How to Implement PVDF in Efficient Drug Delivery Systems?
PVDF in Drug Delivery: Background and Objectives
Polyvinylidene fluoride (PVDF) has emerged as a promising material in the field of drug delivery systems, attracting significant attention from researchers and pharmaceutical companies alike. This synthetic fluoropolymer, known for its exceptional chemical stability, biocompatibility, and unique piezoelectric properties, has opened new avenues for controlled and targeted drug release.
The evolution of PVDF in drug delivery can be traced back to the 1960s when its potential in biomedical applications was first recognized. Initially, PVDF was primarily used in medical devices due to its inertness and durability. However, as nanotechnology and polymer science advanced, researchers began exploring PVDF's potential in drug delivery systems.
The primary objective of implementing PVDF in drug delivery systems is to enhance the efficacy and safety of therapeutic agents. By leveraging PVDF's unique properties, researchers aim to develop smart drug delivery platforms that can respond to external stimuli, such as electrical or mechanical forces, to release drugs in a controlled manner. This approach holds promise for improving patient outcomes by optimizing drug dosage and reducing side effects.
One of the key technological trends driving PVDF's adoption in drug delivery is the development of nanostructured PVDF materials. These include PVDF nanofibers, nanoparticles, and membranes, which offer increased surface area and improved drug loading capacity. The ability to manipulate PVDF at the nanoscale has opened up possibilities for creating highly tailored drug delivery systems that can target specific tissues or cells with unprecedented precision.
Another significant trend is the exploration of PVDF's piezoelectric properties for on-demand drug release. By applying external mechanical or electrical stimuli, researchers can trigger the release of encapsulated drugs from PVDF-based carriers. This approach is particularly promising for developing implantable drug delivery devices that can be remotely controlled, offering new possibilities for personalized medicine.
The integration of PVDF with other materials, such as conductive polymers or hydrogels, represents another frontier in drug delivery research. These composite materials combine the strengths of different components to create multifunctional drug delivery systems capable of responding to multiple stimuli or delivering multiple drugs simultaneously.
As research in this field progresses, the ultimate goal is to develop PVDF-based drug delivery systems that can revolutionize the treatment of various diseases, particularly chronic conditions and cancer. By enabling precise control over drug release kinetics and targeting, these systems have the potential to improve therapeutic outcomes, reduce dosing frequency, and minimize systemic side effects.
Market Analysis for PVDF-based Drug Delivery Systems
The global market for PVDF-based drug delivery systems is experiencing significant growth, driven by the increasing demand for advanced and efficient drug delivery technologies. PVDF (Polyvinylidene fluoride) has emerged as a promising material in this field due to its unique properties, including biocompatibility, chemical resistance, and controllable porosity.
The pharmaceutical industry's focus on targeted drug delivery and personalized medicine has created a favorable environment for PVDF-based systems. These systems offer improved drug efficacy, reduced side effects, and enhanced patient compliance, making them attractive to both healthcare providers and patients. The market is particularly strong in developed regions such as North America and Europe, where there is a high adoption rate of innovative medical technologies.
In terms of application areas, PVDF-based drug delivery systems are finding increasing use in cancer treatment, chronic disease management, and regenerative medicine. The oncology segment, in particular, is expected to be a major driver of market growth, as PVDF-based systems can potentially improve the precision and effectiveness of cancer therapies.
The market is characterized by a mix of established pharmaceutical companies and innovative startups. Major players are investing heavily in research and development to create novel PVDF-based drug delivery platforms. Collaborations between academic institutions and industry partners are also contributing to technological advancements in this field.
Regulatory factors play a crucial role in shaping the market landscape. The stringent approval processes for new drug delivery systems can pose challenges, but they also ensure the safety and efficacy of PVDF-based products. As regulatory bodies become more familiar with these technologies, the approval process is expected to become more streamlined, potentially accelerating market growth.
From a geographical perspective, while North America and Europe currently dominate the market, emerging economies in Asia-Pacific and Latin America are showing increasing interest in PVDF-based drug delivery systems. This is partly due to growing healthcare expenditure and improving research infrastructure in these regions.
Looking ahead, the market for PVDF-based drug delivery systems is projected to continue its upward trajectory. Factors such as the aging population, increasing prevalence of chronic diseases, and the need for more effective drug delivery methods are expected to sustain market growth. However, challenges such as high development costs and competition from alternative drug delivery technologies will need to be addressed for the market to reach its full potential.
Current Challenges in PVDF Drug Delivery Applications
Despite the promising potential of PVDF in drug delivery systems, several challenges hinder its widespread implementation. One of the primary obstacles is the complex processing requirements for PVDF. The polymer's high melting point and strong intermolecular forces make it difficult to form into desired shapes and structures without compromising its properties. This complexity often leads to increased production costs and time, limiting its scalability in pharmaceutical applications.
Another significant challenge lies in controlling the drug release profile from PVDF-based delivery systems. While PVDF's semi-crystalline nature offers unique advantages, it also presents difficulties in achieving precise and sustained drug release. The polymer's hydrophobicity can lead to burst release of hydrophilic drugs, potentially causing toxicity issues and reducing therapeutic efficacy. Conversely, the release of hydrophobic drugs may be too slow, failing to reach therapeutic concentrations.
Biocompatibility and biodegradability concerns also pose challenges for PVDF in drug delivery applications. Although PVDF is generally considered biocompatible, long-term studies on its effects in the human body are limited. The polymer's non-biodegradable nature raises questions about its fate after drug depletion and potential accumulation in tissues, which could lead to adverse effects or complications in long-term treatments.
The surface properties of PVDF present another hurdle in drug delivery applications. Its inherent hydrophobicity can lead to poor wettability and limited cell adhesion, potentially affecting the interaction between the delivery system and biological tissues. This characteristic may impair the efficiency of drug absorption and reduce the overall effectiveness of the delivery system.
Furthermore, the incorporation of drugs into PVDF matrices without altering their chemical structure or activity remains a challenge. The high temperatures and solvents often used in PVDF processing can degrade or denature sensitive therapeutic agents, limiting the range of drugs that can be effectively delivered using this polymer.
Lastly, regulatory hurdles and the lack of standardized manufacturing processes for PVDF-based drug delivery systems present significant obstacles. The novelty of these systems means that regulatory bodies may require extensive safety and efficacy data before approval, potentially prolonging the time-to-market for new PVDF-based drug delivery products.
Addressing these challenges requires interdisciplinary research efforts, combining expertise in polymer science, pharmaceutics, and bioengineering. Innovations in PVDF processing techniques, surface modification strategies, and drug encapsulation methods are crucial for overcoming these obstacles and realizing the full potential of PVDF in efficient drug delivery systems.
Existing PVDF Drug Delivery System Implementations
01 PVDF membrane fabrication and modification
Various techniques are employed to fabricate and modify PVDF membranes to enhance their efficiency. These methods include blending with other polymers, surface modification, and incorporation of nanoparticles. Such modifications can improve the membrane's hydrophilicity, fouling resistance, and overall performance in applications like water treatment and separation processes.- PVDF membrane fabrication and modification: Various techniques are employed to fabricate and modify PVDF membranes to enhance their efficiency. These methods include blending with other polymers, surface modification, and incorporation of nanoparticles. Such modifications can improve the membrane's hydrophilicity, fouling resistance, and overall performance in applications like water treatment and separation processes.
- PVDF in energy storage applications: PVDF is utilized in energy storage devices, particularly in lithium-ion batteries and supercapacitors. Its high dielectric constant and excellent thermal stability make it an effective binder material for electrodes. Research focuses on optimizing PVDF formulations and processing techniques to enhance the overall efficiency and performance of these energy storage systems.
- PVDF in piezoelectric and sensor applications: The piezoelectric properties of PVDF are exploited in various sensor and actuator applications. Researchers are developing novel PVDF-based materials and structures to improve the sensitivity and efficiency of piezoelectric devices. These advancements contribute to the development of more efficient energy harvesters, pressure sensors, and other smart materials.
- PVDF in filtration and separation processes: PVDF membranes are widely used in filtration and separation processes due to their chemical resistance and mechanical strength. Ongoing research focuses on enhancing the efficiency of PVDF membranes through surface modifications, pore size control, and composite formation. These improvements aim to increase flux, reduce fouling, and extend membrane lifespan in applications such as water treatment and gas separation.
- PVDF in coating and protective applications: PVDF is utilized in high-performance coatings and protective films due to its excellent weatherability and chemical resistance. Research in this area focuses on improving the efficiency of PVDF-based coatings by optimizing formulations, incorporating additives, and developing novel application techniques. These advancements aim to enhance durability, adhesion, and overall protective properties in various industrial and architectural applications.
02 PVDF in energy storage applications
PVDF is utilized in energy storage devices, particularly in lithium-ion batteries and supercapacitors. Its high dielectric constant and excellent thermal stability make it an efficient binder material for electrodes. Research focuses on optimizing PVDF formulations and processing techniques to enhance the overall efficiency and performance of these energy storage systems.Expand Specific Solutions03 PVDF-based piezoelectric devices
PVDF's piezoelectric properties are exploited in various sensing and energy harvesting applications. Researchers are developing efficient methods to enhance the piezoelectric response of PVDF films and nanofibers. These improvements lead to more sensitive sensors and higher energy conversion efficiency in piezoelectric generators.Expand Specific Solutions04 PVDF in water treatment and filtration
PVDF membranes are widely used in water treatment and filtration processes. Ongoing research focuses on improving the efficiency of PVDF-based filtration systems by enhancing membrane porosity, reducing fouling, and increasing flux. Novel composite membranes and surface treatments are being developed to achieve better separation performance and longer membrane lifetimes.Expand Specific Solutions05 PVDF in coating and protective applications
PVDF's excellent chemical resistance and weatherability make it an efficient material for protective coatings. Research in this area focuses on improving the adhesion, durability, and performance of PVDF-based coatings. New formulations and application techniques are being developed to enhance the efficiency of PVDF coatings in various industrial and architectural applications.Expand Specific Solutions
Key Players in PVDF-based Drug Delivery Research
The implementation of PVDF in efficient drug delivery systems is currently in a growth phase, with increasing market size and technological advancements. The global drug delivery market is projected to reach significant value, driven by the demand for innovative delivery methods. The technology's maturity varies across applications, with companies like Amgen, Novartis, and FUJIFILM leading in research and development. Emerging players such as Emory University and Georgia Tech Research Corp. are contributing to academic advancements. The competitive landscape is diverse, including pharmaceutical giants, biotechnology firms, and academic institutions, each focusing on different aspects of PVDF-based drug delivery systems. This multifaceted approach is accelerating the technology's evolution and expanding its potential applications in healthcare.
Novartis AG
Genentech, Inc.
Innovative PVDF Modifications for Enhanced Drug Release
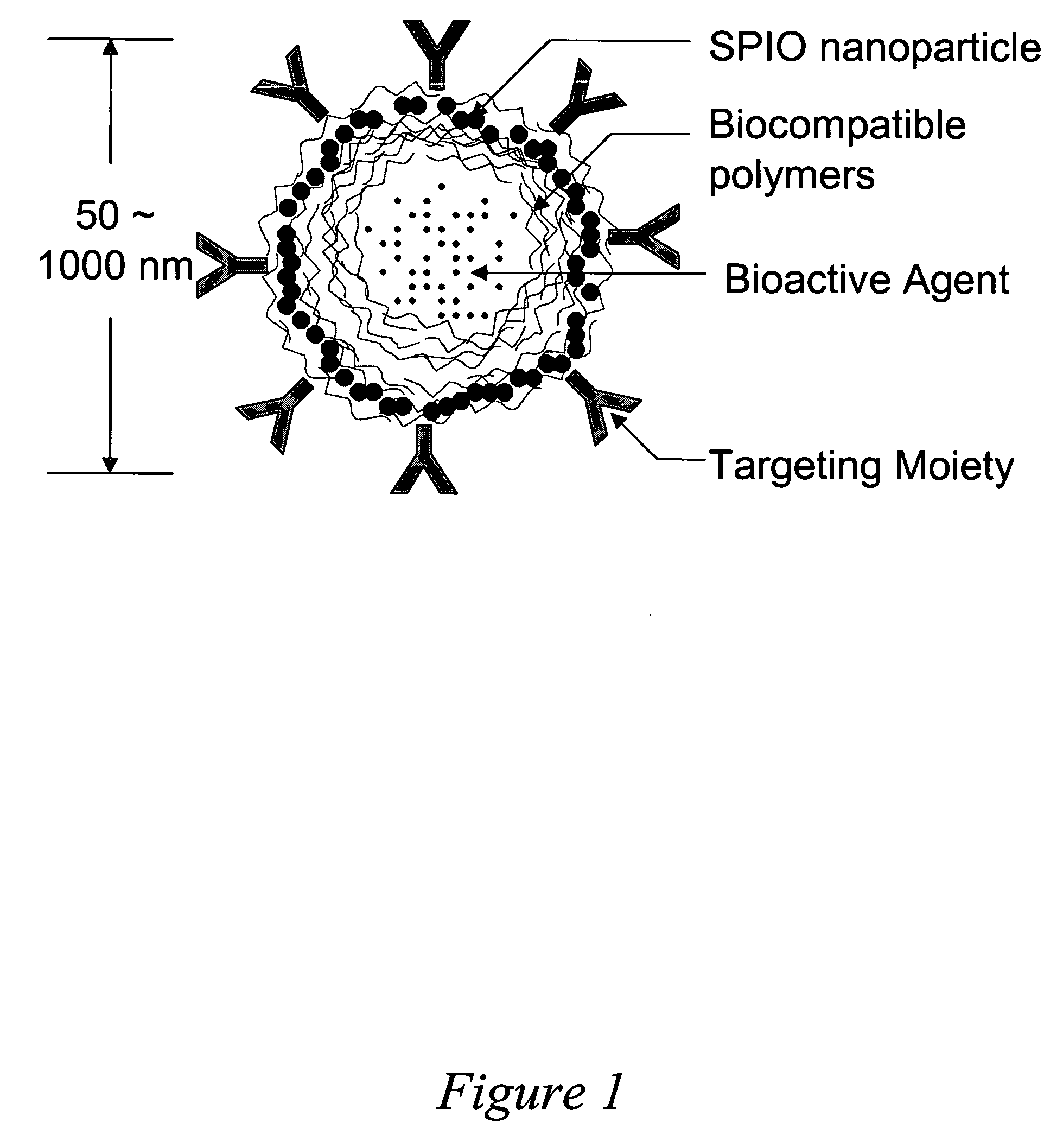
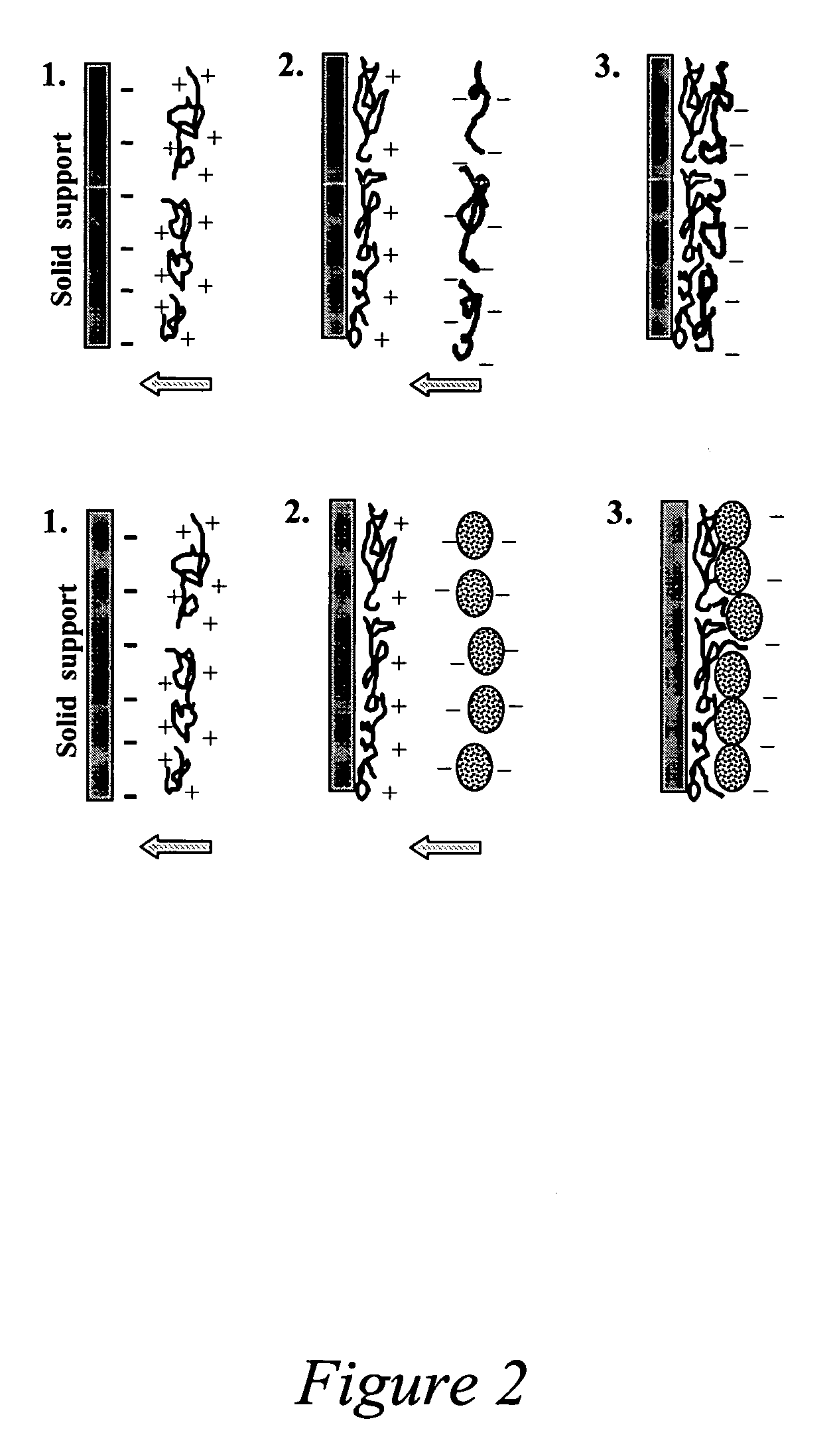
- Development of polymeric nanoshells with diameters between 50 and 1000 nanometers, composed of biocompatible and biodegradable organic polymers, which can be loaded with bioactive agents and equipped with targeting moieties for specific delivery to anatomical sites, using an electrostatic layer-by-layer self-assembly technique to control shell thickness and permeability.
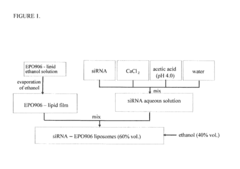
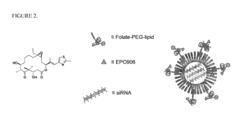
- A vesicular drug delivery system (VDDS) comprising a lipid bilayer with phosphatidylcholine, encapsulating siRNA in an aqueous cavity and embedding hydrophobic drugs like EPO906 within the bilayer, allowing for simultaneous co-delivery and enhanced stability and targeting capabilities through the use of targeting moieties.
Regulatory Considerations for PVDF-based Drug Delivery
The implementation of PVDF in drug delivery systems necessitates careful consideration of regulatory requirements to ensure patient safety and product efficacy. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established stringent guidelines for the use of novel materials in pharmaceutical applications. For PVDF-based drug delivery systems, manufacturers must demonstrate the material's biocompatibility, stability, and safety profile through extensive preclinical and clinical studies.
One of the primary regulatory considerations is the classification of the PVDF-based drug delivery system. Depending on its intended use and mechanism of action, it may be categorized as a drug, medical device, or combination product. This classification determines the regulatory pathway and the specific requirements for approval. For instance, if classified as a combination product, manufacturers must comply with regulations for both drugs and devices, potentially involving multiple FDA centers in the review process.
Quality control and manufacturing standards are crucial aspects of regulatory compliance. Good Manufacturing Practices (GMP) must be strictly adhered to throughout the production process of PVDF-based drug delivery systems. This includes validated methods for material synthesis, purification, and characterization to ensure consistent quality and performance. Regulatory bodies will scrutinize the manufacturing process, including any potential leachables or extractables from the PVDF material that could impact drug stability or patient safety.
Toxicology studies are another critical component of the regulatory process. Manufacturers must conduct comprehensive in vitro and in vivo studies to assess the potential toxicity of PVDF and its degradation products. This includes evaluating systemic toxicity, local tissue reactions, and potential long-term effects. The results of these studies will be crucial in determining the safety profile of the PVDF-based drug delivery system and its suitability for human use.
Clinical trials represent a significant regulatory hurdle for PVDF-based drug delivery systems. Depending on the novelty of the application, regulatory agencies may require extensive clinical data to demonstrate safety and efficacy. This could involve multiple phases of clinical trials, from initial safety studies to large-scale efficacy trials. The design and conduct of these trials must adhere to Good Clinical Practice (GCP) guidelines and receive approval from ethics committees and regulatory authorities.
Post-market surveillance is an ongoing regulatory requirement for PVDF-based drug delivery systems. Manufacturers must implement robust pharmacovigilance systems to monitor and report any adverse events or safety concerns that arise after market approval. This may include conducting long-term follow-up studies to assess the safety and performance of the drug delivery system over extended periods.
In conclusion, navigating the regulatory landscape for PVDF-based drug delivery systems requires a comprehensive understanding of pharmaceutical and medical device regulations. Manufacturers must engage early and frequently with regulatory authorities to ensure alignment on study designs, data requirements, and approval pathways. By addressing these regulatory considerations proactively, developers can streamline the approval process and bring innovative PVDF-based drug delivery systems to market more efficiently.
Biocompatibility and Safety Assessment of PVDF Systems
The biocompatibility and safety assessment of PVDF systems in drug delivery applications is a critical aspect of their implementation. PVDF (polyvinylidene fluoride) has gained significant attention in the pharmaceutical industry due to its unique properties, including chemical resistance, thermal stability, and mechanical strength. However, before widespread adoption in drug delivery systems, rigorous evaluation of its biocompatibility and safety profile is essential.
One of the primary considerations in assessing PVDF biocompatibility is its interaction with biological tissues and fluids. Studies have shown that PVDF exhibits low cytotoxicity and minimal inflammatory responses when in contact with various cell types. This favorable cellular response is attributed to the polymer's inert nature and resistance to degradation in physiological environments. Nevertheless, long-term studies are necessary to fully understand the potential effects of prolonged exposure to PVDF-based drug delivery systems.
The surface properties of PVDF play a crucial role in its biocompatibility. Researchers have explored various surface modification techniques to enhance the material's compatibility with biological systems. These modifications include plasma treatment, grafting of biocompatible polymers, and incorporation of functional groups. Such alterations can improve cell adhesion, reduce protein adsorption, and minimize the risk of adverse immune responses.
In terms of safety assessment, the potential for PVDF to release harmful substances or degrade into toxic byproducts must be thoroughly investigated. While PVDF is generally considered stable, the effects of different drug formulations, sterilization methods, and environmental factors on its integrity need to be evaluated. Comprehensive leachable and extractable studies are essential to identify any potential contaminants that may compromise drug purity or patient safety.
The biocompatibility of PVDF-based drug delivery systems also extends to their interaction with specific drug molecules. It is crucial to assess whether PVDF affects drug stability, potency, or release kinetics. This evaluation involves studying drug-polymer interactions, potential adsorption of active ingredients onto PVDF surfaces, and the impact on drug bioavailability.
Regulatory considerations play a significant role in the safety assessment of PVDF systems. Compliance with guidelines set by regulatory bodies such as the FDA and EMA is paramount. This includes conducting appropriate in vitro and in vivo studies to demonstrate the safety and efficacy of PVDF-based drug delivery devices. Additionally, the manufacturing processes and quality control measures for PVDF systems must adhere to stringent regulatory standards to ensure consistent safety and performance.
As PVDF systems advance towards clinical applications, it is essential to conduct comprehensive preclinical and clinical trials. These studies should evaluate the long-term effects of PVDF exposure, potential systemic toxicity, and any unforeseen interactions with biological systems. The results of these trials will provide valuable insights into the overall safety profile of PVDF-based drug delivery systems and guide their future development and implementation.