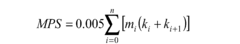How Sodium Percarbonate Influences Laundry Detergents' Cleaning Power
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate in Detergents: Background and Objectives
Sodium percarbonate has emerged as a key component in modern laundry detergents, revolutionizing cleaning power and environmental sustainability. This compound, also known as sodium carbonate peroxyhydrate, is a stable adduct of sodium carbonate and hydrogen peroxide. Its introduction in the detergent industry marks a significant milestone in the evolution of cleaning technologies.
The development of sodium percarbonate can be traced back to the early 20th century, but its widespread use in laundry detergents gained momentum in the 1990s. This shift was driven by increasing consumer demand for more effective and eco-friendly cleaning solutions. As environmental concerns grew, manufacturers sought alternatives to traditional phosphate-based detergents, which were linked to water pollution and eutrophication.
Sodium percarbonate's unique properties make it an ideal candidate for laundry applications. When dissolved in water, it releases oxygen, which acts as a powerful bleaching and stain-removing agent. This oxygen-based bleaching mechanism is effective at lower temperatures compared to traditional chlorine bleaches, aligning with the global trend towards energy-efficient washing practices.
The primary objective of incorporating sodium percarbonate into laundry detergents is to enhance cleaning efficacy while minimizing environmental impact. This dual goal addresses both consumer expectations for superior cleaning performance and regulatory pressures for more sustainable products. Manufacturers aim to optimize the concentration and formulation of sodium percarbonate to achieve maximum cleaning power across a wide range of fabric types and soil conditions.
Another critical objective is to improve the stability of sodium percarbonate within detergent formulations. The compound's tendency to decompose when exposed to moisture presents a challenge for long-term storage and shelf life. Research efforts are focused on developing coating technologies and stabilizing agents to preserve the active oxygen content until the point of use.
The integration of sodium percarbonate into detergents also aims to reduce water and energy consumption in the laundry process. By enabling effective cleaning at lower temperatures, these detergents contribute to energy savings and reduced carbon footprints. This aligns with broader sustainability goals and appeals to environmentally conscious consumers.
As the detergent industry continues to evolve, the role of sodium percarbonate is expected to expand. Future research directions may explore synergistic effects with other cleaning agents, further improvements in stability, and potential applications beyond laundry care. The ongoing development of this technology reflects the industry's commitment to innovation and sustainability in household cleaning products.
The development of sodium percarbonate can be traced back to the early 20th century, but its widespread use in laundry detergents gained momentum in the 1990s. This shift was driven by increasing consumer demand for more effective and eco-friendly cleaning solutions. As environmental concerns grew, manufacturers sought alternatives to traditional phosphate-based detergents, which were linked to water pollution and eutrophication.
Sodium percarbonate's unique properties make it an ideal candidate for laundry applications. When dissolved in water, it releases oxygen, which acts as a powerful bleaching and stain-removing agent. This oxygen-based bleaching mechanism is effective at lower temperatures compared to traditional chlorine bleaches, aligning with the global trend towards energy-efficient washing practices.
The primary objective of incorporating sodium percarbonate into laundry detergents is to enhance cleaning efficacy while minimizing environmental impact. This dual goal addresses both consumer expectations for superior cleaning performance and regulatory pressures for more sustainable products. Manufacturers aim to optimize the concentration and formulation of sodium percarbonate to achieve maximum cleaning power across a wide range of fabric types and soil conditions.
Another critical objective is to improve the stability of sodium percarbonate within detergent formulations. The compound's tendency to decompose when exposed to moisture presents a challenge for long-term storage and shelf life. Research efforts are focused on developing coating technologies and stabilizing agents to preserve the active oxygen content until the point of use.
The integration of sodium percarbonate into detergents also aims to reduce water and energy consumption in the laundry process. By enabling effective cleaning at lower temperatures, these detergents contribute to energy savings and reduced carbon footprints. This aligns with broader sustainability goals and appeals to environmentally conscious consumers.
As the detergent industry continues to evolve, the role of sodium percarbonate is expected to expand. Future research directions may explore synergistic effects with other cleaning agents, further improvements in stability, and potential applications beyond laundry care. The ongoing development of this technology reflects the industry's commitment to innovation and sustainability in household cleaning products.
Market Demand for Effective Laundry Solutions
The global laundry detergent market has been experiencing steady growth, driven by increasing urbanization, rising disposable incomes, and growing awareness of hygiene and cleanliness. Consumers are increasingly demanding more effective and efficient cleaning solutions for their laundry needs, with a particular focus on products that can remove tough stains and maintain fabric quality.
In recent years, there has been a significant shift towards environmentally friendly and sustainable laundry solutions. This trend has led to a growing interest in detergents that are biodegradable, phosphate-free, and contain natural ingredients. Sodium percarbonate, as an eco-friendly bleaching agent, has gained popularity in this context due to its ability to break down into oxygen, water, and sodium carbonate, leaving no harmful residues.
The COVID-19 pandemic has further accelerated the demand for effective laundry solutions, with consumers placing greater emphasis on hygiene and cleanliness. This has resulted in a surge in sales of laundry detergents with enhanced cleaning and disinfecting properties. Sodium percarbonate, known for its ability to release hydrogen peroxide when dissolved in water, has become increasingly attractive to consumers seeking powerful cleaning and sanitizing effects.
Market research indicates that consumers are willing to pay a premium for laundry detergents that offer superior cleaning power and additional benefits such as fabric care, color protection, and long-lasting freshness. This has created opportunities for manufacturers to develop innovative formulations incorporating sodium percarbonate to meet these diverse consumer needs.
The demand for concentrated and compact laundry detergents has also been on the rise, driven by convenience factors and environmental concerns. Sodium percarbonate's stability and compatibility with other detergent ingredients make it an ideal component for these concentrated formulations, allowing manufacturers to create powerful yet compact cleaning solutions.
In the commercial sector, there is a growing demand for industrial-strength laundry detergents in hospitality, healthcare, and institutional settings. These industries require highly effective cleaning solutions that can handle large volumes of laundry while maintaining strict hygiene standards. Sodium percarbonate's powerful oxidizing properties make it a valuable ingredient in meeting these demanding requirements.
As consumers become more educated about laundry care, there is an increasing interest in understanding the science behind cleaning agents. This has led to a demand for transparent labeling and clear communication of product benefits. Manufacturers incorporating sodium percarbonate into their formulations have an opportunity to highlight its eco-friendly nature and superior cleaning capabilities, appealing to environmentally conscious and performance-driven consumers alike.
In recent years, there has been a significant shift towards environmentally friendly and sustainable laundry solutions. This trend has led to a growing interest in detergents that are biodegradable, phosphate-free, and contain natural ingredients. Sodium percarbonate, as an eco-friendly bleaching agent, has gained popularity in this context due to its ability to break down into oxygen, water, and sodium carbonate, leaving no harmful residues.
The COVID-19 pandemic has further accelerated the demand for effective laundry solutions, with consumers placing greater emphasis on hygiene and cleanliness. This has resulted in a surge in sales of laundry detergents with enhanced cleaning and disinfecting properties. Sodium percarbonate, known for its ability to release hydrogen peroxide when dissolved in water, has become increasingly attractive to consumers seeking powerful cleaning and sanitizing effects.
Market research indicates that consumers are willing to pay a premium for laundry detergents that offer superior cleaning power and additional benefits such as fabric care, color protection, and long-lasting freshness. This has created opportunities for manufacturers to develop innovative formulations incorporating sodium percarbonate to meet these diverse consumer needs.
The demand for concentrated and compact laundry detergents has also been on the rise, driven by convenience factors and environmental concerns. Sodium percarbonate's stability and compatibility with other detergent ingredients make it an ideal component for these concentrated formulations, allowing manufacturers to create powerful yet compact cleaning solutions.
In the commercial sector, there is a growing demand for industrial-strength laundry detergents in hospitality, healthcare, and institutional settings. These industries require highly effective cleaning solutions that can handle large volumes of laundry while maintaining strict hygiene standards. Sodium percarbonate's powerful oxidizing properties make it a valuable ingredient in meeting these demanding requirements.
As consumers become more educated about laundry care, there is an increasing interest in understanding the science behind cleaning agents. This has led to a demand for transparent labeling and clear communication of product benefits. Manufacturers incorporating sodium percarbonate into their formulations have an opportunity to highlight its eco-friendly nature and superior cleaning capabilities, appealing to environmentally conscious and performance-driven consumers alike.
Current State and Challenges in Detergent Formulations
The current state of laundry detergent formulations is characterized by a complex interplay of various chemical components, each serving specific functions to enhance cleaning performance. Sodium percarbonate has emerged as a key ingredient in many modern detergent formulations, particularly in powdered and tablet forms. This compound acts as a source of hydrogen peroxide, which is released when the detergent comes into contact with water, providing powerful oxidizing and bleaching capabilities.
One of the primary challenges in detergent formulations is achieving a balance between cleaning efficacy and environmental sustainability. While sodium percarbonate offers excellent stain removal properties, manufacturers must consider its impact on aquatic ecosystems and potential interactions with other detergent components. Additionally, ensuring the stability of sodium percarbonate during storage and transportation poses a significant challenge, as exposure to moisture can prematurely activate its oxidizing properties.
Another critical aspect of current detergent formulations is the need for compatibility with a wide range of fabrics and washing temperatures. Sodium percarbonate's effectiveness can vary depending on water temperature, with optimal performance typically achieved in warm to hot water. This presents a challenge in markets where cold water washing is prevalent due to energy conservation efforts or cultural preferences.
The incorporation of enzymes alongside sodium percarbonate in detergent formulations has become increasingly common. However, maintaining enzyme stability in the presence of strong oxidizing agents like sodium percarbonate remains a technical hurdle. Manufacturers must develop innovative encapsulation techniques or separation methods to prevent premature enzyme deactivation while still allowing for their synergistic action during the washing process.
Consumer preferences for more concentrated detergent formulations have led to increased sodium percarbonate concentrations in some products. This trend necessitates careful consideration of product safety, as higher concentrations may pose risks if mishandled or ingested. Developing child-resistant packaging and clear usage instructions are ongoing challenges for detergent manufacturers.
The global push towards more sustainable and biodegradable cleaning products has spurred research into alternative oxidizing agents that could potentially replace or complement sodium percarbonate. Finding compounds that match its cleaning power while offering improved environmental profiles remains an active area of investigation in the detergent industry.
One of the primary challenges in detergent formulations is achieving a balance between cleaning efficacy and environmental sustainability. While sodium percarbonate offers excellent stain removal properties, manufacturers must consider its impact on aquatic ecosystems and potential interactions with other detergent components. Additionally, ensuring the stability of sodium percarbonate during storage and transportation poses a significant challenge, as exposure to moisture can prematurely activate its oxidizing properties.
Another critical aspect of current detergent formulations is the need for compatibility with a wide range of fabrics and washing temperatures. Sodium percarbonate's effectiveness can vary depending on water temperature, with optimal performance typically achieved in warm to hot water. This presents a challenge in markets where cold water washing is prevalent due to energy conservation efforts or cultural preferences.
The incorporation of enzymes alongside sodium percarbonate in detergent formulations has become increasingly common. However, maintaining enzyme stability in the presence of strong oxidizing agents like sodium percarbonate remains a technical hurdle. Manufacturers must develop innovative encapsulation techniques or separation methods to prevent premature enzyme deactivation while still allowing for their synergistic action during the washing process.
Consumer preferences for more concentrated detergent formulations have led to increased sodium percarbonate concentrations in some products. This trend necessitates careful consideration of product safety, as higher concentrations may pose risks if mishandled or ingested. Developing child-resistant packaging and clear usage instructions are ongoing challenges for detergent manufacturers.
The global push towards more sustainable and biodegradable cleaning products has spurred research into alternative oxidizing agents that could potentially replace or complement sodium percarbonate. Finding compounds that match its cleaning power while offering improved environmental profiles remains an active area of investigation in the detergent industry.
Existing Sodium Percarbonate Integration Methods
01 Composition and preparation of sodium percarbonate
Sodium percarbonate is a compound composed of sodium carbonate and hydrogen peroxide. It is prepared through various methods, including crystallization and spray drying processes. The composition and preparation methods are crucial for determining the stability and effectiveness of sodium percarbonate as a cleaning agent.- Composition and preparation of sodium percarbonate: Sodium percarbonate is a compound formed by combining sodium carbonate and hydrogen peroxide. Its preparation methods and composition are crucial for its cleaning power. The compound is typically produced through various processes, including crystallization or spray drying, to ensure stability and effectiveness in cleaning applications.
- Stabilization of sodium percarbonate: Stabilizing sodium percarbonate is essential to maintain its cleaning power during storage and use. Various stabilizing agents and coating techniques are employed to protect the compound from moisture and premature decomposition. These methods enhance the shelf life and effectiveness of sodium percarbonate in cleaning products.
- Application in laundry and household cleaning: Sodium percarbonate is widely used in laundry detergents and household cleaning products due to its powerful oxidizing properties. It releases oxygen when dissolved in water, effectively removing stains and brightening fabrics. The compound's ability to clean and disinfect makes it a versatile ingredient in various cleaning formulations.
- Environmental and safety considerations: Sodium percarbonate is considered an environmentally friendly cleaning agent as it breaks down into harmless byproducts. Its use in cleaning products reduces the need for harsh chemicals, making it safer for both users and the environment. However, proper handling and storage are necessary to ensure safety and maintain its cleaning power.
- Synergistic effects with other cleaning agents: The cleaning power of sodium percarbonate can be enhanced when combined with other cleaning agents. Synergistic effects are observed when used in conjunction with enzymes, surfactants, or other oxidizing agents. These combinations can improve overall cleaning performance and expand the range of applications for sodium percarbonate-based products.
02 Stabilization of sodium percarbonate
Various techniques are employed to stabilize sodium percarbonate, enhancing its shelf life and cleaning power. These methods include coating the particles with stabilizing agents, incorporating additives, and controlling moisture content. Stabilization is essential for maintaining the active oxygen content and ensuring consistent cleaning performance.Expand Specific Solutions03 Application in cleaning formulations
Sodium percarbonate is widely used in cleaning formulations due to its ability to release active oxygen. It is incorporated into laundry detergents, dishwashing products, and general-purpose cleaners. The cleaning power is enhanced when combined with other ingredients such as surfactants, enzymes, and bleach activators.Expand Specific Solutions04 Environmental and safety considerations
Sodium percarbonate is considered an environmentally friendly alternative to traditional chlorine-based bleaches. It breaks down into harmless substances after use, making it safer for the environment. Safety considerations include proper handling and storage to prevent decomposition and ensure maximum cleaning effectiveness.Expand Specific Solutions05 Synergistic effects with other cleaning agents
The cleaning power of sodium percarbonate can be enhanced through synergistic effects with other cleaning agents. Combinations with enzymes, surfactants, or other oxidizing agents can lead to improved stain removal and overall cleaning performance. These synergistic effects are often exploited in the formulation of advanced cleaning products.Expand Specific Solutions
Key Players in Detergent and Chemical Industries
The market for sodium percarbonate in laundry detergents is in a mature stage, with a global market size estimated to be in the billions of dollars. The technology is well-established, with major players like Solvay SA, Evonik Operations GmbH, and Henkel AG & Co. KGaA leading the industry. These companies have extensive research and development capabilities, driving incremental improvements in product efficiency and environmental sustainability. The competitive landscape is characterized by a mix of large multinational corporations and regional specialists, such as Puyang Hongye Environment Protection New Materials Co., Ltd. and Zhejiang Jinke Daily Chemical Co. Ltd., which are significant players in the Asian market. As environmental concerns grow, there is an increasing focus on developing more eco-friendly formulations, pushing the industry towards further innovation.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate (SPC) formulations for laundry detergents, focusing on stability and efficacy. Their SPC particles are coated with a protective layer that prevents premature decomposition, ensuring longer shelf life and improved performance[1]. The company has also introduced a patented manufacturing process that produces SPC with a controlled particle size distribution, optimizing dissolution rates in various water temperatures[2]. Solvay's SPC technology incorporates oxygen bleach activators, which enhance stain removal at lower temperatures, addressing energy efficiency concerns[3]. Additionally, they have developed eco-friendly SPC variants with reduced environmental impact, aligning with the growing demand for sustainable cleaning solutions[4].
Strengths: Advanced stability technology, optimized particle size distribution for better dissolution, and eco-friendly formulations. Weaknesses: Potentially higher production costs due to specialized coating and manufacturing processes.
Evonik Operations GmbH
Technical Solution: Evonik has innovated in the field of sodium percarbonate for laundry detergents by developing a unique stabilization technology called PERSYNT®. This technology significantly improves the stability of SPC in detergent formulations, especially in humid conditions[1]. Evonik's SPC products feature a core-shell structure, where the sodium percarbonate core is encapsulated in a protective layer that dissolves rapidly when in contact with water[2]. This design ensures both long-term stability and quick release of active oxygen during the washing process. The company has also focused on producing high-purity SPC with minimal impurities, which enhances its compatibility with other detergent ingredients and reduces the risk of fabric damage[3]. Evonik's research has led to the development of SPC formulations that are effective at lower washing temperatures, contributing to energy savings[4].
Strengths: Superior stability in humid conditions, rapid dissolution, and high purity for better compatibility. Weaknesses: May require specialized handling and storage due to enhanced reactivity.
Core Innovations in Sodium Percarbonate Technology
Use of a blend containing percarbonate for detergents and dishwashing formulations
PatentInactiveEP1939276A1
Innovation
- A blend of sodium percarbonate particles with specific particle sizes and additional particles of different chemical compositions, ensuring the blend is classified as a non-oxidizer, reducing segregation risks and enhancing stability, thus minimizing handling and transportation costs.
Coated sodium percarbonate particle
PatentActiveEP1903098A1
Innovation
- Sodium percarbonate particles with a shell layer containing high-temperature phase sodium sulfate and/or a double salt of sodium sulfate and sodium carbonate, which provides improved storage stability by maintaining the active oxygen content and preventing moisture-induced decomposition.
Environmental Impact of Sodium Percarbonate Use
The use of sodium percarbonate in laundry detergents has significant environmental implications, both positive and negative. As a powerful oxidizing agent, sodium percarbonate breaks down into hydrogen peroxide and sodium carbonate when dissolved in water, providing effective cleaning and bleaching properties.
One of the primary environmental benefits of sodium percarbonate is its ability to reduce the need for hot water in laundry processes. By releasing oxygen at lower temperatures, it enables effective cleaning and stain removal even in cold water cycles. This energy-saving potential contributes to reduced carbon emissions associated with water heating, aligning with global efforts to combat climate change.
Furthermore, sodium percarbonate is biodegradable and leaves no harmful residues in the environment. Unlike some traditional bleaching agents, it breaks down into water, oxygen, and sodium carbonate, which are naturally occurring and environmentally benign substances. This characteristic makes it a more eco-friendly alternative to chlorine-based bleaches, which can form harmful chlorinated organic compounds.
However, the environmental impact of sodium percarbonate is not entirely positive. The production process of sodium percarbonate involves energy-intensive steps and the use of chemical precursors. The manufacturing of hydrogen peroxide, a key component in sodium percarbonate production, can have its own environmental footprint, including potential water pollution and greenhouse gas emissions.
Additionally, while sodium percarbonate itself is not toxic to aquatic life, its breakdown product, hydrogen peroxide, can be harmful to some organisms if released in high concentrations. This necessitates proper wastewater treatment to ensure that effluents containing sodium percarbonate or its byproducts are adequately diluted before being released into natural water bodies.
The increased use of sodium percarbonate in detergents may also lead to elevated levels of sodium in wastewater. While sodium is not typically considered a major pollutant, high concentrations can affect soil structure and plant growth in areas where treated wastewater is used for irrigation.
On balance, the environmental impact of sodium percarbonate in laundry detergents is generally considered favorable when compared to more aggressive cleaning agents. Its ability to enable low-temperature washing, coupled with its biodegradability, positions it as a relatively sustainable choice in the context of increasing environmental awareness in consumer products.
One of the primary environmental benefits of sodium percarbonate is its ability to reduce the need for hot water in laundry processes. By releasing oxygen at lower temperatures, it enables effective cleaning and stain removal even in cold water cycles. This energy-saving potential contributes to reduced carbon emissions associated with water heating, aligning with global efforts to combat climate change.
Furthermore, sodium percarbonate is biodegradable and leaves no harmful residues in the environment. Unlike some traditional bleaching agents, it breaks down into water, oxygen, and sodium carbonate, which are naturally occurring and environmentally benign substances. This characteristic makes it a more eco-friendly alternative to chlorine-based bleaches, which can form harmful chlorinated organic compounds.
However, the environmental impact of sodium percarbonate is not entirely positive. The production process of sodium percarbonate involves energy-intensive steps and the use of chemical precursors. The manufacturing of hydrogen peroxide, a key component in sodium percarbonate production, can have its own environmental footprint, including potential water pollution and greenhouse gas emissions.
Additionally, while sodium percarbonate itself is not toxic to aquatic life, its breakdown product, hydrogen peroxide, can be harmful to some organisms if released in high concentrations. This necessitates proper wastewater treatment to ensure that effluents containing sodium percarbonate or its byproducts are adequately diluted before being released into natural water bodies.
The increased use of sodium percarbonate in detergents may also lead to elevated levels of sodium in wastewater. While sodium is not typically considered a major pollutant, high concentrations can affect soil structure and plant growth in areas where treated wastewater is used for irrigation.
On balance, the environmental impact of sodium percarbonate in laundry detergents is generally considered favorable when compared to more aggressive cleaning agents. Its ability to enable low-temperature washing, coupled with its biodegradability, positions it as a relatively sustainable choice in the context of increasing environmental awareness in consumer products.
Consumer Safety and Regulatory Compliance
The incorporation of sodium percarbonate in laundry detergents necessitates careful consideration of consumer safety and regulatory compliance. As an oxidizing agent, sodium percarbonate can pose potential risks if not properly formulated and handled. Manufacturers must adhere to strict safety guidelines and regulations to ensure the protection of consumers and the environment.
Regulatory bodies such as the U.S. Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA) have established specific requirements for the use of sodium percarbonate in household cleaning products. These regulations typically cover aspects such as concentration limits, packaging requirements, and mandatory labeling information. Manufacturers must conduct thorough safety assessments and provide clear usage instructions to mitigate potential risks associated with the product.
One key safety concern is the potential for skin and eye irritation upon direct contact with sodium percarbonate. To address this, detergent formulations must be carefully balanced to minimize irritation while maintaining cleaning efficacy. Additionally, child-resistant packaging is often required to prevent accidental ingestion, which can lead to severe health consequences.
Environmental considerations also play a crucial role in regulatory compliance. Sodium percarbonate breaks down into hydrogen peroxide and sodium carbonate, which are generally considered environmentally friendly. However, manufacturers must still demonstrate that their products do not pose undue risks to aquatic ecosystems when released into wastewater systems.
To ensure compliance with evolving regulations, detergent manufacturers must stay abreast of changes in safety standards and environmental policies across different markets. This often involves ongoing product testing, toxicological assessments, and environmental impact studies. Companies may need to reformulate their products or adjust manufacturing processes to meet new regulatory requirements as they emerge.
Consumer education is another critical aspect of safety and compliance. Clear and accurate product labeling, including proper dosage instructions and safety warnings, is essential. Many regulatory frameworks require specific hazard symbols and precautionary statements on product packaging to inform consumers of potential risks and proper handling procedures.
In the context of international trade, manufacturers must navigate varying regulatory landscapes across different countries and regions. This may involve obtaining certifications or approvals from multiple regulatory agencies, each with its own set of requirements and standards for sodium percarbonate-containing detergents.
As sustainability becomes an increasingly important factor in consumer choices and regulatory policies, manufacturers are also focusing on the environmental profile of their products. This includes considerations such as biodegradability, reduced packaging waste, and the overall carbon footprint of production and distribution processes.
Regulatory bodies such as the U.S. Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA) have established specific requirements for the use of sodium percarbonate in household cleaning products. These regulations typically cover aspects such as concentration limits, packaging requirements, and mandatory labeling information. Manufacturers must conduct thorough safety assessments and provide clear usage instructions to mitigate potential risks associated with the product.
One key safety concern is the potential for skin and eye irritation upon direct contact with sodium percarbonate. To address this, detergent formulations must be carefully balanced to minimize irritation while maintaining cleaning efficacy. Additionally, child-resistant packaging is often required to prevent accidental ingestion, which can lead to severe health consequences.
Environmental considerations also play a crucial role in regulatory compliance. Sodium percarbonate breaks down into hydrogen peroxide and sodium carbonate, which are generally considered environmentally friendly. However, manufacturers must still demonstrate that their products do not pose undue risks to aquatic ecosystems when released into wastewater systems.
To ensure compliance with evolving regulations, detergent manufacturers must stay abreast of changes in safety standards and environmental policies across different markets. This often involves ongoing product testing, toxicological assessments, and environmental impact studies. Companies may need to reformulate their products or adjust manufacturing processes to meet new regulatory requirements as they emerge.
Consumer education is another critical aspect of safety and compliance. Clear and accurate product labeling, including proper dosage instructions and safety warnings, is essential. Many regulatory frameworks require specific hazard symbols and precautionary statements on product packaging to inform consumers of potential risks and proper handling procedures.
In the context of international trade, manufacturers must navigate varying regulatory landscapes across different countries and regions. This may involve obtaining certifications or approvals from multiple regulatory agencies, each with its own set of requirements and standards for sodium percarbonate-containing detergents.
As sustainability becomes an increasingly important factor in consumer choices and regulatory policies, manufacturers are also focusing on the environmental profile of their products. This includes considerations such as biodegradability, reduced packaging waste, and the overall carbon footprint of production and distribution processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!