperature control within the narrow therapeutic ranges required for sensitive biologics like mRNA vaccines (±0.5°C in some cases).
Thermal conductivity limitations represent another major technical hurdle. Most commercially available PCMs exhibit relatively low thermal conductivity (0.2-0.5 W/m·K), resulting in slow heat transfer rates that can compromise temperature uniformity throughout storage containers. This creates potential "hot spots" or "cold spots" that may damage biological materials, especially during temperature fluctuations in the external environment.
Supercooling effects present additional complications, as many PCMs do not crystallize at their theoretical freezing point, leading to unpredictable performance in real-world conditions. This phenomenon can reduce effective holdover time by as much as 15-30% compared to laboratory specifications, creating significant reliability concerns for critical biological shipments.
Encapsulation technologies have advanced to address leakage and material compatibility issues, with multi-layer polymer systems and metal-organic frameworks showing promise. However, these solutions often increase cost and weight while potentially reducing overall thermal efficiency. The trade-off between containment security and thermal performance remains a key challenge for manufacturers.
Geographical distribution of PCM technology development shows concentration in North America, Western Europe, and increasingly in Asia-Pacific regions, particularly Japan and China. Research institutions in these regions are actively pursuing next-generation PCM formulations with enhanced properties, though commercialization pathways remain lengthy due to regulatory requirements for pharmaceutical applications.
Sustainability concerns are emerging as another challenge, with traditional petroleum-based PCMs facing scrutiny for their environmental impact. Bio-based alternatives derived from vegetable oils and sugar alcohols show promise but currently demonstrate lower performance metrics and higher production costs, limiting their widespread adoption in critical biological applications where performance reliability cannot be compromised.
Current PCM Solutions for Biologics Temperature Control
01 PCM applications in thermal management systems
Phase Change Materials (PCMs) are utilized in thermal management systems to maintain stable temperatures during power outages or transportation. The holdover time, which is the duration a system can maintain desired temperature conditions without external power, is enhanced through strategic PCM placement and selection. These systems are particularly valuable in cold chain logistics, medical storage, and electronic cooling applications where temperature stability is critical.- PCM applications in thermal management systems: Phase Change Materials (PCM) are utilized in thermal management systems to maintain stable temperatures for extended periods. These materials absorb and release thermal energy during phase transitions, providing effective temperature control. The holdover time, which is the duration a system can maintain a desired temperature range, is enhanced through strategic PCM implementation in various applications including electronics cooling, building materials, and energy storage systems.
- PCM composition and formulation for extended holdover time: The composition and formulation of Phase Change Materials significantly impact their holdover time performance. By adjusting the chemical composition, incorporating additives, or creating composite materials, the thermal properties can be optimized. Specific formulations can enhance heat capacity, thermal conductivity, and phase transition temperature stability, resulting in prolonged temperature maintenance capabilities and improved overall system efficiency.
- PCM integration in battery and electronic device cooling: Phase Change Materials are increasingly integrated into battery systems and electronic devices to manage thermal issues and extend operational time. By absorbing excess heat during high-load operations and releasing it during idle periods, PCMs help maintain optimal operating temperatures. This thermal buffering effect prevents overheating, extends battery life, and improves device reliability by providing longer holdover times during temperature fluctuations.
- PCM encapsulation techniques for improved holdover performance: Encapsulation techniques for Phase Change Materials enhance their holdover time performance by preventing leakage during the liquid phase and improving heat transfer characteristics. Various encapsulation methods including microencapsulation, macroencapsulation, and shape-stabilized PCMs help maintain material integrity over numerous thermal cycles. These techniques also allow for better integration into host materials and can incorporate additives that enhance thermal conductivity and stability.
- Testing and measurement methods for PCM holdover time: Specialized testing and measurement methods have been developed to accurately evaluate the holdover time performance of Phase Change Materials. These include thermal cycling tests, differential scanning calorimetry, and real-world application simulations. Advanced monitoring systems track temperature profiles during phase transitions to determine effective holdover duration under various conditions. These methodologies help in standardizing PCM performance metrics and optimizing material selection for specific applications.
02 PCM composition and formulation for extended holdover time
The composition and formulation of Phase Change Materials significantly impact holdover time performance. By engineering specific mixtures of organic and inorganic compounds, the melting point and latent heat capacity can be optimized. Additives that enhance thermal conductivity and prevent supercooling can extend the effective duration of temperature maintenance. Encapsulation techniques further improve stability and prevent leakage during phase transitions.Expand Specific Solutions03 PCM integration in battery and electronic systems
Phase Change Materials are increasingly integrated into battery systems and electronics to manage thermal loads and extend operational time. By absorbing excess heat during peak usage and releasing it during idle periods, PCMs help maintain optimal operating temperatures. This thermal buffering effect extends the holdover time of battery systems, prevents thermal runaway, and enhances overall device reliability and lifespan in varying environmental conditions.Expand Specific Solutions04 PCM container and packaging design for holdover time optimization
The design of containers and packaging for Phase Change Materials plays a crucial role in maximizing holdover time. Innovative insulation techniques, multi-layered structures, and vacuum panels can significantly reduce heat transfer rates. Container geometry, wall thickness, and material selection are optimized to enhance thermal performance. Advanced designs incorporate features that facilitate uniform heat distribution and minimize thermal bridges, resulting in extended temperature maintenance periods.Expand Specific Solutions05 PCM testing and performance measurement methodologies
Specialized methodologies have been developed to accurately measure and predict the holdover time of Phase Change Material systems. These include accelerated testing protocols, thermal cycling procedures, and computational modeling approaches. Performance metrics such as effective thermal capacity, phase transition stability over multiple cycles, and response to varying ambient conditions are evaluated. These testing frameworks enable standardized comparison between different PCM solutions and help optimize system designs for specific applications.Expand Specific Solutions
Leading Companies in PCM Cold-Chain Industry
The PCM (Phase Change Materials) cold-chain market for biologics is currently in a growth phase, characterized by increasing adoption across pharmaceutical and healthcare sectors. The global market is expanding rapidly due to growing demand for temperature-sensitive biologic transportation solutions, estimated to reach several billion dollars by 2025. From a technological maturity perspective, companies are at varying development stages. Established players like Softbox Systems and Dr. Reddy's Laboratories offer commercialized PCM solutions, while innovative startups such as Tan90 Thermal Solutions and Nostromo are advancing next-generation technologies. Research institutions including Shanghai Ocean University and Fudan University are contributing fundamental research. Companies like Immunomedics and Hombrechtikon Systems Engineering are integrating PCM technologies into specialized biologics delivery systems, demonstrating the technology's transition from emerging to mainstream adoption in critical cold-chain applications.
Tan90 Thermal Solutions Pte Ltd.
Technical Solution: Tan90 Thermal Solutions has developed innovative PCM-based cold chain solutions specifically targeting biologics transportation challenges. Their proprietary PCM formulations utilize plant-based materials engineered to maintain precise temperature ranges (2-8°C, -20°C) critical for biological products. The company's modular PCM panels feature a unique phase transition stabilization technology that prevents temperature spikes during the phase change process, ensuring more consistent temperature maintenance[3]. Tan90's solutions incorporate a multi-layer PCM approach where different formulations with staggered phase change temperatures work together to extend holdover times up to 100+ hours. Their PCM technology also features specialized nucleating agents that reduce supercooling effects, allowing for more predictable performance in varying ambient conditions. The company has developed portable PCM-based containers with IoT-enabled temperature monitoring that provides real-time alerts and location tracking for high-value biological shipments.
Strengths: Plant-based sustainable PCM formulations, excellent cost-effectiveness compared to traditional solutions, and innovative multi-layer PCM technology for extended temperature stability. Weaknesses: Relatively newer market entrant with less extensive field validation data compared to established players, and current limitations in ultra-low temperature (-80°C) applications.
Softbox Systems Ltd.
Technical Solution: Softbox Systems has pioneered the Tempcell™ ECO and Silverpod™ pallet shipper systems that incorporate advanced PCM technology specifically for biologics cold-chain management. Their PCM solutions feature proprietary formulations that provide precise temperature control at multiple set points (2-8°C, 15-25°C, and frozen applications). The company's PCM panels are designed with a honeycomb structure that optimizes thermal energy storage and release, resulting in holdover times of up to 96+ hours in challenging ambient conditions[2]. Softbox's PCM technology includes their patented Tempaphase™ materials, which undergo rigorous stability testing to ensure consistent performance over hundreds of freeze-thaw cycles. Their systems also incorporate recyclable and biodegradable components, addressing sustainability concerns while maintaining thermal performance for sensitive biological products during global distribution.
Strengths: Exceptional thermal stability with documented performance in extreme conditions, environmentally sustainable design elements, and versatile packaging options for different payload sizes. Weaknesses: Requires careful pre-conditioning of PCM elements before use, and some solutions may have higher volume-to-payload ratios compared to active cooling systems.
Key PCM Materials and Thermal Properties Analysis
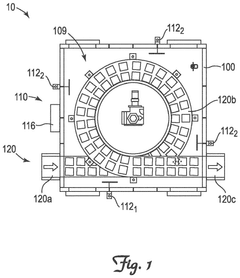
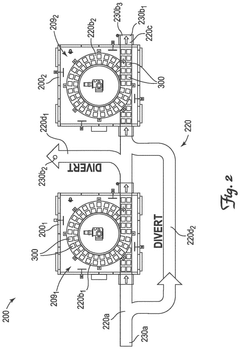
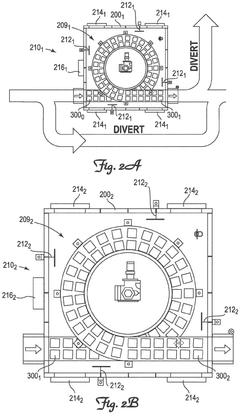
System and method for cryogenic thermal conditioning of cold chain phase change material panels
PatentPendingEP4571219A1
Innovation
- A cryogenic freezer and a two-stage inline thermal conditioning system are used to expedite the thermal conditioning of thermally-spent phase change material panels. The system includes a conveyor system with intermediate platform lengths for dwell times and spray nozzles for controlled cryogenic fluid injection, allowing for rapid transition from thermally spent to deep-frozen and then to thermally tempered panels.
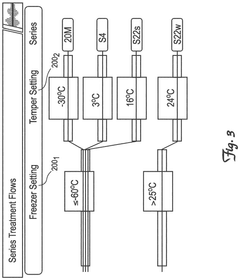
Phase change material composition and method of preparation thereof
PatentActiveUS11692116B2
Innovation
- A phase change material (PCM) composition is developed, incorporating glass fibers and xanthan gum, which reduces overall thermal conductivity and increases latent heat, allowing for extended phase change duration and improved insulation performance, along with a stackable, sealable package design that minimizes air gaps with glass microparticles for enhanced thermal insulation.
Regulatory Standards for Biologics Transportation
The transportation of biologics is governed by stringent regulatory frameworks designed to ensure product integrity throughout the cold chain. The World Health Organization (WHO) has established comprehensive guidelines for the storage and transportation of temperature-sensitive pharmaceutical products, including biologics. These guidelines specify temperature ranges, monitoring requirements, and validation protocols that must be adhered to when implementing Phase Change Material (PCM) solutions.
In the United States, the Food and Drug Administration (FDA) enforces regulations through 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals) and 21 CFR Part 203 (Prescription Drug Marketing), which include provisions for proper storage and handling of temperature-sensitive products. The FDA's guidance document "Stability Testing of Drug Substances and Drug Products" specifically addresses temperature excursion management, where PCM technology plays a crucial role in maintaining compliance.
The European Medicines Agency (EMA) has implemented Guidelines on Good Distribution Practice (GDP) that mandate temperature control during transportation. These guidelines require pharmaceutical companies to validate their cold chain processes, including the performance of PCM-based packaging systems under various environmental conditions and durations.
International Air Transport Association (IATA) Temperature Control Regulations provide standards for air shipment of biologics, including specific requirements for passive cooling systems utilizing PCMs. These regulations define acceptable temperature monitoring practices and documentation requirements for international shipments.
The International Safe Transit Association (ISTA) has developed standardized testing protocols (particularly ISTA 7D) for evaluating temperature-controlled packaging performance. These protocols are widely used to validate PCM-based solutions against regulatory requirements before implementation in actual supply chains.
Regulatory bodies increasingly require real-time temperature monitoring and data logging capabilities to be integrated with cold chain packaging solutions. This has led to the development of smart PCM systems that combine phase change materials with IoT-enabled monitoring devices to ensure continuous compliance documentation.
Recent regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing for greater innovation in PCM technologies while maintaining strict focus on product quality and patient safety. Companies must demonstrate through validation studies that their PCM solutions can reliably maintain required temperatures throughout the expected duration of transportation, including accounting for potential delays and extreme environmental conditions.
In the United States, the Food and Drug Administration (FDA) enforces regulations through 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals) and 21 CFR Part 203 (Prescription Drug Marketing), which include provisions for proper storage and handling of temperature-sensitive products. The FDA's guidance document "Stability Testing of Drug Substances and Drug Products" specifically addresses temperature excursion management, where PCM technology plays a crucial role in maintaining compliance.
The European Medicines Agency (EMA) has implemented Guidelines on Good Distribution Practice (GDP) that mandate temperature control during transportation. These guidelines require pharmaceutical companies to validate their cold chain processes, including the performance of PCM-based packaging systems under various environmental conditions and durations.
International Air Transport Association (IATA) Temperature Control Regulations provide standards for air shipment of biologics, including specific requirements for passive cooling systems utilizing PCMs. These regulations define acceptable temperature monitoring practices and documentation requirements for international shipments.
The International Safe Transit Association (ISTA) has developed standardized testing protocols (particularly ISTA 7D) for evaluating temperature-controlled packaging performance. These protocols are widely used to validate PCM-based solutions against regulatory requirements before implementation in actual supply chains.
Regulatory bodies increasingly require real-time temperature monitoring and data logging capabilities to be integrated with cold chain packaging solutions. This has led to the development of smart PCM systems that combine phase change materials with IoT-enabled monitoring devices to ensure continuous compliance documentation.
Recent regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing for greater innovation in PCM technologies while maintaining strict focus on product quality and patient safety. Companies must demonstrate through validation studies that their PCM solutions can reliably maintain required temperatures throughout the expected duration of transportation, including accounting for potential delays and extreme environmental conditions.
Sustainability Aspects of PCM Cold-Chain Solutions
The sustainability profile of Phase Change Materials (PCM) in cold-chain applications represents a critical consideration as the biopharmaceutical industry seeks more environmentally responsible temperature control solutions. PCM-based systems offer significant sustainability advantages over traditional cold-chain methods, particularly in reducing carbon footprint and energy consumption during the transportation and storage of temperature-sensitive biologics.
PCM solutions dramatically reduce dependency on active cooling systems that require continuous power input. By utilizing stored thermal energy through phase transitions, these materials can maintain stable temperatures for extended periods without electricity, resulting in energy savings of up to 40% compared to conventional refrigeration systems. This passive cooling approach is particularly valuable in regions with unreliable power infrastructure, where it simultaneously addresses both sustainability and practical logistics challenges.
The recyclability of many modern PCMs further enhances their environmental credentials. Unlike single-use cooling solutions such as dry ice or gel packs, properly designed PCM systems can be reused hundreds or even thousands of times without significant degradation in performance. This characteristic substantially reduces waste generation across the cold-chain lifecycle, with some manufacturers reporting up to 95% reduction in packaging waste when implementing reusable PCM solutions.
Life cycle assessment (LCA) studies indicate that bio-based PCMs derived from sustainable sources offer additional environmental benefits. These materials, often manufactured from agricultural byproducts or renewable resources, demonstrate lower embodied carbon compared to petroleum-based alternatives. Recent innovations have produced PCMs with biodegradable properties, addressing end-of-life environmental concerns that have historically limited widespread adoption of certain thermal management solutions.
Water consumption metrics also favor PCM technology, with studies showing 30-50% reduction in water usage across the cold-chain when compared to traditional cooling methods. This advantage becomes particularly significant when considering the water-intensive nature of biopharmaceutical manufacturing processes, allowing companies to improve their overall environmental performance while maintaining product integrity.
The extended holdover times provided by PCM technology contribute to sustainability by reducing the frequency of shipments and associated transportation emissions. By enabling fewer, more efficient deliveries of temperature-sensitive biologics, PCM solutions help minimize the carbon footprint associated with global pharmaceutical distribution networks, potentially reducing transportation-related emissions by 15-25% according to industry analyses.
PCM solutions dramatically reduce dependency on active cooling systems that require continuous power input. By utilizing stored thermal energy through phase transitions, these materials can maintain stable temperatures for extended periods without electricity, resulting in energy savings of up to 40% compared to conventional refrigeration systems. This passive cooling approach is particularly valuable in regions with unreliable power infrastructure, where it simultaneously addresses both sustainability and practical logistics challenges.
The recyclability of many modern PCMs further enhances their environmental credentials. Unlike single-use cooling solutions such as dry ice or gel packs, properly designed PCM systems can be reused hundreds or even thousands of times without significant degradation in performance. This characteristic substantially reduces waste generation across the cold-chain lifecycle, with some manufacturers reporting up to 95% reduction in packaging waste when implementing reusable PCM solutions.
Life cycle assessment (LCA) studies indicate that bio-based PCMs derived from sustainable sources offer additional environmental benefits. These materials, often manufactured from agricultural byproducts or renewable resources, demonstrate lower embodied carbon compared to petroleum-based alternatives. Recent innovations have produced PCMs with biodegradable properties, addressing end-of-life environmental concerns that have historically limited widespread adoption of certain thermal management solutions.
Water consumption metrics also favor PCM technology, with studies showing 30-50% reduction in water usage across the cold-chain when compared to traditional cooling methods. This advantage becomes particularly significant when considering the water-intensive nature of biopharmaceutical manufacturing processes, allowing companies to improve their overall environmental performance while maintaining product integrity.
The extended holdover times provided by PCM technology contribute to sustainability by reducing the frequency of shipments and associated transportation emissions. By enabling fewer, more efficient deliveries of temperature-sensitive biologics, PCM solutions help minimize the carbon footprint associated with global pharmaceutical distribution networks, potentially reducing transportation-related emissions by 15-25% according to industry analyses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!