Integration of High Pass Filters in Bioprocess Control Systems
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioprocess Control HPF Background and Objectives
Bioprocess control systems have undergone significant evolution over the past few decades, driven by the increasing complexity and precision requirements of modern biotechnology and pharmaceutical manufacturing. The integration of high pass filters (HPFs) into these systems represents a critical advancement in enhancing the accuracy and reliability of bioprocess monitoring and control.
High pass filters, originally developed for signal processing in electronics, have found a crucial role in bioprocess control due to their ability to attenuate low-frequency noise while allowing higher frequency signals to pass through. This characteristic is particularly valuable in bioprocessing environments where slow-changing background signals or drift can obscure important rapid fluctuations in process parameters.
The historical development of HPFs in bioprocess control can be traced back to the early 2000s when the biopharmaceutical industry began to adopt more sophisticated process analytical technologies (PAT). As the demand for real-time monitoring and control of critical process parameters increased, the limitations of traditional sensing and control methods became apparent. HPFs emerged as a solution to improve signal quality and enable more responsive control strategies.
The primary objective of integrating HPFs into bioprocess control systems is to enhance the detection and measurement of rapid changes in key process variables such as pH, dissolved oxygen, temperature, and metabolite concentrations. By filtering out low-frequency noise and baseline drift, HPFs allow for more precise identification of process deviations and faster response times in control loops.
Another crucial goal is to improve the overall robustness and reliability of bioprocess control systems. HPFs contribute to this by reducing the impact of sensor fouling, environmental fluctuations, and other sources of low-frequency interference that can compromise the accuracy of measurements over extended periods.
Furthermore, the integration of HPFs aims to support the implementation of advanced control strategies, such as model predictive control and adaptive control algorithms. These sophisticated approaches rely on high-quality, real-time data to make accurate predictions and adjustments, making the signal enhancement provided by HPFs invaluable.
As the bioprocessing industry continues to move towards continuous manufacturing and increased automation, the role of HPFs in control systems is expected to grow. Future objectives include the development of more adaptive and intelligent filtering techniques that can automatically adjust to changing process conditions and optimize filter performance in real-time.
High pass filters, originally developed for signal processing in electronics, have found a crucial role in bioprocess control due to their ability to attenuate low-frequency noise while allowing higher frequency signals to pass through. This characteristic is particularly valuable in bioprocessing environments where slow-changing background signals or drift can obscure important rapid fluctuations in process parameters.
The historical development of HPFs in bioprocess control can be traced back to the early 2000s when the biopharmaceutical industry began to adopt more sophisticated process analytical technologies (PAT). As the demand for real-time monitoring and control of critical process parameters increased, the limitations of traditional sensing and control methods became apparent. HPFs emerged as a solution to improve signal quality and enable more responsive control strategies.
The primary objective of integrating HPFs into bioprocess control systems is to enhance the detection and measurement of rapid changes in key process variables such as pH, dissolved oxygen, temperature, and metabolite concentrations. By filtering out low-frequency noise and baseline drift, HPFs allow for more precise identification of process deviations and faster response times in control loops.
Another crucial goal is to improve the overall robustness and reliability of bioprocess control systems. HPFs contribute to this by reducing the impact of sensor fouling, environmental fluctuations, and other sources of low-frequency interference that can compromise the accuracy of measurements over extended periods.
Furthermore, the integration of HPFs aims to support the implementation of advanced control strategies, such as model predictive control and adaptive control algorithms. These sophisticated approaches rely on high-quality, real-time data to make accurate predictions and adjustments, making the signal enhancement provided by HPFs invaluable.
As the bioprocessing industry continues to move towards continuous manufacturing and increased automation, the role of HPFs in control systems is expected to grow. Future objectives include the development of more adaptive and intelligent filtering techniques that can automatically adjust to changing process conditions and optimize filter performance in real-time.
Market Demand for Advanced Bioprocess Control
The bioprocess industry is experiencing a significant surge in demand for advanced control systems, particularly those incorporating high pass filters. This growing market need is driven by several key factors that are reshaping the landscape of bioprocess manufacturing.
Firstly, the increasing complexity of bioprocesses, especially in the pharmaceutical and biotechnology sectors, necessitates more sophisticated control mechanisms. As companies strive to produce more complex biologics and personalized medicines, the need for precise control over process parameters becomes paramount. High pass filters, when integrated into bioprocess control systems, offer enhanced ability to remove low-frequency noise and baseline drift, thereby improving the accuracy of measurements and control actions.
Secondly, the push for higher productivity and efficiency in bioprocess operations is fueling the demand for advanced control solutions. Manufacturers are seeking ways to optimize their processes, reduce variability, and increase yields. Advanced control systems with high pass filters can help achieve these goals by providing more stable and responsive control, leading to improved process consistency and product quality.
The growing emphasis on quality assurance and regulatory compliance in the biopharmaceutical industry is another significant driver. Regulatory bodies are increasingly focusing on process analytical technology (PAT) and quality by design (QbD) approaches. Advanced bioprocess control systems that incorporate high pass filters align well with these initiatives, offering better real-time monitoring and control capabilities that can help ensure consistent product quality and regulatory compliance.
Moreover, the trend towards continuous bioprocessing is creating new opportunities for advanced control systems. Continuous processes require more sophisticated control strategies compared to traditional batch processes. High pass filters in control systems can play a crucial role in managing the dynamic nature of continuous bioprocessing, helping to maintain steady-state conditions and respond quickly to process disturbances.
The increasing adoption of Industry 4.0 concepts in bioprocessing is also driving demand for more advanced control solutions. As bioprocess facilities move towards greater automation and data integration, there is a growing need for control systems that can handle complex data streams and make real-time adjustments. High pass filters can be instrumental in processing these data streams, filtering out noise and providing cleaner signals for decision-making algorithms.
Lastly, the global expansion of the biopharmaceutical industry, particularly in emerging markets, is creating new demand for advanced bioprocess control systems. As new facilities are built and existing ones are upgraded, there is a significant opportunity for the implementation of state-of-the-art control technologies, including those incorporating high pass filters.
Firstly, the increasing complexity of bioprocesses, especially in the pharmaceutical and biotechnology sectors, necessitates more sophisticated control mechanisms. As companies strive to produce more complex biologics and personalized medicines, the need for precise control over process parameters becomes paramount. High pass filters, when integrated into bioprocess control systems, offer enhanced ability to remove low-frequency noise and baseline drift, thereby improving the accuracy of measurements and control actions.
Secondly, the push for higher productivity and efficiency in bioprocess operations is fueling the demand for advanced control solutions. Manufacturers are seeking ways to optimize their processes, reduce variability, and increase yields. Advanced control systems with high pass filters can help achieve these goals by providing more stable and responsive control, leading to improved process consistency and product quality.
The growing emphasis on quality assurance and regulatory compliance in the biopharmaceutical industry is another significant driver. Regulatory bodies are increasingly focusing on process analytical technology (PAT) and quality by design (QbD) approaches. Advanced bioprocess control systems that incorporate high pass filters align well with these initiatives, offering better real-time monitoring and control capabilities that can help ensure consistent product quality and regulatory compliance.
Moreover, the trend towards continuous bioprocessing is creating new opportunities for advanced control systems. Continuous processes require more sophisticated control strategies compared to traditional batch processes. High pass filters in control systems can play a crucial role in managing the dynamic nature of continuous bioprocessing, helping to maintain steady-state conditions and respond quickly to process disturbances.
The increasing adoption of Industry 4.0 concepts in bioprocessing is also driving demand for more advanced control solutions. As bioprocess facilities move towards greater automation and data integration, there is a growing need for control systems that can handle complex data streams and make real-time adjustments. High pass filters can be instrumental in processing these data streams, filtering out noise and providing cleaner signals for decision-making algorithms.
Lastly, the global expansion of the biopharmaceutical industry, particularly in emerging markets, is creating new demand for advanced bioprocess control systems. As new facilities are built and existing ones are upgraded, there is a significant opportunity for the implementation of state-of-the-art control technologies, including those incorporating high pass filters.
Current HPF Integration Challenges in Bioprocessing
The integration of High Pass Filters (HPFs) in bioprocess control systems presents several significant challenges that hinder their widespread adoption and optimal performance. One of the primary obstacles is the complexity of bioprocesses, which often involve multiple interacting variables and non-linear dynamics. This complexity makes it difficult to design and implement HPFs that can effectively filter out low-frequency noise while preserving critical high-frequency signals relevant to bioprocess control.
Another major challenge is the sensitivity of bioprocesses to environmental fluctuations and process variations. HPFs must be carefully tuned to accommodate these variations without compromising the stability of the control system. This requires sophisticated adaptive algorithms and robust filter designs that can maintain performance across a wide range of operating conditions.
The real-time processing requirements of bioprocess control systems also pose significant challenges for HPF integration. Bioprocesses often demand rapid response times, necessitating high-speed signal processing and minimal latency in filter operations. Achieving this level of performance while maintaining accuracy and reliability can be technically demanding and resource-intensive.
Furthermore, the integration of HPFs must contend with the inherent noise characteristics of bioprocess sensors and measurement systems. Biological systems often generate signals with complex noise profiles that can be difficult to distinguish from genuine process variations. Designing HPFs that can effectively separate these signals without introducing artifacts or distortions is a persistent challenge in the field.
Scalability and flexibility are additional concerns in HPF integration for bioprocess control. As bioprocesses scale up from laboratory to industrial levels, the filtering requirements may change dramatically. Developing HPF solutions that can adapt to different scales and process configurations without requiring extensive redesign or recalibration remains a significant technical hurdle.
The regulatory environment surrounding bioprocessing also impacts HPF integration. Strict validation and documentation requirements for control systems in regulated industries like pharmaceuticals can make it challenging to implement and update advanced filtering techniques. Ensuring that HPF implementations comply with regulatory standards while maintaining optimal performance adds another layer of complexity to their integration.
Lastly, the interdisciplinary nature of bioprocess control systems presents challenges in terms of expertise and collaboration. Effective HPF integration requires a combination of skills in signal processing, control theory, and bioprocess engineering. Bridging these diverse fields and fostering effective communication between specialists can be a significant organizational and technical challenge for many companies and research institutions.
Another major challenge is the sensitivity of bioprocesses to environmental fluctuations and process variations. HPFs must be carefully tuned to accommodate these variations without compromising the stability of the control system. This requires sophisticated adaptive algorithms and robust filter designs that can maintain performance across a wide range of operating conditions.
The real-time processing requirements of bioprocess control systems also pose significant challenges for HPF integration. Bioprocesses often demand rapid response times, necessitating high-speed signal processing and minimal latency in filter operations. Achieving this level of performance while maintaining accuracy and reliability can be technically demanding and resource-intensive.
Furthermore, the integration of HPFs must contend with the inherent noise characteristics of bioprocess sensors and measurement systems. Biological systems often generate signals with complex noise profiles that can be difficult to distinguish from genuine process variations. Designing HPFs that can effectively separate these signals without introducing artifacts or distortions is a persistent challenge in the field.
Scalability and flexibility are additional concerns in HPF integration for bioprocess control. As bioprocesses scale up from laboratory to industrial levels, the filtering requirements may change dramatically. Developing HPF solutions that can adapt to different scales and process configurations without requiring extensive redesign or recalibration remains a significant technical hurdle.
The regulatory environment surrounding bioprocessing also impacts HPF integration. Strict validation and documentation requirements for control systems in regulated industries like pharmaceuticals can make it challenging to implement and update advanced filtering techniques. Ensuring that HPF implementations comply with regulatory standards while maintaining optimal performance adds another layer of complexity to their integration.
Lastly, the interdisciplinary nature of bioprocess control systems presents challenges in terms of expertise and collaboration. Effective HPF integration requires a combination of skills in signal processing, control theory, and bioprocess engineering. Bridging these diverse fields and fostering effective communication between specialists can be a significant organizational and technical challenge for many companies and research institutions.
Existing HPF Integration Solutions
01 Circuit design for high pass filters
High pass filters are designed using various circuit configurations to attenuate low-frequency signals while allowing high-frequency signals to pass through. These designs often involve the use of capacitors and resistors in specific arrangements to achieve the desired frequency response. Advanced designs may incorporate active components like operational amplifiers to enhance performance and provide additional functionality.- Circuit design for high pass filters: High pass filters are designed using various circuit configurations to attenuate low-frequency signals while allowing high-frequency signals to pass through. These designs often involve the use of capacitors and resistors in specific arrangements to achieve the desired frequency response. Advanced designs may incorporate active components like operational amplifiers to enhance performance and provide additional control over the filter characteristics.
- Digital implementation of high pass filters: High pass filters can be implemented digitally using signal processing techniques. This approach involves the use of digital signal processors (DSPs) or field-programmable gate arrays (FPGAs) to perform the filtering operations. Digital high pass filters offer advantages such as programmability, adaptability, and the ability to implement complex filter designs without the need for precise analog components.
- Application in image and video processing: High pass filters play a crucial role in image and video processing applications. They are used to enhance edge detection, improve image sharpness, and remove low-frequency noise from visual data. In video systems, high pass filters can be employed for various purposes, including motion detection, artifact reduction, and improving overall video quality.
- Integration with other filter types: High pass filters are often combined with other filter types to create more complex filtering systems. This integration can include the development of band-pass filters, which combine high pass and low pass characteristics, or the creation of adaptive filtering systems that can dynamically adjust their frequency response based on input signals or environmental conditions.
- Noise reduction and signal conditioning: High pass filters are utilized in various applications for noise reduction and signal conditioning. They can effectively remove low-frequency noise, DC offset, and unwanted baseline drift from signals. This is particularly useful in audio systems, sensor interfaces, and communication systems where maintaining signal integrity and improving signal-to-noise ratio is critical.
02 Application in image and video processing
High pass filters play a crucial role in image and video processing applications. They are used to enhance edge detection, improve image sharpness, and remove low-frequency noise from visual data. These filters can be implemented in both hardware and software solutions for various imaging devices and systems, including digital cameras, video processors, and display technologies.Expand Specific Solutions03 Integration with communication systems
High pass filters are essential components in communication systems, particularly in signal processing and noise reduction. They are used to eliminate low-frequency interference, improve signal quality, and optimize bandwidth utilization in various communication technologies such as wireless networks, satellite communications, and cellular systems.Expand Specific Solutions04 Adaptive and tunable high pass filters
Advanced high pass filter designs incorporate adaptive and tunable features, allowing for dynamic adjustment of filter characteristics based on changing signal conditions or user requirements. These filters may use digital control mechanisms, variable components, or software algorithms to modify their frequency response in real-time, enhancing their versatility and performance across different applications.Expand Specific Solutions05 High pass filters in analog-to-digital conversion
High pass filters are utilized in analog-to-digital conversion processes to improve the accuracy and efficiency of signal digitization. They help remove DC offsets and low-frequency noise before the analog signal is converted to digital form, ensuring better resolution and dynamic range in the resulting digital signal. This application is particularly important in high-precision measurement and data acquisition systems.Expand Specific Solutions
Key Players in Bioprocess Control Systems
The integration of high pass filters in bioprocess control systems is currently in a growth phase, with increasing market demand driven by advancements in biopharmaceutical manufacturing. The global market size for bioprocess control systems is expanding rapidly, reflecting the industry's maturation and the growing need for sophisticated filtration technologies. Technologically, the field is progressing steadily, with companies like Sartorius Stedim Biotech GmbH and Cytiva (formerly Global Life Sciences Solutions USA LLC) leading innovation. These firms, along with emerging players such as Microfluidx Ltd., are developing increasingly advanced and efficient high pass filter solutions, indicating a moderate to high level of technological maturity in this sector.
Sartorius Stedim Biotech GmbH
Technical Solution: Sartorius Stedim Biotech has developed advanced high pass filter integration systems for bioprocess control. Their technology utilizes a combination of hardware and software solutions to effectively implement high pass filters in various bioprocessing applications. The company's approach involves the use of smart sensors and real-time data processing algorithms to continuously monitor and adjust filter parameters[1]. This allows for dynamic optimization of the filtration process, enhancing the overall efficiency and reliability of bioprocess control systems. Sartorius has also implemented machine learning techniques to predict and prevent filter fouling, thereby extending the operational lifespan of the filtration components[3].
Strengths: Advanced sensor technology and real-time data processing capabilities. Weaknesses: May require significant initial investment and specialized training for operators.
Global Life Sciences Solutions USA LLC
Technical Solution: Global Life Sciences Solutions USA LLC, a part of Cytiva (formerly GE Healthcare Life Sciences), has developed innovative high pass filter integration solutions for bioprocess control systems. Their approach focuses on modular and scalable filter designs that can be easily integrated into existing bioprocessing equipment[2]. The company utilizes advanced materials and membrane technologies to create high-performance filters capable of handling a wide range of biomolecules and process conditions. Additionally, they have implemented smart control systems that allow for real-time monitoring and adjustment of filter parameters, ensuring optimal performance throughout the bioprocess[4].
Strengths: Modular and scalable filter designs, wide range of compatibility with existing systems. Weaknesses: May have higher operational costs due to advanced materials and technologies used.
Core Innovations in HPF for Bioprocessing
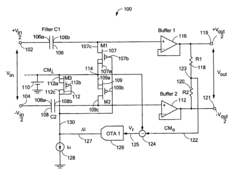
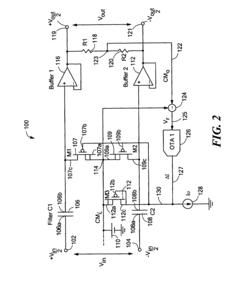
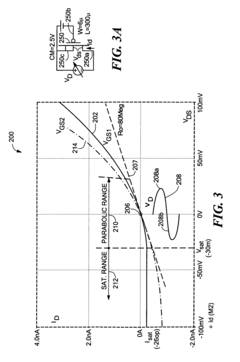
High pass filter using insulated gate field effect transistors
PatentInactiveUS6995606B2
Innovation
- A high pass filter circuit utilizing a differential arrangement of capacitors and insulated gate field effect transistors (IGFETs) as effective resistors, with a feedback mechanism to control gate voltages and compensate for DC shifted voltages, ensuring reduced distortion and maintaining a low frequency break frequency.
Filter, filter module, and electronic device
PatentWO2022049927A1
Innovation
- A high-pass filter configuration with a first capacitor connected in series between terminal pairs and magnetically coupled inductors, where the parasitic capacitance of the inductors is connected in parallel to the capacitor, reducing self-resonance and improving passband characteristics, and a filter module composed of this filter and other filters for broader frequency coverage.
Regulatory Compliance for Bioprocess Control Systems
Regulatory compliance is a critical aspect of bioprocess control systems, particularly when integrating high pass filters. The bioprocessing industry is subject to stringent regulations set by various governing bodies, including the Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Conference on Harmonisation (ICH). These regulations aim to ensure product quality, safety, and efficacy in the production of biopharmaceuticals.
When implementing high pass filters in bioprocess control systems, manufacturers must adhere to Good Manufacturing Practice (GMP) guidelines. These guidelines encompass a wide range of requirements, including equipment validation, process control, and documentation. The integration of high pass filters must be thoroughly documented, with detailed standard operating procedures (SOPs) outlining their use, maintenance, and calibration.
Validation is a key component of regulatory compliance for bioprocess control systems. This involves demonstrating that the high pass filters consistently perform as intended within the specified operating parameters. Manufacturers must conduct extensive testing and provide evidence that the filters effectively remove unwanted low-frequency signals without compromising the integrity of the bioprocess data.
Data integrity is another crucial aspect of regulatory compliance. The use of high pass filters in bioprocess control systems must not compromise the accuracy, completeness, or traceability of data. Manufacturers need to implement robust data management systems that ensure the preservation of raw data alongside filtered data, allowing for retrospective analysis and auditing.
Risk management is an integral part of regulatory compliance in bioprocessing. The integration of high pass filters must be assessed for potential risks to product quality and process consistency. Manufacturers are required to conduct thorough risk assessments and implement appropriate mitigation strategies to address any identified risks associated with the use of these filters.
Continuous monitoring and periodic review of the bioprocess control system, including the high pass filters, are essential for maintaining regulatory compliance. This involves regular calibration, performance checks, and system audits to ensure ongoing adherence to regulatory standards. Any deviations or non-conformities must be promptly addressed and documented.
Training and personnel qualification are also critical components of regulatory compliance. Operators and technicians responsible for managing bioprocess control systems with integrated high pass filters must receive comprehensive training on their proper use, maintenance, and troubleshooting. This training should be documented and regularly updated to reflect any changes in technology or regulatory requirements.
When implementing high pass filters in bioprocess control systems, manufacturers must adhere to Good Manufacturing Practice (GMP) guidelines. These guidelines encompass a wide range of requirements, including equipment validation, process control, and documentation. The integration of high pass filters must be thoroughly documented, with detailed standard operating procedures (SOPs) outlining their use, maintenance, and calibration.
Validation is a key component of regulatory compliance for bioprocess control systems. This involves demonstrating that the high pass filters consistently perform as intended within the specified operating parameters. Manufacturers must conduct extensive testing and provide evidence that the filters effectively remove unwanted low-frequency signals without compromising the integrity of the bioprocess data.
Data integrity is another crucial aspect of regulatory compliance. The use of high pass filters in bioprocess control systems must not compromise the accuracy, completeness, or traceability of data. Manufacturers need to implement robust data management systems that ensure the preservation of raw data alongside filtered data, allowing for retrospective analysis and auditing.
Risk management is an integral part of regulatory compliance in bioprocessing. The integration of high pass filters must be assessed for potential risks to product quality and process consistency. Manufacturers are required to conduct thorough risk assessments and implement appropriate mitigation strategies to address any identified risks associated with the use of these filters.
Continuous monitoring and periodic review of the bioprocess control system, including the high pass filters, are essential for maintaining regulatory compliance. This involves regular calibration, performance checks, and system audits to ensure ongoing adherence to regulatory standards. Any deviations or non-conformities must be promptly addressed and documented.
Training and personnel qualification are also critical components of regulatory compliance. Operators and technicians responsible for managing bioprocess control systems with integrated high pass filters must receive comprehensive training on their proper use, maintenance, and troubleshooting. This training should be documented and regularly updated to reflect any changes in technology or regulatory requirements.
Economic Impact of HPF Integration in Bioprocessing
The integration of High Pass Filters (HPFs) in bioprocess control systems has significant economic implications for the bioprocessing industry. This technological advancement offers substantial cost savings and efficiency improvements across various aspects of bioprocessing operations. Primarily, HPFs enhance the quality control processes by effectively filtering out low-frequency noise and baseline drift in sensor signals, leading to more accurate measurements and reduced error rates in production.
The implementation of HPFs in bioprocess control systems results in a notable reduction in raw material waste. By improving the precision of measurements and control mechanisms, manufacturers can optimize their resource utilization, minimizing overuse of expensive biological materials and reagents. This optimization directly translates to lower production costs and improved profit margins for bioprocessing companies.
Furthermore, the enhanced process control enabled by HPFs contributes to increased product yield and quality. The more stable and accurate control of critical process parameters ensures consistent product characteristics, reducing the likelihood of batch rejections and the associated economic losses. This improvement in product quality can lead to a stronger market position and potentially higher pricing power for manufacturers.
The adoption of HPF technology also impacts operational efficiency. By reducing signal noise and improving data quality, bioprocess engineers can make more informed decisions in real-time, leading to faster troubleshooting and reduced downtime. This increased operational efficiency translates to higher throughput and better asset utilization, ultimately improving the return on investment for bioprocessing equipment.
In terms of regulatory compliance, the integration of HPFs can lead to cost savings by reducing the risk of non-compliance. The improved accuracy and reliability of process data facilitate easier adherence to Good Manufacturing Practices (GMP) and simplify the documentation process for regulatory submissions. This can potentially accelerate time-to-market for new biopharmaceutical products and reduce the costs associated with regulatory delays.
The economic benefits of HPF integration extend to the broader supply chain as well. Improved process control and product consistency can lead to more predictable production schedules, enabling better inventory management and logistics planning. This optimization can result in reduced inventory holding costs and improved cash flow for bioprocessing companies and their partners in the pharmaceutical supply chain.
The implementation of HPFs in bioprocess control systems results in a notable reduction in raw material waste. By improving the precision of measurements and control mechanisms, manufacturers can optimize their resource utilization, minimizing overuse of expensive biological materials and reagents. This optimization directly translates to lower production costs and improved profit margins for bioprocessing companies.
Furthermore, the enhanced process control enabled by HPFs contributes to increased product yield and quality. The more stable and accurate control of critical process parameters ensures consistent product characteristics, reducing the likelihood of batch rejections and the associated economic losses. This improvement in product quality can lead to a stronger market position and potentially higher pricing power for manufacturers.
The adoption of HPF technology also impacts operational efficiency. By reducing signal noise and improving data quality, bioprocess engineers can make more informed decisions in real-time, leading to faster troubleshooting and reduced downtime. This increased operational efficiency translates to higher throughput and better asset utilization, ultimately improving the return on investment for bioprocessing equipment.
In terms of regulatory compliance, the integration of HPFs can lead to cost savings by reducing the risk of non-compliance. The improved accuracy and reliability of process data facilitate easier adherence to Good Manufacturing Practices (GMP) and simplify the documentation process for regulatory submissions. This can potentially accelerate time-to-market for new biopharmaceutical products and reduce the costs associated with regulatory delays.
The economic benefits of HPF integration extend to the broader supply chain as well. Improved process control and product consistency can lead to more predictable production schedules, enabling better inventory management and logistics planning. This optimization can result in reduced inventory holding costs and improved cash flow for bioprocessing companies and their partners in the pharmaceutical supply chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!