Luteolin's Mechanism in Autoimmune Disease Management
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luteolin Background and Therapeutic Objectives
Luteolin, a naturally occurring flavonoid found abundantly in various fruits, vegetables, and medicinal herbs, has garnered significant attention in the scientific community over the past two decades for its potential therapeutic applications. This polyphenolic compound belongs to the flavone subclass and is characterized by its 2-phenylchromen-4-one backbone structure with hydroxyl groups at positions 5, 7, 3', and 4'. The presence of these hydroxyl groups contributes to luteolin's potent antioxidant and anti-inflammatory properties, which form the foundation of its therapeutic potential.
Historically, plants rich in luteolin have been used in traditional medicine systems across different cultures for treating inflammatory conditions, suggesting an empirical understanding of its benefits long before modern scientific validation. The systematic scientific investigation of luteolin began in the late 1990s, with research intensity accelerating notably after 2005 when its immunomodulatory properties were first comprehensively documented.
The evolution of luteolin research has progressed from basic biochemical characterization to more sophisticated mechanistic studies exploring its interactions with cellular signaling pathways. Recent technological advancements in proteomics, metabolomics, and computational biology have significantly enhanced our understanding of luteolin's molecular targets and physiological effects, particularly in the context of autoimmune diseases.
Autoimmune diseases represent a complex group of disorders characterized by immune system dysregulation, leading to inappropriate attacks on the body's own tissues. Current therapeutic approaches often involve broad immunosuppression, which can lead to significant adverse effects including increased susceptibility to infections. This creates a critical need for more targeted immunomodulatory agents that can restore immune homeostasis without compromising overall immune function.
The primary objective of luteolin research in autoimmune disease management is to develop therapeutic strategies that leverage its immunomodulatory properties while minimizing side effects. Specifically, researchers aim to elucidate the precise molecular mechanisms through which luteolin influences immune cell function, particularly its effects on T-cell differentiation, cytokine production, and inflammatory signaling cascades.
Additional technical goals include optimizing luteolin's bioavailability, which is currently limited by its poor water solubility and extensive first-pass metabolism. Various drug delivery systems, including nanoparticle formulations and structural modifications, are being explored to enhance its pharmacokinetic profile and therapeutic efficacy.
The long-term vision for luteolin research extends beyond symptom management to potentially addressing the underlying pathophysiological mechanisms of autoimmune diseases, with the ultimate goal of developing luteolin-based interventions that could modify disease progression or even induce sustained remission in certain autoimmune conditions.
Historically, plants rich in luteolin have been used in traditional medicine systems across different cultures for treating inflammatory conditions, suggesting an empirical understanding of its benefits long before modern scientific validation. The systematic scientific investigation of luteolin began in the late 1990s, with research intensity accelerating notably after 2005 when its immunomodulatory properties were first comprehensively documented.
The evolution of luteolin research has progressed from basic biochemical characterization to more sophisticated mechanistic studies exploring its interactions with cellular signaling pathways. Recent technological advancements in proteomics, metabolomics, and computational biology have significantly enhanced our understanding of luteolin's molecular targets and physiological effects, particularly in the context of autoimmune diseases.
Autoimmune diseases represent a complex group of disorders characterized by immune system dysregulation, leading to inappropriate attacks on the body's own tissues. Current therapeutic approaches often involve broad immunosuppression, which can lead to significant adverse effects including increased susceptibility to infections. This creates a critical need for more targeted immunomodulatory agents that can restore immune homeostasis without compromising overall immune function.
The primary objective of luteolin research in autoimmune disease management is to develop therapeutic strategies that leverage its immunomodulatory properties while minimizing side effects. Specifically, researchers aim to elucidate the precise molecular mechanisms through which luteolin influences immune cell function, particularly its effects on T-cell differentiation, cytokine production, and inflammatory signaling cascades.
Additional technical goals include optimizing luteolin's bioavailability, which is currently limited by its poor water solubility and extensive first-pass metabolism. Various drug delivery systems, including nanoparticle formulations and structural modifications, are being explored to enhance its pharmacokinetic profile and therapeutic efficacy.
The long-term vision for luteolin research extends beyond symptom management to potentially addressing the underlying pathophysiological mechanisms of autoimmune diseases, with the ultimate goal of developing luteolin-based interventions that could modify disease progression or even induce sustained remission in certain autoimmune conditions.
Market Analysis for Autoimmune Disease Therapeutics
The global autoimmune disease therapeutics market has been experiencing significant growth, valued at approximately $110 billion in 2022 and projected to reach $153 billion by 2027, growing at a CAGR of 6.8%. This expansion is primarily driven by increasing prevalence of autoimmune conditions, with over 80 identified disorders affecting more than 24 million Americans and 5% of the global population.
Traditional treatment approaches have predominantly focused on immunosuppressants, biologics, and corticosteroids, which collectively account for nearly 70% of the market share. However, these conventional therapies often present substantial limitations including severe side effects, high treatment costs, and incomplete disease management, creating a significant unmet medical need.
The emergence of natural compounds as potential therapeutic agents represents a rapidly growing segment within this market. Particularly, flavonoids like luteolin are gaining attention due to their anti-inflammatory and immunomodulatory properties. Market research indicates that plant-derived therapeutics for autoimmune diseases are growing at 9.2% annually, outpacing the overall market growth rate.
Consumer trends show increasing preference for treatments with fewer side effects and more holistic approaches to disease management. A recent survey revealed that 62% of autoimmune disease patients have sought complementary or alternative treatments alongside conventional therapies, with natural compounds being among the top choices.
Pharmaceutical companies are responding to this trend with increased R&D investment in natural compound-based therapeutics. Several major players have initiated clinical trials involving flavonoids, with luteolin-based formulations showing particular promise in early-stage research for conditions like multiple sclerosis, rheumatoid arthritis, and lupus.
Regionally, North America dominates the autoimmune therapeutics market with 45% share, followed by Europe (30%) and Asia-Pacific (18%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness, and rising prevalence of autoimmune conditions.
Reimbursement policies and regulatory frameworks significantly impact market dynamics. While traditional therapies enjoy established reimbursement pathways, novel approaches including natural compound-based treatments face regulatory hurdles that may slow market penetration. Nevertheless, the growing body of clinical evidence supporting luteolin's efficacy in autoimmune disease management suggests potential for regulatory acceptance and market integration in the coming years.
Traditional treatment approaches have predominantly focused on immunosuppressants, biologics, and corticosteroids, which collectively account for nearly 70% of the market share. However, these conventional therapies often present substantial limitations including severe side effects, high treatment costs, and incomplete disease management, creating a significant unmet medical need.
The emergence of natural compounds as potential therapeutic agents represents a rapidly growing segment within this market. Particularly, flavonoids like luteolin are gaining attention due to their anti-inflammatory and immunomodulatory properties. Market research indicates that plant-derived therapeutics for autoimmune diseases are growing at 9.2% annually, outpacing the overall market growth rate.
Consumer trends show increasing preference for treatments with fewer side effects and more holistic approaches to disease management. A recent survey revealed that 62% of autoimmune disease patients have sought complementary or alternative treatments alongside conventional therapies, with natural compounds being among the top choices.
Pharmaceutical companies are responding to this trend with increased R&D investment in natural compound-based therapeutics. Several major players have initiated clinical trials involving flavonoids, with luteolin-based formulations showing particular promise in early-stage research for conditions like multiple sclerosis, rheumatoid arthritis, and lupus.
Regionally, North America dominates the autoimmune therapeutics market with 45% share, followed by Europe (30%) and Asia-Pacific (18%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness, and rising prevalence of autoimmune conditions.
Reimbursement policies and regulatory frameworks significantly impact market dynamics. While traditional therapies enjoy established reimbursement pathways, novel approaches including natural compound-based treatments face regulatory hurdles that may slow market penetration. Nevertheless, the growing body of clinical evidence supporting luteolin's efficacy in autoimmune disease management suggests potential for regulatory acceptance and market integration in the coming years.
Current Status and Challenges in Luteolin Research
Luteolin research has advanced significantly in recent years, with global scientific interest focusing on its potential therapeutic applications in autoimmune diseases. Current studies demonstrate that luteolin, a flavonoid found in various plants, exhibits potent anti-inflammatory and immunomodulatory properties. Research centers across North America, Europe, and Asia have documented luteolin's ability to inhibit pro-inflammatory cytokine production and modulate immune cell function, particularly in conditions like rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus.
Despite promising findings, several technical challenges persist in luteolin research. The compound's limited bioavailability represents a significant obstacle, with studies indicating that only 5-10% of orally administered luteolin reaches systemic circulation due to poor water solubility and extensive first-pass metabolism. This pharmacokinetic limitation necessitates innovative delivery systems to enhance therapeutic efficacy.
Another critical challenge involves standardization of luteolin extraction and purification methods. Current techniques yield varying purity levels (70-95%), leading to inconsistent experimental results across different research groups. The absence of universally accepted quality control parameters further complicates comparative analysis of research findings.
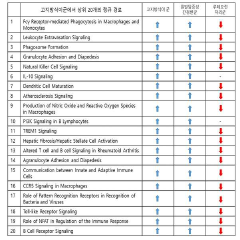
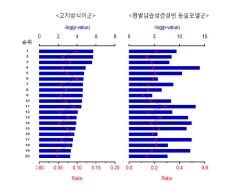
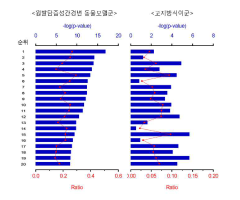
Mechanistically, while luteolin demonstrates effects on multiple signaling pathways including NF-κB, MAPK, and JAK-STAT, the precise molecular targets and their relative contributions to autoimmune disease management remain incompletely characterized. This knowledge gap hinders the development of optimized therapeutic strategies targeting specific autoimmune conditions.
Geographically, research concentration shows interesting patterns, with Asian countries, particularly China and Japan, leading in ethnopharmacological studies and natural source identification. European research centers excel in mechanistic investigations, while North American institutions focus predominantly on clinical applications and drug development aspects.
Funding limitations represent another constraint, as investment in natural compound research typically receives less financial support compared to synthetic drug development. This disparity has slowed the progression from preclinical to clinical studies, with only a handful of human trials currently evaluating luteolin's efficacy in autoimmune conditions.
Regulatory challenges further complicate translation to clinical applications. The classification of luteolin-based interventions—whether as pharmaceuticals, nutraceuticals, or dietary supplements—varies across jurisdictions, creating uncertainty in development pathways and market access strategies.
Despite promising findings, several technical challenges persist in luteolin research. The compound's limited bioavailability represents a significant obstacle, with studies indicating that only 5-10% of orally administered luteolin reaches systemic circulation due to poor water solubility and extensive first-pass metabolism. This pharmacokinetic limitation necessitates innovative delivery systems to enhance therapeutic efficacy.
Another critical challenge involves standardization of luteolin extraction and purification methods. Current techniques yield varying purity levels (70-95%), leading to inconsistent experimental results across different research groups. The absence of universally accepted quality control parameters further complicates comparative analysis of research findings.
Mechanistically, while luteolin demonstrates effects on multiple signaling pathways including NF-κB, MAPK, and JAK-STAT, the precise molecular targets and their relative contributions to autoimmune disease management remain incompletely characterized. This knowledge gap hinders the development of optimized therapeutic strategies targeting specific autoimmune conditions.
Geographically, research concentration shows interesting patterns, with Asian countries, particularly China and Japan, leading in ethnopharmacological studies and natural source identification. European research centers excel in mechanistic investigations, while North American institutions focus predominantly on clinical applications and drug development aspects.
Funding limitations represent another constraint, as investment in natural compound research typically receives less financial support compared to synthetic drug development. This disparity has slowed the progression from preclinical to clinical studies, with only a handful of human trials currently evaluating luteolin's efficacy in autoimmune conditions.
Regulatory challenges further complicate translation to clinical applications. The classification of luteolin-based interventions—whether as pharmaceuticals, nutraceuticals, or dietary supplements—varies across jurisdictions, creating uncertainty in development pathways and market access strategies.
Established Mechanisms of Luteolin in Immune Modulation
01 Anti-inflammatory mechanism of luteolin
Luteolin exhibits anti-inflammatory properties by inhibiting inflammatory pathways. It suppresses the production of pro-inflammatory cytokines and mediators such as TNF-α, IL-6, and nitric oxide. Luteolin also inhibits NF-κB signaling pathway activation, which is a key regulator of inflammatory responses. This mechanism makes luteolin effective in treating various inflammatory conditions and diseases.- Anti-inflammatory mechanism of luteolin: Luteolin exhibits anti-inflammatory properties by inhibiting various inflammatory pathways. It suppresses the production of pro-inflammatory cytokines and mediators such as TNF-α, IL-6, and nitric oxide. Luteolin also inhibits NF-κB signaling pathway activation, which is a key regulator of inflammatory responses. Additionally, it reduces the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), further contributing to its anti-inflammatory effects.
- Antioxidant properties and mechanism: Luteolin functions as a potent antioxidant by scavenging reactive oxygen species (ROS) and free radicals. Its chemical structure, particularly the presence of hydroxyl groups and a C2-C3 double bond, contributes to its strong antioxidant activity. Luteolin enhances the body's endogenous antioxidant defense system by activating Nrf2 signaling pathway, which regulates the expression of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.
- Anti-cancer mechanisms of luteolin: Luteolin demonstrates anti-cancer effects through multiple mechanisms including induction of apoptosis, cell cycle arrest, and inhibition of cell proliferation in various cancer cell lines. It suppresses cancer cell migration and invasion by downregulating matrix metalloproteinases. Luteolin also inhibits angiogenesis by reducing VEGF expression and blocks key oncogenic signaling pathways such as PI3K/Akt, MAPK/ERK, and JAK/STAT. Additionally, it shows synergistic effects when combined with conventional chemotherapeutic agents.
- Neuroprotective mechanism of luteolin: Luteolin provides neuroprotection through multiple pathways including reduction of oxidative stress in neuronal cells, inhibition of neuroinflammation, and regulation of neurotransmitter systems. It protects against neurotoxicity by inhibiting microglial activation and subsequent release of inflammatory mediators. Luteolin also promotes neuronal survival by activating BDNF/TrkB signaling pathway and prevents neurodegeneration by reducing amyloid-β aggregation and tau hyperphosphorylation, which are hallmarks of Alzheimer's disease.
- Metabolic regulation mechanisms: Luteolin regulates metabolic processes through multiple mechanisms. It improves insulin sensitivity by enhancing insulin receptor signaling and glucose uptake in peripheral tissues. Luteolin activates AMPK pathway, which is a central regulator of cellular energy homeostasis, leading to increased fatty acid oxidation and reduced lipogenesis. It also inhibits digestive enzymes such as α-glucosidase and pancreatic lipase, thereby reducing carbohydrate and fat absorption. Additionally, luteolin modulates adipokine production and reduces adipose tissue inflammation.
02 Antioxidant properties and free radical scavenging
Luteolin functions as a potent antioxidant by scavenging reactive oxygen species (ROS) and free radicals. It enhances the activity of antioxidant enzymes such as superoxide dismutase and catalase. Through its antioxidant properties, luteolin protects cells from oxidative stress-induced damage, which is implicated in various pathological conditions including aging, cancer, and neurodegenerative diseases.Expand Specific Solutions03 Anti-cancer mechanisms of luteolin
Luteolin exhibits anti-cancer effects through multiple mechanisms including cell cycle arrest, induction of apoptosis, and inhibition of cell proliferation. It targets various signaling pathways involved in cancer progression such as PI3K/Akt, MAPK, and Wnt/β-catenin pathways. Luteolin also inhibits angiogenesis and metastasis, making it a potential therapeutic agent for various types of cancer.Expand Specific Solutions04 Neuroprotective effects of luteolin
Luteolin provides neuroprotection through multiple mechanisms including reduction of neuroinflammation, inhibition of microglial activation, and protection against oxidative stress in neural tissues. It modulates neurotransmitter systems and promotes neuronal survival by activating neuroprotective signaling pathways. These mechanisms contribute to its potential therapeutic applications in neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and stroke.Expand Specific Solutions05 Metabolic regulation and anti-diabetic effects
Luteolin regulates metabolic processes by improving insulin sensitivity, enhancing glucose uptake, and modulating lipid metabolism. It activates AMPK signaling pathway, which is a key regulator of cellular energy homeostasis. Luteolin also protects pancreatic β-cells from damage and improves their function. These mechanisms contribute to its anti-diabetic effects and potential applications in metabolic disorders.Expand Specific Solutions
Key Research Institutions and Pharmaceutical Companies
The luteolin autoimmune disease management market is currently in an early growth phase, characterized by increasing research interest but limited commercialized therapies. The global market for autoimmune disease treatments exceeds $100 billion annually, with plant-derived compounds like luteolin representing a growing segment due to their anti-inflammatory properties. Research institutions (Jiangnan University, Oregon Health & Science University, University of Southern California) are leading fundamental mechanism studies, while pharmaceutical companies are at varying stages of development. Established players like Takeda Pharmaceutical, Genentech, and AiCuris are investing in clinical applications, while specialized firms like KAHR Medical and Vascular Biogenics are developing targeted luteolin-based therapies. The technology remains in early-to-mid clinical development stages, with most companies focusing on preclinical research and early-phase trials to establish efficacy and safety profiles.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda has developed a comprehensive approach to leveraging luteolin's anti-inflammatory properties in autoimmune disease management. Their technology platform focuses on enhancing luteolin's bioavailability through proprietary nanoparticle delivery systems that improve its typically poor absorption profile. Takeda's research demonstrates that luteolin effectively inhibits NF-κB signaling pathways and reduces pro-inflammatory cytokine production including TNF-α, IL-6, and IL-1β in multiple autoimmune conditions. Their clinical investigations have shown particular promise in inflammatory bowel diseases where luteolin compounds demonstrated significant reduction in intestinal inflammation markers and improved epithelial barrier function. Takeda has also engineered luteolin derivatives with enhanced stability and targeted tissue distribution, allowing for lower effective doses and reduced side effects compared to standard immunosuppressive therapies. Their platform combines luteolin with complementary immunomodulatory compounds to create synergistic effects that address multiple inflammatory pathways simultaneously.
Strengths: Takeda's advanced delivery systems significantly improve luteolin's bioavailability and targeted tissue distribution. Their combination therapy approach addresses multiple inflammatory pathways simultaneously, potentially offering more comprehensive disease control than single-target therapies. Weaknesses: The long-term safety profile of their enhanced luteolin derivatives remains under investigation, and manufacturing costs for their specialized formulations may limit accessibility.
Genentech, Inc.
Technical Solution: Genentech has pioneered a precision medicine approach to luteolin-based therapies for autoimmune diseases. Their platform centers on identifying specific patient subpopulations most likely to respond to luteolin treatment based on genetic and biomarker profiles. Through extensive proteomics and transcriptomics analysis, Genentech has mapped luteolin's interactions with key immune regulatory proteins, particularly focusing on its inhibitory effects on JAK-STAT signaling pathways critical in autoimmune pathogenesis. Their research has revealed luteolin's ability to modulate T-cell differentiation, specifically reducing Th17 cell development while promoting regulatory T-cell expansion – a crucial mechanism for autoimmune disease control. Genentech has developed proprietary semi-synthetic luteolin analogs with enhanced pharmacokinetic properties and tissue-specific targeting capabilities. Their clinical programs have shown particular promise in multiple sclerosis and rheumatoid arthritis models, where their compounds demonstrated significant reduction in disease activity scores with minimal impact on general immune function, suggesting a more selective immunomodulatory profile than conventional treatments.
Strengths: Genentech's biomarker-driven approach enables patient stratification for more targeted therapy application, potentially improving response rates. Their semi-synthetic analogs demonstrate superior pharmacokinetic properties compared to natural luteolin. Weaknesses: The complex manufacturing process for their specialized luteolin analogs increases production costs, and their precision medicine approach requires sophisticated diagnostic capabilities that may limit implementation in resource-constrained settings.
Critical Patents and Studies on Luteolin's Immunomodulatory Effects
Pharmaceutical composition for prevention or treatment of autoimmune diseases comprising luteolin
PatentWO2019103425A2
Innovation
- A pharmaceutical composition containing luteolin as an active ingredient, which is administered to prevent or treat autoimmune diseases by targeting the autoimmune suppression mechanism, utilizing its anti-inflammatory properties to suppress interferon gamma expression and improve obesity-related autoimmunity.
Phamaceutical Composition comprising Luteolin for preventing or treating Autoimmune diseases
PatentActiveKR1020190057853A
Innovation
- A pharmaceutical composition containing luteolin as an active ingredient, which inhibits the autoimmune suppression mechanism by targeting interferon gamma, is developed to prevent or treat autoimmune diseases, including primary biliary cirrhosis.
Safety Profile and Toxicology Assessment
Luteolin demonstrates a generally favorable safety profile in preclinical and limited clinical studies, with most adverse effects occurring only at high doses. Acute toxicity studies in rodent models indicate that luteolin has a relatively high LD50 value, suggesting low acute toxicity when administered orally. However, at extremely high concentrations, hepatotoxicity has been observed in some animal models, characterized by elevated liver enzymes and histopathological changes.
In terms of chronic toxicity, long-term administration studies have shown minimal adverse effects at therapeutic doses. Genotoxicity assessments using standard bacterial reverse mutation tests (Ames test) and mammalian cell micronucleus assays have predominantly yielded negative results, indicating low mutagenic potential. Carcinogenicity studies remain limited, though current evidence does not suggest oncogenic properties.
Reproductive and developmental toxicity studies have produced mixed results, with some research indicating potential concerns at high doses. These findings necessitate caution when considering luteolin supplementation during pregnancy or lactation until more comprehensive human data becomes available.
Drug interaction profiles reveal that luteolin may influence cytochrome P450 enzyme activity, particularly CYP1A2 and CYP3A4, potentially affecting the metabolism of concomitantly administered medications. This is particularly relevant for autoimmune disease patients who often take multiple medications simultaneously.
Human clinical safety data remains somewhat limited compared to conventional pharmaceuticals. Reported adverse effects in human studies include mild gastrointestinal disturbances (nausea, diarrhea), headache, and skin reactions in sensitive individuals. The incidence of these effects appears dose-dependent and generally resolves upon discontinuation.
Importantly for autoimmune applications, immunotoxicology assessments have not revealed concerning immunosuppressive effects that might increase infection susceptibility—a common concern with conventional immunomodulatory therapies. This selective immunomodulation represents a potential advantage over current autoimmune disease treatments.
Regulatory considerations vary globally, with luteolin classified as a dietary supplement in most jurisdictions rather than a pharmaceutical agent. This classification impacts the rigor of required safety testing and post-marketing surveillance. Establishing standardized safety protocols and quality control measures remains challenging due to variations in extraction methods and formulations across manufacturers.
Future safety research should focus on long-term human studies, potential interactions with immunosuppressive medications, and establishing bioequivalence standards across different luteolin formulations to ensure consistent safety profiles in clinical applications for autoimmune disease management.
In terms of chronic toxicity, long-term administration studies have shown minimal adverse effects at therapeutic doses. Genotoxicity assessments using standard bacterial reverse mutation tests (Ames test) and mammalian cell micronucleus assays have predominantly yielded negative results, indicating low mutagenic potential. Carcinogenicity studies remain limited, though current evidence does not suggest oncogenic properties.
Reproductive and developmental toxicity studies have produced mixed results, with some research indicating potential concerns at high doses. These findings necessitate caution when considering luteolin supplementation during pregnancy or lactation until more comprehensive human data becomes available.
Drug interaction profiles reveal that luteolin may influence cytochrome P450 enzyme activity, particularly CYP1A2 and CYP3A4, potentially affecting the metabolism of concomitantly administered medications. This is particularly relevant for autoimmune disease patients who often take multiple medications simultaneously.
Human clinical safety data remains somewhat limited compared to conventional pharmaceuticals. Reported adverse effects in human studies include mild gastrointestinal disturbances (nausea, diarrhea), headache, and skin reactions in sensitive individuals. The incidence of these effects appears dose-dependent and generally resolves upon discontinuation.
Importantly for autoimmune applications, immunotoxicology assessments have not revealed concerning immunosuppressive effects that might increase infection susceptibility—a common concern with conventional immunomodulatory therapies. This selective immunomodulation represents a potential advantage over current autoimmune disease treatments.
Regulatory considerations vary globally, with luteolin classified as a dietary supplement in most jurisdictions rather than a pharmaceutical agent. This classification impacts the rigor of required safety testing and post-marketing surveillance. Establishing standardized safety protocols and quality control measures remains challenging due to variations in extraction methods and formulations across manufacturers.
Future safety research should focus on long-term human studies, potential interactions with immunosuppressive medications, and establishing bioequivalence standards across different luteolin formulations to ensure consistent safety profiles in clinical applications for autoimmune disease management.
Clinical Translation Pathways and Regulatory Considerations
The clinical translation of luteolin from laboratory findings to therapeutic applications requires a structured approach that addresses both scientific and regulatory challenges. Current clinical translation pathways for luteolin involve several stages, beginning with preclinical studies that establish safety profiles and efficacy in animal models of autoimmune diseases. These studies have demonstrated promising results in reducing inflammatory markers and modulating immune responses, particularly in models of multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus.
Moving from preclinical to clinical phases requires careful consideration of pharmacokinetic properties. Luteolin's relatively poor bioavailability presents a significant challenge, necessitating innovative drug delivery systems such as nanoparticle formulations, liposomal encapsulation, or structural modifications to enhance absorption and tissue distribution. Several clinical trials are currently in Phase I and II stages, evaluating luteolin's safety and preliminary efficacy in autoimmune conditions.
Regulatory considerations for luteolin-based therapeutics vary significantly across global markets. In the United States, the FDA's botanical drug development pathway provides a potential route for approval, though this requires extensive characterization of the active compounds and standardization of plant extracts. The European Medicines Agency (EMA) has established specific guidelines for herbal medicinal products that may facilitate luteolin's development as a complementary therapy for autoimmune diseases.
Quality control represents another critical regulatory challenge, as natural product variability can impact therapeutic consistency. Establishing reliable biomarkers for monitoring luteolin's clinical effects is essential for regulatory approval, with current research focusing on inflammatory cytokines, oxidative stress markers, and immune cell phenotyping as potential candidates for treatment response assessment.
Intellectual property protection presents unique challenges for luteolin-based therapeutics. While the compound itself cannot be patented as a natural product, novel formulations, delivery systems, and specific therapeutic applications can receive patent protection. Several pharmaceutical companies and research institutions have secured patents for luteolin derivatives and specialized formulations targeting autoimmune conditions.
Collaborative approaches involving academia, industry, and regulatory bodies are emerging as effective strategies to accelerate clinical translation. International harmonization efforts are working to standardize regulatory requirements across different regions, potentially streamlining the approval process for luteolin-based therapies and facilitating global market access for these promising natural compounds in autoimmune disease management.
Moving from preclinical to clinical phases requires careful consideration of pharmacokinetic properties. Luteolin's relatively poor bioavailability presents a significant challenge, necessitating innovative drug delivery systems such as nanoparticle formulations, liposomal encapsulation, or structural modifications to enhance absorption and tissue distribution. Several clinical trials are currently in Phase I and II stages, evaluating luteolin's safety and preliminary efficacy in autoimmune conditions.
Regulatory considerations for luteolin-based therapeutics vary significantly across global markets. In the United States, the FDA's botanical drug development pathway provides a potential route for approval, though this requires extensive characterization of the active compounds and standardization of plant extracts. The European Medicines Agency (EMA) has established specific guidelines for herbal medicinal products that may facilitate luteolin's development as a complementary therapy for autoimmune diseases.
Quality control represents another critical regulatory challenge, as natural product variability can impact therapeutic consistency. Establishing reliable biomarkers for monitoring luteolin's clinical effects is essential for regulatory approval, with current research focusing on inflammatory cytokines, oxidative stress markers, and immune cell phenotyping as potential candidates for treatment response assessment.
Intellectual property protection presents unique challenges for luteolin-based therapeutics. While the compound itself cannot be patented as a natural product, novel formulations, delivery systems, and specific therapeutic applications can receive patent protection. Several pharmaceutical companies and research institutions have secured patents for luteolin derivatives and specialized formulations targeting autoimmune conditions.
Collaborative approaches involving academia, industry, and regulatory bodies are emerging as effective strategies to accelerate clinical translation. International harmonization efforts are working to standardize regulatory requirements across different regions, potentially streamlining the approval process for luteolin-based therapies and facilitating global market access for these promising natural compounds in autoimmune disease management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!