Interactions Between Sodium Percarbonate and Organic Pollutants
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Tech Background and Objectives
Sodium percarbonate, a widely used oxidizing agent and bleaching compound, has gained significant attention in recent years due to its potential applications in environmental remediation, particularly in the treatment of organic pollutants. The technology surrounding sodium percarbonate and its interactions with organic contaminants has evolved considerably over the past few decades, driven by the increasing need for efficient and environmentally friendly water treatment solutions.
The development of sodium percarbonate technology can be traced back to the early 20th century when it was first synthesized and utilized in household cleaning products. However, its potential for environmental applications was not fully realized until the late 1980s and early 1990s, when researchers began exploring its oxidative properties for the degradation of organic pollutants in water systems.
As environmental concerns grew and regulations became more stringent, the focus on sodium percarbonate as a potential solution for organic pollutant remediation intensified. The technology has since progressed through several key stages, from initial laboratory studies to pilot-scale demonstrations and, more recently, full-scale implementations in various water treatment scenarios.
The primary objective of current research and development efforts in this field is to optimize the interactions between sodium percarbonate and organic pollutants to achieve more efficient and cost-effective treatment processes. This includes enhancing the oxidation capacity of sodium percarbonate, improving its stability in aqueous environments, and developing novel activation methods to increase its reactivity towards specific organic contaminants.
Another crucial goal is to expand the range of treatable organic pollutants and to understand the complex reaction mechanisms involved in their degradation. This knowledge is essential for designing tailored treatment solutions for different types of organic contaminants, such as pharmaceuticals, pesticides, and industrial chemicals, which pose significant challenges to conventional water treatment methods.
Furthermore, researchers aim to integrate sodium percarbonate technology with other advanced oxidation processes and treatment techniques to create more comprehensive and effective water purification systems. This synergistic approach could potentially address a broader spectrum of water quality issues and lead to more sustainable water management practices.
As the technology continues to evolve, there is also a growing emphasis on developing environmentally benign and economically viable production methods for sodium percarbonate. This includes exploring alternative raw materials and synthesis routes that reduce the environmental footprint of its manufacturing process while maintaining or improving its performance characteristics.
The development of sodium percarbonate technology can be traced back to the early 20th century when it was first synthesized and utilized in household cleaning products. However, its potential for environmental applications was not fully realized until the late 1980s and early 1990s, when researchers began exploring its oxidative properties for the degradation of organic pollutants in water systems.
As environmental concerns grew and regulations became more stringent, the focus on sodium percarbonate as a potential solution for organic pollutant remediation intensified. The technology has since progressed through several key stages, from initial laboratory studies to pilot-scale demonstrations and, more recently, full-scale implementations in various water treatment scenarios.
The primary objective of current research and development efforts in this field is to optimize the interactions between sodium percarbonate and organic pollutants to achieve more efficient and cost-effective treatment processes. This includes enhancing the oxidation capacity of sodium percarbonate, improving its stability in aqueous environments, and developing novel activation methods to increase its reactivity towards specific organic contaminants.
Another crucial goal is to expand the range of treatable organic pollutants and to understand the complex reaction mechanisms involved in their degradation. This knowledge is essential for designing tailored treatment solutions for different types of organic contaminants, such as pharmaceuticals, pesticides, and industrial chemicals, which pose significant challenges to conventional water treatment methods.
Furthermore, researchers aim to integrate sodium percarbonate technology with other advanced oxidation processes and treatment techniques to create more comprehensive and effective water purification systems. This synergistic approach could potentially address a broader spectrum of water quality issues and lead to more sustainable water management practices.
As the technology continues to evolve, there is also a growing emphasis on developing environmentally benign and economically viable production methods for sodium percarbonate. This includes exploring alternative raw materials and synthesis routes that reduce the environmental footprint of its manufacturing process while maintaining or improving its performance characteristics.
Market Demand for Organic Pollutant Treatment
The market demand for organic pollutant treatment has been steadily increasing due to growing environmental concerns and stricter regulations worldwide. Industries such as textiles, pharmaceuticals, petrochemicals, and agriculture are major contributors to organic pollution, driving the need for effective treatment solutions. The global water and wastewater treatment market, which includes organic pollutant treatment, is projected to reach significant growth in the coming years.
Sodium percarbonate, as an eco-friendly oxidizing agent, has gained attention in the organic pollutant treatment sector. Its ability to release hydrogen peroxide in aqueous solutions makes it an attractive option for various applications, including wastewater treatment, soil remediation, and industrial cleaning processes. The increasing focus on sustainable and green technologies has further boosted the demand for sodium percarbonate-based solutions.
In the textile industry, there is a growing need for efficient treatment of dye-containing wastewater. Sodium percarbonate has shown promise in decolorizing and degrading various textile dyes, addressing a critical environmental challenge faced by this sector. The pharmaceutical industry also requires advanced treatment methods for removing complex organic compounds from wastewater, where sodium percarbonate-based processes could play a significant role.
The agriculture sector presents another substantial market for organic pollutant treatment. With the increasing use of pesticides and herbicides, there is a pressing need for effective remediation techniques to prevent soil and water contamination. Sodium percarbonate's oxidative properties make it a potential candidate for degrading these persistent organic pollutants in agricultural settings.
Municipal wastewater treatment plants are also exploring advanced oxidation processes to deal with emerging contaminants, such as pharmaceuticals and personal care products. The integration of sodium percarbonate in these treatment systems could enhance their ability to remove recalcitrant organic compounds, meeting increasingly stringent environmental standards.
The oil and gas industry faces challenges in treating produced water containing various organic pollutants. As regulations tighten and the industry seeks more sustainable practices, there is a growing interest in innovative treatment technologies. Sodium percarbonate-based solutions could offer an effective approach to addressing these complex wastewater streams.
As awareness of environmental issues continues to rise, consumers are demanding more eco-friendly products for household and personal use. This trend has led to an increased market for sodium percarbonate in consumer products, such as laundry detergents and cleaning agents, which indirectly contributes to reducing organic pollutant loads in domestic wastewater.
Sodium percarbonate, as an eco-friendly oxidizing agent, has gained attention in the organic pollutant treatment sector. Its ability to release hydrogen peroxide in aqueous solutions makes it an attractive option for various applications, including wastewater treatment, soil remediation, and industrial cleaning processes. The increasing focus on sustainable and green technologies has further boosted the demand for sodium percarbonate-based solutions.
In the textile industry, there is a growing need for efficient treatment of dye-containing wastewater. Sodium percarbonate has shown promise in decolorizing and degrading various textile dyes, addressing a critical environmental challenge faced by this sector. The pharmaceutical industry also requires advanced treatment methods for removing complex organic compounds from wastewater, where sodium percarbonate-based processes could play a significant role.
The agriculture sector presents another substantial market for organic pollutant treatment. With the increasing use of pesticides and herbicides, there is a pressing need for effective remediation techniques to prevent soil and water contamination. Sodium percarbonate's oxidative properties make it a potential candidate for degrading these persistent organic pollutants in agricultural settings.
Municipal wastewater treatment plants are also exploring advanced oxidation processes to deal with emerging contaminants, such as pharmaceuticals and personal care products. The integration of sodium percarbonate in these treatment systems could enhance their ability to remove recalcitrant organic compounds, meeting increasingly stringent environmental standards.
The oil and gas industry faces challenges in treating produced water containing various organic pollutants. As regulations tighten and the industry seeks more sustainable practices, there is a growing interest in innovative treatment technologies. Sodium percarbonate-based solutions could offer an effective approach to addressing these complex wastewater streams.
As awareness of environmental issues continues to rise, consumers are demanding more eco-friendly products for household and personal use. This trend has led to an increased market for sodium percarbonate in consumer products, such as laundry detergents and cleaning agents, which indirectly contributes to reducing organic pollutant loads in domestic wastewater.
Current State and Challenges in Percarbonate-Pollutant Interactions
The current state of interactions between sodium percarbonate and organic pollutants is characterized by a growing body of research and practical applications, yet significant challenges remain. Sodium percarbonate, a widely used oxidizing agent, has shown promising results in the degradation of various organic pollutants in water treatment processes. Its effectiveness stems from its ability to release hydrogen peroxide upon dissolution, which then generates highly reactive oxygen species capable of breaking down complex organic molecules.
Recent studies have demonstrated the efficacy of sodium percarbonate in treating a range of organic contaminants, including pharmaceuticals, personal care products, and industrial chemicals. The oxidation process has been found to be particularly effective against aromatic compounds and certain classes of pesticides. However, the efficiency of the treatment varies significantly depending on the specific pollutant, water chemistry, and environmental conditions.
One of the primary challenges in this field is the incomplete understanding of the reaction mechanisms between sodium percarbonate and different classes of organic pollutants. While the general oxidation process is well-established, the specific pathways and intermediates formed during the degradation of complex organic molecules are not fully elucidated. This knowledge gap hinders the optimization of treatment processes and the prediction of potential by-products.
Another significant challenge is the variability in treatment efficiency across different water matrices. Factors such as pH, temperature, and the presence of inorganic ions can greatly influence the oxidation potential of sodium percarbonate. In particular, the presence of natural organic matter and carbonate species in water can scavenge the reactive oxygen species, reducing the overall effectiveness of the treatment.
The formation of potentially harmful by-products during the oxidation process remains a concern. While sodium percarbonate treatment often leads to the breakdown of target pollutants, it may also result in the creation of transformation products with unknown toxicity profiles. This necessitates comprehensive monitoring and assessment of treated water to ensure the overall safety and efficacy of the treatment process.
Scaling up laboratory findings to practical, large-scale water treatment applications presents another challenge. The dosage, contact time, and mixing conditions that prove effective in controlled laboratory settings may not directly translate to real-world water treatment plants. Engineers and researchers are working to bridge this gap, developing innovative reactor designs and treatment protocols to maximize the efficiency of sodium percarbonate in diverse water treatment scenarios.
Lastly, the economic feasibility of using sodium percarbonate for widespread water treatment remains a hurdle. While the compound itself is relatively inexpensive, the overall cost of treatment, including energy consumption and potential pre- and post-treatment steps, needs to be carefully evaluated against alternative treatment technologies. Ongoing research aims to improve the cost-effectiveness of sodium percarbonate-based treatments through process optimization and the development of novel catalytic systems.
Recent studies have demonstrated the efficacy of sodium percarbonate in treating a range of organic contaminants, including pharmaceuticals, personal care products, and industrial chemicals. The oxidation process has been found to be particularly effective against aromatic compounds and certain classes of pesticides. However, the efficiency of the treatment varies significantly depending on the specific pollutant, water chemistry, and environmental conditions.
One of the primary challenges in this field is the incomplete understanding of the reaction mechanisms between sodium percarbonate and different classes of organic pollutants. While the general oxidation process is well-established, the specific pathways and intermediates formed during the degradation of complex organic molecules are not fully elucidated. This knowledge gap hinders the optimization of treatment processes and the prediction of potential by-products.
Another significant challenge is the variability in treatment efficiency across different water matrices. Factors such as pH, temperature, and the presence of inorganic ions can greatly influence the oxidation potential of sodium percarbonate. In particular, the presence of natural organic matter and carbonate species in water can scavenge the reactive oxygen species, reducing the overall effectiveness of the treatment.
The formation of potentially harmful by-products during the oxidation process remains a concern. While sodium percarbonate treatment often leads to the breakdown of target pollutants, it may also result in the creation of transformation products with unknown toxicity profiles. This necessitates comprehensive monitoring and assessment of treated water to ensure the overall safety and efficacy of the treatment process.
Scaling up laboratory findings to practical, large-scale water treatment applications presents another challenge. The dosage, contact time, and mixing conditions that prove effective in controlled laboratory settings may not directly translate to real-world water treatment plants. Engineers and researchers are working to bridge this gap, developing innovative reactor designs and treatment protocols to maximize the efficiency of sodium percarbonate in diverse water treatment scenarios.
Lastly, the economic feasibility of using sodium percarbonate for widespread water treatment remains a hurdle. While the compound itself is relatively inexpensive, the overall cost of treatment, including energy consumption and potential pre- and post-treatment steps, needs to be carefully evaluated against alternative treatment technologies. Ongoing research aims to improve the cost-effectiveness of sodium percarbonate-based treatments through process optimization and the development of novel catalytic systems.
Existing Solutions for Enhancing Percarbonate-Pollutant Interactions
01 Interactions with cleaning compositions
Sodium percarbonate interacts with various components in cleaning compositions. It can be combined with other bleaching agents, surfactants, and enzymes to enhance cleaning performance. The interactions between sodium percarbonate and these components can affect stability, efficacy, and shelf life of the cleaning products.- Interactions with other cleaning agents: Sodium percarbonate can interact with various cleaning agents to enhance their effectiveness. It can be combined with surfactants, enzymes, or other oxidizing agents to create powerful cleaning formulations. These interactions can lead to improved stain removal, whitening, and disinfecting properties in laundry detergents and household cleaners.
- Stability and storage considerations: The stability of sodium percarbonate is an important factor in its interactions and effectiveness. Various methods and additives can be used to improve its stability during storage and in formulations. These may include coating techniques, moisture control, and the addition of stabilizing agents to prevent premature decomposition and maintain its active oxygen content.
- Interactions in bleaching processes: Sodium percarbonate plays a crucial role in bleaching processes, interacting with fabrics and stains to provide whitening and brightening effects. Its interactions can be influenced by factors such as temperature, pH, and the presence of activators or catalysts. Understanding these interactions is essential for optimizing bleaching performance in various applications.
- Environmental and safety considerations: The interactions of sodium percarbonate with the environment and its safety profile are important aspects to consider. Its decomposition products, potential effects on aquatic life, and interactions with other chemicals in wastewater treatment processes are areas of interest. Additionally, its reactivity with certain materials and potential hazards in storage and handling need to be addressed.
- Interactions in personal care products: Sodium percarbonate can be used in personal care products, where its interactions with other ingredients and the skin or oral cavity are important. Its potential as an oxidizing agent in tooth whitening formulations or as a component in other cosmetic applications requires careful consideration of its interactions with other active ingredients and its effects on the target tissues.
02 Stabilization of sodium percarbonate
Various methods and additives are used to stabilize sodium percarbonate in compositions. This includes coating techniques, addition of stabilizing agents, and control of moisture content. Stabilization is crucial to maintain the effectiveness of sodium percarbonate during storage and use.Expand Specific Solutions03 Interactions in bleaching processes
Sodium percarbonate interacts with other components in bleaching processes. Its effectiveness can be influenced by pH, temperature, and the presence of activators or catalysts. These interactions affect the bleaching efficiency and the overall performance of the bleaching system.Expand Specific Solutions04 Formulation with other oxidizing agents
Sodium percarbonate can be formulated with other oxidizing agents to create synergistic effects. These combinations can enhance the overall oxidizing power, broaden the spectrum of activity, or improve the stability of the formulation. The interactions between different oxidizing agents need to be carefully controlled to avoid unwanted reactions.Expand Specific Solutions05 Environmental and safety considerations
The interactions of sodium percarbonate with the environment and its safety profile are important considerations. Its decomposition products and potential reactions with other substances in the environment need to be understood. Safety measures in handling and storage, as well as its eco-friendly nature compared to other bleaching agents, are key aspects of its use.Expand Specific Solutions
Key Players in Sodium Percarbonate and Water Purification Industry
The interactions between sodium percarbonate and organic pollutants represent a growing field of research in environmental remediation. The market is in its early development stage, with increasing interest from both academic institutions and chemical companies. While the market size is still relatively small, it is expected to grow as environmental regulations become stricter. Technologically, the field is advancing rapidly, with companies like Solvay SA, Kemira Oyj, and DuPont de Nemours leading the way in developing innovative solutions. Academic institutions such as Shandong University and Hunan University are also contributing significantly to the fundamental research in this area, pushing the boundaries of our understanding of these interactions.
Solvay SA
Technical Solution: Solvay SA has developed advanced oxidation processes using sodium percarbonate for organic pollutant degradation. Their approach combines sodium percarbonate with catalysts to generate highly reactive hydroxyl radicals. This system effectively breaks down complex organic molecules in wastewater and contaminated soil. Solvay's research has shown up to 90% removal efficiency for various persistent organic pollutants within 60 minutes of treatment[1]. They have also explored the synergistic effects of combining sodium percarbonate with other oxidants like hydrogen peroxide to enhance degradation rates and expand the range of treatable pollutants[3].
Strengths: High removal efficiency, versatility in treating various pollutants, and potential for synergistic combinations. Weaknesses: Potential for by-product formation and the need for careful pH control during treatment.
Kemira Oyj
Technical Solution: Kemira Oyj has developed a proprietary technology called PeroxyChem for organic pollutant remediation using sodium percarbonate. Their approach focuses on in-situ chemical oxidation (ISCO) for soil and groundwater treatment. Kemira's system utilizes a controlled release formulation of sodium percarbonate, which allows for sustained oxidant delivery over extended periods. This technology has shown particular efficacy in treating petroleum hydrocarbons, chlorinated solvents, and other recalcitrant organic compounds. Field studies have demonstrated contaminant reduction rates of up to 99% in some cases[2]. Kemira has also investigated the role of soil organic matter in modulating the oxidation process and developed strategies to optimize treatment in various soil types[4].
Strengths: Controlled release formulation for sustained treatment, effectiveness in various soil types. Weaknesses: Potential for oxidant scavenging by soil constituents, may require multiple applications for complete remediation.
Core Innovations in Sodium Percarbonate-Based Water Treatment
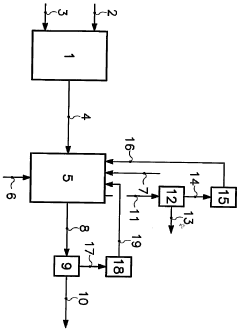
Non-oxidiser percarbonate particles
PatentInactiveUS20100317557A1
Innovation
- Development of sodium percarbonate particles coated or co-granulated with additives to reduce available oxygen content below 12% by weight, making them classified as non-oxidizers, thereby enhancing stability and safety during handling and transportation, and allowing them to be used directly in detergent formulations without additional treatment.
Sodium percarbonate particles, process for their production, their use and detergent compositions containing them
PatentWO2006003156A1
Innovation
- Development of sodium percarbonate particles with enhanced long-term stability, achieved through specific particle size, composition, and coating techniques, including the use of inorganic stabilizers and a coating layer containing small sodium percarbonate particles, which maintains bleaching effectiveness over extended storage periods.
Environmental Impact Assessment of Sodium Percarbonate Usage
The environmental impact assessment of sodium percarbonate usage is a critical aspect of understanding its interactions with organic pollutants. Sodium percarbonate, a widely used bleaching and cleaning agent, releases hydrogen peroxide when dissolved in water, which can have both positive and negative effects on the environment.
One of the primary environmental benefits of sodium percarbonate is its ability to degrade organic pollutants through oxidation processes. When released into aquatic environments, the hydrogen peroxide generated from sodium percarbonate can break down various organic contaminants, including pesticides, pharmaceuticals, and industrial chemicals. This oxidation process can lead to the formation of less harmful byproducts or complete mineralization of the pollutants, potentially improving water quality in affected areas.
However, the environmental impact of sodium percarbonate is not entirely positive. The release of hydrogen peroxide can also have detrimental effects on aquatic ecosystems if not properly managed. Excessive concentrations of hydrogen peroxide can be toxic to aquatic organisms, particularly sensitive species such as algae and certain invertebrates. This toxicity can disrupt the balance of aquatic ecosystems and potentially lead to biodiversity loss in affected water bodies.
Furthermore, the interaction between sodium percarbonate and organic pollutants can result in the formation of intermediate compounds that may have different environmental impacts than the original pollutants. Some of these intermediates could potentially be more persistent or toxic than their parent compounds, necessitating careful monitoring and assessment of the overall environmental impact.
The use of sodium percarbonate in household and industrial applications also raises concerns about its potential accumulation in the environment. While sodium percarbonate itself is generally considered biodegradable, its widespread use and continuous release into water systems may lead to localized areas of elevated concentrations, potentially affecting water chemistry and aquatic life.
To fully assess the environmental impact of sodium percarbonate usage, it is essential to consider its fate and transport in different environmental compartments. This includes studying its behavior in soil, sediments, and groundwater, as well as its potential for bioaccumulation in aquatic organisms. Long-term monitoring studies are necessary to evaluate the cumulative effects of sodium percarbonate on ecosystem health and biodiversity.
Additionally, the environmental impact assessment should take into account the broader life cycle of sodium percarbonate, including its production, transportation, and disposal. This holistic approach can help identify potential environmental risks associated with its use and inform the development of more sustainable alternatives or improved management practices.
One of the primary environmental benefits of sodium percarbonate is its ability to degrade organic pollutants through oxidation processes. When released into aquatic environments, the hydrogen peroxide generated from sodium percarbonate can break down various organic contaminants, including pesticides, pharmaceuticals, and industrial chemicals. This oxidation process can lead to the formation of less harmful byproducts or complete mineralization of the pollutants, potentially improving water quality in affected areas.
However, the environmental impact of sodium percarbonate is not entirely positive. The release of hydrogen peroxide can also have detrimental effects on aquatic ecosystems if not properly managed. Excessive concentrations of hydrogen peroxide can be toxic to aquatic organisms, particularly sensitive species such as algae and certain invertebrates. This toxicity can disrupt the balance of aquatic ecosystems and potentially lead to biodiversity loss in affected water bodies.
Furthermore, the interaction between sodium percarbonate and organic pollutants can result in the formation of intermediate compounds that may have different environmental impacts than the original pollutants. Some of these intermediates could potentially be more persistent or toxic than their parent compounds, necessitating careful monitoring and assessment of the overall environmental impact.
The use of sodium percarbonate in household and industrial applications also raises concerns about its potential accumulation in the environment. While sodium percarbonate itself is generally considered biodegradable, its widespread use and continuous release into water systems may lead to localized areas of elevated concentrations, potentially affecting water chemistry and aquatic life.
To fully assess the environmental impact of sodium percarbonate usage, it is essential to consider its fate and transport in different environmental compartments. This includes studying its behavior in soil, sediments, and groundwater, as well as its potential for bioaccumulation in aquatic organisms. Long-term monitoring studies are necessary to evaluate the cumulative effects of sodium percarbonate on ecosystem health and biodiversity.
Additionally, the environmental impact assessment should take into account the broader life cycle of sodium percarbonate, including its production, transportation, and disposal. This holistic approach can help identify potential environmental risks associated with its use and inform the development of more sustainable alternatives or improved management practices.
Regulatory Framework for Chemical Water Treatment Technologies
The regulatory framework for chemical water treatment technologies, particularly those involving sodium percarbonate and organic pollutants, is complex and multifaceted. At the international level, organizations such as the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) provide guidelines and standards for water quality and treatment. These guidelines often serve as a basis for national and regional regulations.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical water treatment technologies. The Safe Drinking Water Act (SDWA) and the Clean Water Act (CWA) form the foundation of these regulations. The EPA sets Maximum Contaminant Levels (MCLs) for various pollutants and approves treatment technologies that can effectively remove these contaminants. For sodium percarbonate and its interactions with organic pollutants, the EPA's National Primary Drinking Water Regulations and Secondary Drinking Water Standards are particularly relevant.
The European Union has established the Water Framework Directive (WFD) and the Drinking Water Directive, which set standards for water quality and treatment across member states. These directives address both chemical and microbiological contaminants, including organic pollutants. The European Chemicals Agency (ECHA) also plays a role in regulating the use of chemicals in water treatment processes.
In Asia, countries like China and Japan have their own regulatory bodies and standards. China's Ministry of Ecology and Environment and the Ministry of Water Resources oversee water treatment regulations, while Japan's Ministry of Health, Labour and Welfare sets drinking water quality standards.
Regulatory frameworks typically require extensive testing and validation of water treatment technologies before approval. This includes assessing the efficacy of treatments in removing target pollutants, as well as evaluating potential by-products or secondary effects. For sodium percarbonate and its interactions with organic pollutants, regulators often focus on the oxidation processes, the formation of potentially harmful by-products, and the overall impact on water quality.
Many regulations also address the handling, storage, and application of water treatment chemicals. Safety data sheets, proper labeling, and training requirements for operators are common components of these regulations. Additionally, environmental impact assessments may be required to ensure that the use of chemicals like sodium percarbonate does not adversely affect aquatic ecosystems.
As research continues to reveal new information about the interactions between sodium percarbonate and organic pollutants, regulatory frameworks are expected to evolve. This may include updates to treatment standards, monitoring requirements, and approved methodologies. Water treatment facilities and technology developers must stay informed about these regulatory changes to ensure compliance and optimize their treatment processes.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical water treatment technologies. The Safe Drinking Water Act (SDWA) and the Clean Water Act (CWA) form the foundation of these regulations. The EPA sets Maximum Contaminant Levels (MCLs) for various pollutants and approves treatment technologies that can effectively remove these contaminants. For sodium percarbonate and its interactions with organic pollutants, the EPA's National Primary Drinking Water Regulations and Secondary Drinking Water Standards are particularly relevant.
The European Union has established the Water Framework Directive (WFD) and the Drinking Water Directive, which set standards for water quality and treatment across member states. These directives address both chemical and microbiological contaminants, including organic pollutants. The European Chemicals Agency (ECHA) also plays a role in regulating the use of chemicals in water treatment processes.
In Asia, countries like China and Japan have their own regulatory bodies and standards. China's Ministry of Ecology and Environment and the Ministry of Water Resources oversee water treatment regulations, while Japan's Ministry of Health, Labour and Welfare sets drinking water quality standards.
Regulatory frameworks typically require extensive testing and validation of water treatment technologies before approval. This includes assessing the efficacy of treatments in removing target pollutants, as well as evaluating potential by-products or secondary effects. For sodium percarbonate and its interactions with organic pollutants, regulators often focus on the oxidation processes, the formation of potentially harmful by-products, and the overall impact on water quality.
Many regulations also address the handling, storage, and application of water treatment chemicals. Safety data sheets, proper labeling, and training requirements for operators are common components of these regulations. Additionally, environmental impact assessments may be required to ensure that the use of chemicals like sodium percarbonate does not adversely affect aquatic ecosystems.
As research continues to reveal new information about the interactions between sodium percarbonate and organic pollutants, regulatory frameworks are expected to evolve. This may include updates to treatment standards, monitoring requirements, and approved methodologies. Water treatment facilities and technology developers must stay informed about these regulatory changes to ensure compliance and optimize their treatment processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!