How to Use Gel Electrophoresis for Gene Editing Success?
Gene Editing Background and Objectives
Gene editing has emerged as a revolutionary technology in molecular biology, offering unprecedented precision in modifying genetic material. The field has evolved rapidly since the discovery of DNA structure in the 1950s, progressing through various techniques such as zinc finger nucleases and TALENs, to the current state-of-the-art CRISPR-Cas9 system. This evolution has been driven by the need for more efficient, accurate, and versatile tools to manipulate genes for research, medical, and biotechnological applications.
The primary objective of gene editing is to introduce specific modifications to DNA sequences within living organisms. These modifications can range from single nucleotide changes to the insertion or deletion of larger genetic segments. The goals of gene editing are diverse, including understanding gene function, developing disease models, creating genetically modified organisms for agriculture, and potentially treating genetic disorders in humans.
In the context of using gel electrophoresis for gene editing success, the technology aims to provide a crucial analytical tool for verifying and characterizing the results of gene editing experiments. Gel electrophoresis allows researchers to separate DNA fragments based on their size, enabling the visualization and analysis of edited genetic material. This technique is essential for confirming successful gene modifications, detecting unintended edits, and assessing the efficiency of various gene editing protocols.
The development of gene editing technologies has been accompanied by significant advancements in supporting techniques, including DNA sequencing, PCR amplification, and bioinformatics tools. These complementary technologies have enhanced the precision and scope of gene editing applications, allowing researchers to design more targeted and effective editing strategies.
As the field progresses, there is a growing focus on improving the specificity and efficiency of gene editing tools while minimizing off-target effects. Researchers are exploring new Cas proteins, engineered guide RNAs, and delivery methods to enhance the capabilities of gene editing systems. Additionally, there is increasing interest in developing methods for multiplexed editing, allowing simultaneous modifications of multiple genes.
The integration of gel electrophoresis into gene editing workflows represents a critical step in ensuring the accuracy and reliability of genetic modifications. By providing a means to visualize and analyze edited DNA fragments, gel electrophoresis contributes to the overall success and validation of gene editing experiments. As gene editing technologies continue to advance, the role of analytical techniques like gel electrophoresis in verifying and optimizing editing outcomes remains paramount.
Market Analysis for Gene Editing Technologies
The gene editing market has experienced significant growth in recent years, driven by advancements in CRISPR technology and increasing applications in healthcare, agriculture, and biotechnology. The global gene editing market size was valued at $5.2 billion in 2020 and is projected to reach $11.2 billion by 2025, growing at a CAGR of 16.7% during the forecast period.
Gel electrophoresis plays a crucial role in gene editing success, particularly in the analysis and validation of gene editing outcomes. As gene editing technologies continue to evolve, the demand for reliable and efficient gel electrophoresis methods is expected to increase proportionally. The market for gel electrophoresis equipment and consumables is anticipated to grow in tandem with the gene editing market.
Key factors driving the market growth include the rising prevalence of genetic disorders, increasing investments in genomics research, and growing applications of gene editing in drug discovery and development. The pharmaceutical and biotechnology sectors are the largest end-users of gene editing technologies, accounting for approximately 40% of the market share.
North America dominates the gene editing market, followed by Europe and Asia-Pacific. The United States leads in terms of research and development activities, with major biotechnology companies and academic institutions driving innovation in the field. However, Asia-Pacific is expected to witness the highest growth rate due to increasing investments in life sciences research and favorable government initiatives.
The gene editing market is highly competitive, with key players including Thermo Fisher Scientific, CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics. These companies are actively developing new gene editing tools and techniques, including improved methods for analyzing gene editing outcomes using gel electrophoresis.
Challenges in the market include ethical concerns surrounding gene editing, regulatory hurdles, and technical limitations. However, ongoing research and development efforts are addressing these challenges, with a focus on improving the accuracy, efficiency, and safety of gene editing technologies.
In conclusion, the market analysis for gene editing technologies indicates a robust growth trajectory, with gel electrophoresis playing a vital role in ensuring gene editing success. As the field continues to advance, opportunities for innovation in gel electrophoresis techniques and equipment are likely to emerge, further supporting the expansion of the gene editing market.
Current Challenges in Gel Electrophoresis for Gene Editing
Gel electrophoresis has been a cornerstone technique in molecular biology and gene editing, but its application in modern gene editing workflows faces several challenges. One of the primary issues is the limited resolution for detecting small genetic modifications. As gene editing techniques like CRISPR-Cas9 often introduce minor changes, traditional gel electrophoresis may struggle to distinguish these subtle alterations from wild-type sequences.
Another significant challenge is the time-consuming nature of the process. Gel electrophoresis typically requires several hours to complete, which can be a bottleneck in high-throughput gene editing experiments. This prolonged duration not only slows down the overall workflow but also increases the risk of DNA degradation, potentially compromising the accuracy of results.
The sensitivity of gel electrophoresis in detecting low abundance DNA fragments presents another hurdle. In gene editing applications, where the efficiency of editing may be low, the ability to detect rare edited sequences among a background of unedited DNA is crucial. Current gel electrophoresis methods often lack the sensitivity required for this level of detection.
Reproducibility and standardization across different laboratories remain ongoing challenges. Variations in gel composition, running conditions, and imaging techniques can lead to inconsistent results, making it difficult to compare data between experiments or research groups. This lack of standardization hampers the broader applicability and reliability of gel electrophoresis in gene editing success evaluation.
The quantitative analysis of gene editing outcomes using gel electrophoresis is also problematic. While the technique can provide a visual representation of DNA fragments, accurate quantification of editing efficiency often requires more sophisticated methods. This limitation restricts the ability to precisely measure the success rate of gene editing experiments.
Environmental concerns associated with the use of potentially harmful chemicals in gel electrophoresis, such as ethidium bromide for DNA staining, pose additional challenges. The need for safer alternatives that maintain sensitivity and ease of use is becoming increasingly important in modern laboratory settings.
Lastly, the integration of gel electrophoresis results with advanced bioinformatics tools for comprehensive analysis of gene editing outcomes remains a challenge. As the field of gene editing becomes more complex, there is a growing need for seamless integration between traditional molecular biology techniques and computational analysis methods to fully interpret experimental results.
Existing Gel Electrophoresis Protocols for Gene Editing
01 Optimization of gel composition and conditions
Improving the success rate of gel electrophoresis involves optimizing the gel composition and running conditions. This includes adjusting the concentration of agarose or polyacrylamide, buffer composition, and electric field strength. Proper optimization can lead to better resolution, separation, and overall performance of the electrophoresis process.- Optimization of gel composition and conditions: Improving the success rate of gel electrophoresis involves optimizing the gel composition and running conditions. This includes adjusting the agarose concentration, buffer composition, and voltage settings to achieve better separation and resolution of DNA or protein samples. Proper optimization can lead to clearer bands and more accurate results.
- Sample preparation techniques: Effective sample preparation is crucial for successful gel electrophoresis. This includes proper DNA or protein extraction, purification, and loading techniques. Using appropriate loading buffers, dyes, and sample volumes can significantly improve the quality of results and increase the success rate of the electrophoresis process.
- Advanced detection and imaging methods: Implementing advanced detection and imaging techniques can enhance the success rate of gel electrophoresis. This includes using fluorescent dyes, chemiluminescent markers, or specialized imaging systems to improve the visibility and analysis of separated bands. These methods can increase sensitivity and allow for better quantification of results.
- Microfluidic and automated systems: Incorporating microfluidic technologies and automated systems can improve the reproducibility and success rate of gel electrophoresis. These systems offer precise control over sample loading, separation, and detection processes, reducing human error and increasing consistency across experiments.
- Quality control and troubleshooting strategies: Implementing robust quality control measures and effective troubleshooting strategies is essential for maintaining a high success rate in gel electrophoresis. This includes regular calibration of equipment, using appropriate controls, and developing standardized protocols for addressing common issues such as smearing, faint bands, or uneven migration.
02 Sample preparation techniques
Effective sample preparation is crucial for successful gel electrophoresis. This involves proper DNA or protein extraction, purification, and loading techniques. Improved sample preparation methods can enhance the quality of results and increase the success rate of the electrophoresis process.Expand Specific Solutions03 Advanced detection and imaging methods
Implementing advanced detection and imaging techniques can significantly improve the success rate of gel electrophoresis. This includes using fluorescent dyes, chemiluminescent markers, and high-resolution imaging systems to enhance the visibility and analysis of separated molecules.Expand Specific Solutions04 Microfluidic and miniaturized systems
Developing microfluidic and miniaturized gel electrophoresis systems can lead to improved success rates. These systems offer better control over sample handling, reduced sample volumes, and faster analysis times, resulting in more consistent and reliable results.Expand Specific Solutions05 Automation and robotics integration
Integrating automation and robotics into gel electrophoresis processes can enhance reproducibility and success rates. Automated systems can perform precise sample loading, gel preparation, and result analysis, reducing human error and improving overall efficiency.Expand Specific Solutions
Key Players in Gene Editing and Electrophoresis
The gene editing field, particularly in the context of gel electrophoresis, is in a growth phase with increasing market size and technological advancements. The competitive landscape is diverse, featuring established biotech companies like Life Technologies Corp. and Exact Sciences Corp., alongside academic institutions such as the University of California and Yale University. The technology's maturity is evolving, with companies like Natera, Inc. and Iovance Biotherapeutics, Inc. pushing boundaries in clinical applications. Research institutions like RIKEN and Harbin Medical University contribute to fundamental advancements, while agricultural giants like KWS SAAT SE & Co. KGaA and Syngenta Crop Protection AG explore applications in crop improvement. This multifaceted competition drives innovation and expands the technology's reach across various sectors.
Life Technologies Corp.
The Regents of the University of California
Innovations in Gel Electrophoresis for Gene Editing
- A single system for gel electrophoresis, electrotransfer, and detection that includes a device with a biomolecule receiving material and electrodes arranged to allow current flow in multiple directions, enabling efficient transfer and detection of biomolecules with reduced buffer volumes and hazardous waste, and improved antibody recognition.
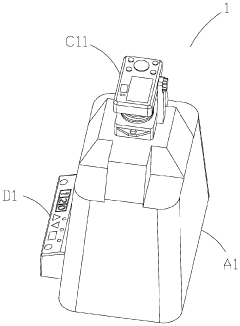
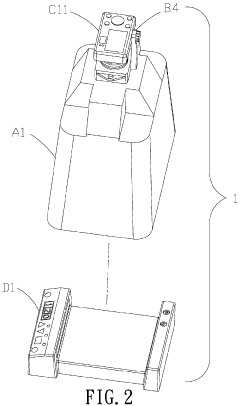
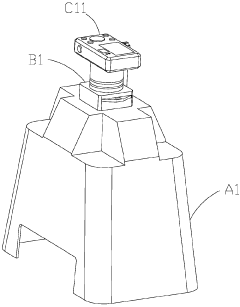
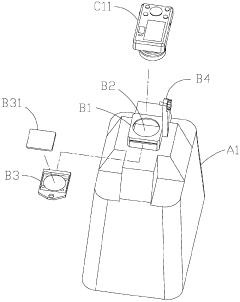
- An electrophoresis system equipped with a pedestal, LED lamps emitting blue light, a gel block, a covering member with a hollow mount and tray, and a picture taking device, including a digital camera or CMOS/CCD image sensor, allowing for visual observation and recording of DNA sample state changes through fluorescent dye color changes, with a control panel and photographic filters for safety and accuracy.
Regulatory Framework for Gene Editing Technologies
The regulatory framework for gene editing technologies, particularly in the context of gel electrophoresis for gene editing success, is a complex and evolving landscape. As gene editing techniques continue to advance, governments and international bodies are working to establish comprehensive guidelines to ensure responsible research and application.
At the forefront of this regulatory framework is the need to balance scientific progress with ethical considerations and public safety. Many countries have implemented strict oversight mechanisms for gene editing research, requiring institutional review boards and ethics committees to approve studies before they can proceed. These bodies evaluate the potential risks and benefits of proposed gene editing experiments, including those utilizing gel electrophoresis techniques.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating gene editing technologies. The FDA has established guidelines for the development and testing of gene therapies, which include gene editing approaches. These guidelines cover various aspects of the research process, from preclinical studies to clinical trials, and emphasize the importance of rigorous safety assessments.
The European Union has also developed a robust regulatory framework for gene editing technologies. The European Medicines Agency (EMA) oversees the evaluation and authorization of gene therapy products, including those developed using gene editing techniques. The EU's regulatory approach emphasizes the precautionary principle, requiring thorough risk assessments and long-term monitoring of gene editing applications.
Internationally, organizations such as the World Health Organization (WHO) have been working to establish global standards for gene editing research and applications. The WHO has called for the creation of a global registry for human genome editing research and has emphasized the need for harmonized regulations across countries to prevent regulatory arbitrage.
One of the key challenges in regulating gene editing technologies is keeping pace with rapid scientific advancements. Regulatory bodies must continually update their guidelines to address new techniques and applications, including improvements in gel electrophoresis methodologies for gene editing success. This requires ongoing collaboration between scientists, policymakers, and ethicists to ensure that regulations remain relevant and effective.
As the field of gene editing continues to evolve, there is growing recognition of the need for a more nuanced regulatory approach that distinguishes between different types of gene editing applications. For instance, regulations governing somatic cell gene editing may differ from those applied to germline editing, which has more far-reaching implications for future generations.
Ethical Implications of Gene Editing Advancements
The rapid advancements in gene editing technologies, particularly CRISPR-Cas9, have raised significant ethical concerns within the scientific community and society at large. As gel electrophoresis plays a crucial role in verifying gene editing success, it is imperative to consider the ethical implications of these advancements. One primary concern is the potential for unintended consequences and off-target effects in gene editing procedures. While gel electrophoresis can help identify successful edits, it may not always detect subtle genetic changes that could have far-reaching impacts on an organism's health and function.
The ability to make precise genetic modifications also raises questions about the boundaries of human intervention in nature. As gene editing becomes more accessible and efficient, there are concerns about its potential misuse for enhancing human traits beyond medical necessity. This could lead to increased societal inequalities if genetic enhancements become available only to those who can afford them. Furthermore, the use of gene editing in embryos and germline cells presents complex ethical dilemmas regarding the rights of future generations and the potential for irreversible changes to the human gene pool.
Another critical ethical consideration is the impact of gene editing on biodiversity and ecosystems. While gel electrophoresis can confirm successful genetic modifications in plants and animals, the long-term ecological consequences of releasing genetically edited organisms into the environment remain uncertain. This raises concerns about the potential disruption of natural ecosystems and the unintended effects on non-target species.
The ethical implications extend to the realm of intellectual property and commercialization of gene editing technologies. As companies and research institutions race to patent gene editing techniques and their applications, questions arise about equitable access to these technologies and the potential for monopolization of genetic resources. This could have significant implications for global health equity and agricultural practices, particularly in developing countries.
Lastly, the rapid pace of gene editing advancements outstrips the development of regulatory frameworks and ethical guidelines. While gel electrophoresis provides a means to verify gene editing success, there is a pressing need for comprehensive ethical oversight and international cooperation to address the complex moral and societal implications of these powerful technologies. Balancing the potential benefits of gene editing with the ethical concerns it raises will be crucial in shaping the responsible development and application of these transformative technologies in the years to come.