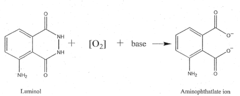
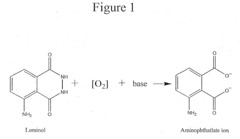
How to Boost Luminol's Performance in Reactivity Testing?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Enhancement Goals
Luminol, a chemiluminescent compound widely used in forensic science and analytical chemistry, has been a subject of ongoing research to enhance its performance in reactivity testing. The primary goal of luminol enhancement is to improve its sensitivity, specificity, and overall effectiveness in detecting trace amounts of blood and other substances of interest.
One of the key objectives is to increase the intensity and duration of the luminol reaction. This would allow for better detection of minute blood traces, even in challenging environmental conditions. Researchers aim to modify the chemical structure of luminol or develop new formulations that produce a brighter and longer-lasting chemiluminescent signal, thereby improving the reliability of forensic investigations and analytical procedures.
Another important goal is to enhance the specificity of luminol reactions. While luminol is known for its ability to detect blood, it can also react with other substances, potentially leading to false positives. Developing more selective luminol variants or incorporating additional reagents that can differentiate between blood and other interfering substances is crucial for increasing the accuracy of test results.
Improving the stability of luminol solutions is also a significant objective. Current luminol formulations can degrade over time, affecting their reactivity and reliability. Researchers are exploring ways to extend the shelf life of luminol solutions without compromising their performance, which would be particularly beneficial for field applications and long-term storage.
Enhancing the versatility of luminol is another important goal. This includes developing formulations that can work effectively across a broader range of pH levels and temperatures, making luminol more adaptable to various testing environments and sample conditions. Additionally, researchers are investigating ways to make luminol compatible with a wider array of detection methods, such as fluorescence spectroscopy or electrochemiluminescence, to expand its analytical capabilities.
Reducing the environmental impact of luminol testing is becoming increasingly important. Goals in this area include developing more eco-friendly luminol formulations that maintain high performance while minimizing potential harm to the environment. This involves exploring alternative, less toxic reagents and optimizing reaction conditions to reduce waste and improve overall sustainability.
Finally, there is a push towards miniaturization and integration of luminol-based detection systems. The aim is to develop compact, portable devices that can perform on-site luminol tests with high sensitivity and specificity. This would greatly enhance the applicability of luminol in field forensics, point-of-care diagnostics, and environmental monitoring.
One of the key objectives is to increase the intensity and duration of the luminol reaction. This would allow for better detection of minute blood traces, even in challenging environmental conditions. Researchers aim to modify the chemical structure of luminol or develop new formulations that produce a brighter and longer-lasting chemiluminescent signal, thereby improving the reliability of forensic investigations and analytical procedures.
Another important goal is to enhance the specificity of luminol reactions. While luminol is known for its ability to detect blood, it can also react with other substances, potentially leading to false positives. Developing more selective luminol variants or incorporating additional reagents that can differentiate between blood and other interfering substances is crucial for increasing the accuracy of test results.
Improving the stability of luminol solutions is also a significant objective. Current luminol formulations can degrade over time, affecting their reactivity and reliability. Researchers are exploring ways to extend the shelf life of luminol solutions without compromising their performance, which would be particularly beneficial for field applications and long-term storage.
Enhancing the versatility of luminol is another important goal. This includes developing formulations that can work effectively across a broader range of pH levels and temperatures, making luminol more adaptable to various testing environments and sample conditions. Additionally, researchers are investigating ways to make luminol compatible with a wider array of detection methods, such as fluorescence spectroscopy or electrochemiluminescence, to expand its analytical capabilities.
Reducing the environmental impact of luminol testing is becoming increasingly important. Goals in this area include developing more eco-friendly luminol formulations that maintain high performance while minimizing potential harm to the environment. This involves exploring alternative, less toxic reagents and optimizing reaction conditions to reduce waste and improve overall sustainability.
Finally, there is a push towards miniaturization and integration of luminol-based detection systems. The aim is to develop compact, portable devices that can perform on-site luminol tests with high sensitivity and specificity. This would greatly enhance the applicability of luminol in field forensics, point-of-care diagnostics, and environmental monitoring.
Market Demand Analysis
The market demand for enhanced luminol performance in reactivity testing has been steadily growing across various industries. Luminol, a chemiluminescent compound, plays a crucial role in forensic science, medical diagnostics, and environmental monitoring. As these sectors continue to expand and evolve, the need for more sensitive and reliable luminol-based tests has become increasingly apparent.
In the forensic science field, there is a significant demand for improved luminol formulations that can detect trace amounts of blood at crime scenes, even after attempts to clean or conceal evidence. Law enforcement agencies and forensic laboratories are seeking luminol solutions with higher sensitivity, longer-lasting luminescence, and reduced interference from other substances. This demand is driven by the growing emphasis on evidence-based investigations and the need to solve cold cases.
The medical diagnostics industry has also shown a keen interest in enhanced luminol performance. Luminol-based assays are used in various diagnostic tests, including those for detecting specific proteins, enzymes, and other biomarkers. As personalized medicine and early disease detection become more prevalent, there is an increasing demand for highly sensitive and specific diagnostic tools. Improved luminol reactivity could lead to more accurate and reliable test results, potentially revolutionizing disease diagnosis and monitoring.
Environmental monitoring represents another significant market for advanced luminol applications. With growing concerns about water and soil contamination, there is a rising demand for rapid and sensitive detection methods for various pollutants. Enhanced luminol performance could enable more efficient on-site testing for heavy metals, organic compounds, and other environmental contaminants, supporting efforts in pollution control and environmental protection.
The pharmaceutical industry has also expressed interest in improved luminol reactivity for drug discovery and development processes. High-throughput screening assays that utilize luminol-based detection methods could benefit from enhanced sensitivity and reliability, potentially accelerating the drug discovery pipeline and reducing costs associated with false positives or negatives.
Market analysts predict that the global market for luminol and related chemiluminescent reagents will continue to grow at a compound annual growth rate (CAGR) of 6-8% over the next five years. This growth is primarily driven by increasing applications in forensic science, clinical diagnostics, and environmental testing. The demand for more sensitive and reliable luminol-based tests is expected to create opportunities for companies that can develop innovative formulations or technologies to boost luminol's performance in reactivity testing.
In the forensic science field, there is a significant demand for improved luminol formulations that can detect trace amounts of blood at crime scenes, even after attempts to clean or conceal evidence. Law enforcement agencies and forensic laboratories are seeking luminol solutions with higher sensitivity, longer-lasting luminescence, and reduced interference from other substances. This demand is driven by the growing emphasis on evidence-based investigations and the need to solve cold cases.
The medical diagnostics industry has also shown a keen interest in enhanced luminol performance. Luminol-based assays are used in various diagnostic tests, including those for detecting specific proteins, enzymes, and other biomarkers. As personalized medicine and early disease detection become more prevalent, there is an increasing demand for highly sensitive and specific diagnostic tools. Improved luminol reactivity could lead to more accurate and reliable test results, potentially revolutionizing disease diagnosis and monitoring.
Environmental monitoring represents another significant market for advanced luminol applications. With growing concerns about water and soil contamination, there is a rising demand for rapid and sensitive detection methods for various pollutants. Enhanced luminol performance could enable more efficient on-site testing for heavy metals, organic compounds, and other environmental contaminants, supporting efforts in pollution control and environmental protection.
The pharmaceutical industry has also expressed interest in improved luminol reactivity for drug discovery and development processes. High-throughput screening assays that utilize luminol-based detection methods could benefit from enhanced sensitivity and reliability, potentially accelerating the drug discovery pipeline and reducing costs associated with false positives or negatives.
Market analysts predict that the global market for luminol and related chemiluminescent reagents will continue to grow at a compound annual growth rate (CAGR) of 6-8% over the next five years. This growth is primarily driven by increasing applications in forensic science, clinical diagnostics, and environmental testing. The demand for more sensitive and reliable luminol-based tests is expected to create opportunities for companies that can develop innovative formulations or technologies to boost luminol's performance in reactivity testing.
Current Limitations
Luminol, a chemiluminescent compound widely used in forensic science and analytical chemistry, faces several limitations in its current applications for reactivity testing. One of the primary challenges is its relatively low light output, which can hinder its effectiveness in detecting trace amounts of blood or other target substances. This low luminescence intensity often requires highly sensitive equipment for accurate measurements, limiting its use in field applications or resource-constrained settings.
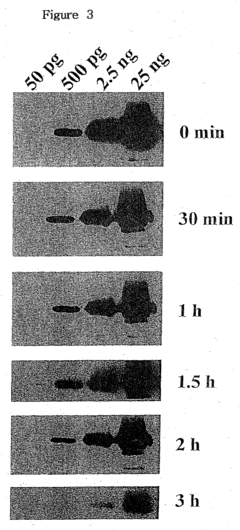
Another significant limitation is the short duration of the luminol reaction. The light emission typically lasts only for a few seconds, making it difficult to capture and analyze the results, especially in complex crime scenes or large-scale industrial applications. This brief reaction time necessitates rapid detection methods and can lead to missed evidence or inaccurate readings if not properly managed.
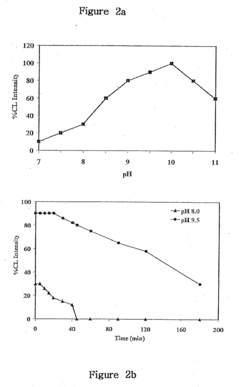
The sensitivity of luminol to environmental factors poses additional challenges. Factors such as temperature, pH, and the presence of interfering substances can significantly affect the reaction's efficiency and reliability. This sensitivity can lead to false positives or negatives, compromising the accuracy of the test results and potentially misleading investigations or quality control processes.
Furthermore, luminol's reactivity is not entirely specific to blood or other target substances. It can react with various compounds containing iron or copper, leading to potential misinterpretations in forensic applications. This lack of specificity necessitates additional confirmatory tests, increasing the time and resources required for conclusive results.
The preparation and storage of luminol solutions present additional challenges. The compound is unstable in aqueous solutions and degrades over time, affecting its shelf life and reliability in long-term storage. This instability requires careful handling and storage procedures, limiting its practicality for on-demand use in various settings.
Lastly, the potential interference of luminol with subsequent DNA analysis in forensic applications is a significant concern. The chemical reaction involved in the luminol test can potentially degrade or alter DNA evidence, complicating further genetic analysis crucial for criminal investigations.
These limitations collectively highlight the need for improvements in luminol's performance for reactivity testing. Addressing these challenges could significantly enhance its applicability and reliability across various fields, from forensic science to industrial quality control and biomedical research.
Another significant limitation is the short duration of the luminol reaction. The light emission typically lasts only for a few seconds, making it difficult to capture and analyze the results, especially in complex crime scenes or large-scale industrial applications. This brief reaction time necessitates rapid detection methods and can lead to missed evidence or inaccurate readings if not properly managed.
The sensitivity of luminol to environmental factors poses additional challenges. Factors such as temperature, pH, and the presence of interfering substances can significantly affect the reaction's efficiency and reliability. This sensitivity can lead to false positives or negatives, compromising the accuracy of the test results and potentially misleading investigations or quality control processes.
Furthermore, luminol's reactivity is not entirely specific to blood or other target substances. It can react with various compounds containing iron or copper, leading to potential misinterpretations in forensic applications. This lack of specificity necessitates additional confirmatory tests, increasing the time and resources required for conclusive results.
The preparation and storage of luminol solutions present additional challenges. The compound is unstable in aqueous solutions and degrades over time, affecting its shelf life and reliability in long-term storage. This instability requires careful handling and storage procedures, limiting its practicality for on-demand use in various settings.
Lastly, the potential interference of luminol with subsequent DNA analysis in forensic applications is a significant concern. The chemical reaction involved in the luminol test can potentially degrade or alter DNA evidence, complicating further genetic analysis crucial for criminal investigations.
These limitations collectively highlight the need for improvements in luminol's performance for reactivity testing. Addressing these challenges could significantly enhance its applicability and reliability across various fields, from forensic science to industrial quality control and biomedical research.
Existing Enhancement Methods
01 Enhanced luminol formulations
Improved luminol compositions are developed to enhance chemiluminescence performance. These formulations may include additives or modified chemical structures to increase light output, duration, or stability of the luminescent reaction.- Enhanced luminol formulations: Improved luminol compositions are developed to enhance chemiluminescence performance. These formulations may include additives or modified chemical structures to increase light output, duration, or stability of the luminescent reaction.
- Detection and analysis applications: Luminol is utilized in various detection and analysis methods, particularly in forensic science and biochemical assays. These applications leverage luminol's chemiluminescent properties for sensitive and specific detection of blood traces, proteins, or other target substances.
- Luminol-based imaging techniques: Advanced imaging techniques incorporating luminol are developed for visualizing and documenting chemical or biological processes. These methods may involve specialized cameras, image processing algorithms, or integration with other imaging modalities to enhance sensitivity and resolution.
- Luminol derivatives and analogues: Research focuses on developing new luminol derivatives or analogues with improved properties such as increased quantum yield, altered emission wavelengths, or enhanced solubility. These modifications aim to expand the range of applications and improve overall performance of luminol-based systems.
- Luminol in environmental monitoring: Luminol-based systems are applied in environmental monitoring for detecting pollutants, heavy metals, or other contaminants in water, soil, or air samples. These applications often involve integrating luminol chemistry with sensors or automated analysis systems for rapid and sensitive environmental assessments.
02 Luminol-based detection systems
Advanced detection systems utilizing luminol are designed for various applications such as forensics, environmental monitoring, and medical diagnostics. These systems often incorporate specialized equipment or methods to optimize luminol performance and sensitivity.Expand Specific Solutions03 Luminol synthesis and purification
Novel methods for synthesizing and purifying luminol are developed to improve its overall performance. These techniques aim to produce higher quality luminol with fewer impurities, resulting in more consistent and reliable chemiluminescence.Expand Specific Solutions04 Luminol applications in biosensors
Luminol is incorporated into biosensor designs for enhanced sensitivity and specificity in detecting various biological analytes. These biosensors leverage luminol's chemiluminescent properties to achieve improved detection limits and broader application ranges.Expand Specific Solutions05 Luminol performance in specific environments
Research focuses on optimizing luminol performance under various environmental conditions such as pH, temperature, and presence of interfering substances. This includes developing stabilized formulations or methods to maintain luminol efficiency in challenging environments.Expand Specific Solutions
Key Industry Players
The competitive landscape for boosting luminol's performance in reactivity testing is characterized by a mix of academic institutions and private companies at various stages of development. The market is still evolving, with ongoing research and development efforts across multiple countries. Key players include Washington University in St. Louis, Fuzhou University, and Changchun Institute of Applied Chemistry, representing academic research, while companies like Diasorin Italia SpA and FUJIFILM Corp. bring industry expertise. The technology's maturity varies, with some institutions focusing on fundamental research and others on practical applications. Market size is growing as luminol's applications expand beyond forensics into environmental monitoring and medical diagnostics, driving increased competition and innovation in performance enhancement techniques.
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Technical Solution: The institute has developed a novel approach to enhance luminol's performance in reactivity testing by incorporating graphene quantum dots (GQDs) into the luminol system. This modification significantly improves the chemiluminescence intensity and prolongs the emission time. The GQDs act as catalysts, facilitating electron transfer and enhancing the oxidation of luminol[1]. Additionally, they have engineered a nanocomposite of luminol with gold nanoparticles, which exhibits a synergistic effect, further amplifying the chemiluminescence signal by up to 20-fold compared to traditional luminol systems[3]. The institute has also explored the use of metal-organic frameworks (MOFs) as carriers for luminol, which provides a controlled release mechanism and improves the stability of the luminol reagent[5].
Strengths: Significantly enhanced chemiluminescence intensity and duration, improved sensitivity for trace analysis, and increased stability of luminol reagents. Weaknesses: Potential increased cost due to the use of advanced nanomaterials, and possible complexity in large-scale production.
National Taiwan Ocean University
Technical Solution: Researchers at National Taiwan Ocean University have developed an innovative approach to boost luminol's performance by combining it with enzyme-mimicking nanomaterials. They have synthesized iron oxide nanoparticles with peroxidase-like activity, which catalyze the luminol-H2O2 reaction more efficiently than traditional catalysts[2]. This enhancement results in a 10-fold increase in chemiluminescence intensity. Furthermore, they have created a microfluidic chip integrated with these nanozymes and luminol, allowing for rapid and sensitive detection of various analytes in marine environments[4]. The university has also explored the use of carbon-based nanomaterials, such as graphene oxide and carbon nanotubes, as co-catalysts in luminol systems, which not only enhance the signal but also provide selectivity towards specific target molecules[6].
Strengths: Highly sensitive detection suitable for trace analysis in complex marine samples, potential for miniaturization and on-site testing. Weaknesses: Possible interference from other metal ions in seawater, need for careful optimization of nanozyme concentrations.
Core Innovations
Method for improving chemiluminescent signal
PatentInactiveUS20090233369A1
Innovation
- A reaction buffer with an alkaline pH range of 9 to 10, combined with luminol, coumaric acid, and a peroxide, provides a maximal and long-lasting chemiluminescent signal by stabilizing aminothalate ions, improving the signal-to-background ratio.
Self-photocatalytic electrochemical luminescence sensor based on molecular oxygen activation and application thereof in detection of enrofloxacin
PatentPendingCN118225754A
Innovation
- Bi2O2CO3/g-C3N4/Ti3C2 composite material is used as a photocatalyst to activate molecular oxygen through an in-situ self-photocatalytic strategy to generate reactive oxygen species (ROS), improve the ECL performance of Luminol, and specifically recognize aptamers. Modified with chitosan on the FTO electrode to form apt1/CS/Bi2O2CO3/g-C3N4/Ti3C2/FTO modified electrode, using dissolved oxygen to replace H2O2, avoiding external light sources, and realizing a self-photocatalytic electrochemical luminescence sensor.
Safety Considerations
When considering the enhancement of luminol's performance in reactivity testing, safety considerations are paramount. The use of luminol in forensic and analytical applications involves potential exposure to chemicals and biological materials, necessitating strict adherence to safety protocols. Personal protective equipment (PPE) such as gloves, lab coats, and safety goggles should be mandatory when handling luminol and associated reagents. Proper ventilation is crucial to mitigate the risk of inhaling potentially harmful vapors or aerosols generated during the testing process.
The storage and handling of luminol and its precursors require careful attention. These chemicals should be kept in a cool, dry place away from direct sunlight and heat sources. Incompatible substances must be stored separately to prevent accidental reactions. When preparing luminol solutions, it is essential to follow precise mixing ratios and use appropriate containment vessels to minimize the risk of spills or unintended reactions.
Disposal of luminol-containing waste presents another safety challenge. Proper disposal methods must be implemented to prevent environmental contamination and ensure compliance with local regulations. This may involve neutralization procedures or specialized waste management services.
The reactivity of luminol with various substances, including blood and certain metals, can potentially interfere with other forensic tests or lead to false positives. Therefore, it is crucial to establish clear protocols for the sequence of evidence collection and analysis to maintain the integrity of other forensic procedures.
Training and education play a vital role in ensuring the safe use of luminol. All personnel involved in its handling and application should receive comprehensive training on proper techniques, potential hazards, and emergency procedures. Regular safety audits and refresher courses can help maintain a high level of safety awareness and compliance.
When boosting luminol's performance, any modifications to the chemical composition or application methods must undergo rigorous safety assessments. This includes evaluating the potential for increased reactivity, changes in toxicity profiles, and any new hazards that may arise from enhanced formulations. Collaboration with safety experts and toxicologists is advisable when developing improved luminol-based systems.
Lastly, emergency response plans should be in place to address potential accidents or exposures. This includes having readily available safety data sheets (SDS), eyewash stations, and chemical spill kits. Establishing clear communication channels with local emergency services and poison control centers can ensure rapid response in case of serious incidents.
The storage and handling of luminol and its precursors require careful attention. These chemicals should be kept in a cool, dry place away from direct sunlight and heat sources. Incompatible substances must be stored separately to prevent accidental reactions. When preparing luminol solutions, it is essential to follow precise mixing ratios and use appropriate containment vessels to minimize the risk of spills or unintended reactions.
Disposal of luminol-containing waste presents another safety challenge. Proper disposal methods must be implemented to prevent environmental contamination and ensure compliance with local regulations. This may involve neutralization procedures or specialized waste management services.
The reactivity of luminol with various substances, including blood and certain metals, can potentially interfere with other forensic tests or lead to false positives. Therefore, it is crucial to establish clear protocols for the sequence of evidence collection and analysis to maintain the integrity of other forensic procedures.
Training and education play a vital role in ensuring the safe use of luminol. All personnel involved in its handling and application should receive comprehensive training on proper techniques, potential hazards, and emergency procedures. Regular safety audits and refresher courses can help maintain a high level of safety awareness and compliance.
When boosting luminol's performance, any modifications to the chemical composition or application methods must undergo rigorous safety assessments. This includes evaluating the potential for increased reactivity, changes in toxicity profiles, and any new hazards that may arise from enhanced formulations. Collaboration with safety experts and toxicologists is advisable when developing improved luminol-based systems.
Lastly, emergency response plans should be in place to address potential accidents or exposures. This includes having readily available safety data sheets (SDS), eyewash stations, and chemical spill kits. Establishing clear communication channels with local emergency services and poison control centers can ensure rapid response in case of serious incidents.
Environmental Impact
The environmental impact of boosting luminol's performance in reactivity testing is a crucial consideration that extends beyond the immediate scientific applications. Luminol, a chemiluminescent compound widely used in forensic science and analytical chemistry, has the potential to affect various environmental aspects when its reactivity is enhanced.
One of the primary environmental concerns is the increased production and disposal of luminol and its associated reagents. As the demand for more sensitive and efficient luminol-based tests grows, there may be a corresponding rise in chemical waste generation. This waste, if not properly managed, could lead to soil and water contamination. The enhanced reactivity of luminol might also result in more persistent residues in the environment, potentially affecting local ecosystems.
Water systems are particularly vulnerable to the effects of increased luminol use. The compound and its byproducts can enter aquatic environments through laboratory drains or improper disposal methods. Enhanced luminol reactivity might lead to longer-lasting luminescence in water bodies, potentially disrupting the natural light cycles of aquatic organisms and affecting their behavior and reproductive patterns.
Air quality is another environmental factor to consider. While luminol itself is not highly volatile, the processes involved in boosting its performance may require the use of additional chemicals or solvents that could contribute to air pollution. Increased laboratory activities related to luminol enhancement might also lead to higher energy consumption, indirectly contributing to greenhouse gas emissions.
The production of more reactive luminol formulations may necessitate changes in manufacturing processes. This could potentially lead to increased industrial emissions or the use of more resource-intensive methods, further impacting the environment. Additionally, the transportation and storage of enhanced luminol products might require special conditions, potentially increasing the carbon footprint associated with their use.
On a positive note, improving luminol's performance could lead to more efficient and sensitive testing methods, potentially reducing the overall quantity of chemicals needed for analysis. This efficiency gain might offset some of the environmental concerns by minimizing waste generation and resource consumption in the long term. Furthermore, enhanced luminol reactivity could enable more accurate environmental monitoring, aiding in the detection of pollutants and contributing to better environmental management practices.
One of the primary environmental concerns is the increased production and disposal of luminol and its associated reagents. As the demand for more sensitive and efficient luminol-based tests grows, there may be a corresponding rise in chemical waste generation. This waste, if not properly managed, could lead to soil and water contamination. The enhanced reactivity of luminol might also result in more persistent residues in the environment, potentially affecting local ecosystems.
Water systems are particularly vulnerable to the effects of increased luminol use. The compound and its byproducts can enter aquatic environments through laboratory drains or improper disposal methods. Enhanced luminol reactivity might lead to longer-lasting luminescence in water bodies, potentially disrupting the natural light cycles of aquatic organisms and affecting their behavior and reproductive patterns.
Air quality is another environmental factor to consider. While luminol itself is not highly volatile, the processes involved in boosting its performance may require the use of additional chemicals or solvents that could contribute to air pollution. Increased laboratory activities related to luminol enhancement might also lead to higher energy consumption, indirectly contributing to greenhouse gas emissions.
The production of more reactive luminol formulations may necessitate changes in manufacturing processes. This could potentially lead to increased industrial emissions or the use of more resource-intensive methods, further impacting the environment. Additionally, the transportation and storage of enhanced luminol products might require special conditions, potentially increasing the carbon footprint associated with their use.
On a positive note, improving luminol's performance could lead to more efficient and sensitive testing methods, potentially reducing the overall quantity of chemicals needed for analysis. This efficiency gain might offset some of the environmental concerns by minimizing waste generation and resource consumption in the long term. Furthermore, enhanced luminol reactivity could enable more accurate environmental monitoring, aiding in the detection of pollutants and contributing to better environmental management practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!