MSH-assisted heavy metal extraction from aqueous solutions.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MSH-assisted Extraction Background and Objectives
The extraction of heavy metals from aqueous solutions has become a critical environmental and industrial challenge in recent years. As global industrialization continues to expand, the release of heavy metals into water bodies has increased significantly, posing severe threats to ecosystems and human health. Traditional methods of heavy metal removal, such as chemical precipitation, ion exchange, and membrane filtration, often face limitations in efficiency, cost-effectiveness, and environmental sustainability.
In this context, the emergence of MSH-assisted heavy metal extraction represents a promising technological advancement. MSH, or Magnetic Separable Hydroxyapatite, combines the unique properties of hydroxyapatite with magnetic characteristics, offering a novel approach to heavy metal removal. This technology leverages the high adsorption capacity of hydroxyapatite for various metal ions and the ease of separation provided by magnetic properties.
The development of MSH-assisted extraction techniques has evolved over the past decade, driven by the need for more efficient and environmentally friendly water treatment methods. Initial research focused on synthesizing MSH particles with optimal adsorption and magnetic properties. Subsequent studies have explored various modifications to enhance performance, including doping with other elements and surface functionalization.
The primary objective of MSH-assisted heavy metal extraction is to achieve high removal efficiency of a wide range of heavy metals from aqueous solutions while maintaining ease of operation and cost-effectiveness. Researchers aim to develop MSH materials that exhibit strong affinity for multiple heavy metal ions, rapid adsorption kinetics, and excellent magnetic responsiveness for easy separation.
Another crucial goal is to enhance the reusability of MSH adsorbents, as this significantly impacts the economic viability and sustainability of the technology. Efforts are being made to improve the regeneration processes and maintain high adsorption capacity over multiple cycles of use.
Furthermore, the technology seeks to address the challenges of scalability and integration into existing water treatment systems. This includes optimizing particle size and morphology for improved performance in various water conditions and developing methods for large-scale synthesis of MSH materials.
As environmental regulations become increasingly stringent, there is also a focus on ensuring that MSH-assisted extraction meets or exceeds current and future regulatory standards for water quality. This drives research towards achieving ultra-low residual concentrations of heavy metals in treated water.
In this context, the emergence of MSH-assisted heavy metal extraction represents a promising technological advancement. MSH, or Magnetic Separable Hydroxyapatite, combines the unique properties of hydroxyapatite with magnetic characteristics, offering a novel approach to heavy metal removal. This technology leverages the high adsorption capacity of hydroxyapatite for various metal ions and the ease of separation provided by magnetic properties.
The development of MSH-assisted extraction techniques has evolved over the past decade, driven by the need for more efficient and environmentally friendly water treatment methods. Initial research focused on synthesizing MSH particles with optimal adsorption and magnetic properties. Subsequent studies have explored various modifications to enhance performance, including doping with other elements and surface functionalization.
The primary objective of MSH-assisted heavy metal extraction is to achieve high removal efficiency of a wide range of heavy metals from aqueous solutions while maintaining ease of operation and cost-effectiveness. Researchers aim to develop MSH materials that exhibit strong affinity for multiple heavy metal ions, rapid adsorption kinetics, and excellent magnetic responsiveness for easy separation.
Another crucial goal is to enhance the reusability of MSH adsorbents, as this significantly impacts the economic viability and sustainability of the technology. Efforts are being made to improve the regeneration processes and maintain high adsorption capacity over multiple cycles of use.
Furthermore, the technology seeks to address the challenges of scalability and integration into existing water treatment systems. This includes optimizing particle size and morphology for improved performance in various water conditions and developing methods for large-scale synthesis of MSH materials.
As environmental regulations become increasingly stringent, there is also a focus on ensuring that MSH-assisted extraction meets or exceeds current and future regulatory standards for water quality. This drives research towards achieving ultra-low residual concentrations of heavy metals in treated water.
Market Analysis for Heavy Metal Removal Solutions
The global market for heavy metal removal solutions has been experiencing significant growth due to increasing environmental concerns and stringent regulations regarding water pollution. The demand for effective and efficient heavy metal extraction technologies, such as MSH-assisted methods, is driven by the growing awareness of the detrimental effects of heavy metal contamination on human health and ecosystems.
Industrial sectors, including mining, metallurgy, electronics manufacturing, and chemical processing, are the primary contributors to heavy metal pollution in aqueous environments. These industries are increasingly seeking advanced solutions to meet regulatory requirements and improve their environmental performance. The market for heavy metal removal technologies is expected to expand further as developing countries implement more stringent environmental policies and developed nations continue to upgrade their water treatment infrastructure.
The Asia-Pacific region, particularly China and India, represents the fastest-growing market for heavy metal removal solutions due to rapid industrialization and urbanization. North America and Europe remain significant markets, driven by the need to replace aging water treatment infrastructure and comply with increasingly strict environmental regulations.
MSH-assisted heavy metal extraction technology is positioned to capture a growing share of this market due to its potential advantages in efficiency, cost-effectiveness, and environmental friendliness compared to traditional methods. The technology's ability to selectively remove heavy metals from complex aqueous solutions makes it particularly attractive for industrial wastewater treatment applications.
Key market drivers include the rising global population, increasing water scarcity, and the growing emphasis on circular economy principles in industrial processes. These factors are pushing industries to adopt more sustainable water management practices, including the recovery and reuse of valuable metals from wastewater streams.
However, the market also faces challenges, such as high initial investment costs for advanced treatment technologies and the need for specialized expertise in implementing and operating these systems. Additionally, the variability in regulatory standards across different regions can impact the adoption rates of new technologies like MSH-assisted extraction.
Emerging trends in the heavy metal removal market include the integration of nanotechnology, the development of bio-based adsorbents, and the application of artificial intelligence for process optimization. These innovations are expected to further enhance the efficiency and cost-effectiveness of heavy metal extraction processes, potentially accelerating market growth in the coming years.
Industrial sectors, including mining, metallurgy, electronics manufacturing, and chemical processing, are the primary contributors to heavy metal pollution in aqueous environments. These industries are increasingly seeking advanced solutions to meet regulatory requirements and improve their environmental performance. The market for heavy metal removal technologies is expected to expand further as developing countries implement more stringent environmental policies and developed nations continue to upgrade their water treatment infrastructure.
The Asia-Pacific region, particularly China and India, represents the fastest-growing market for heavy metal removal solutions due to rapid industrialization and urbanization. North America and Europe remain significant markets, driven by the need to replace aging water treatment infrastructure and comply with increasingly strict environmental regulations.
MSH-assisted heavy metal extraction technology is positioned to capture a growing share of this market due to its potential advantages in efficiency, cost-effectiveness, and environmental friendliness compared to traditional methods. The technology's ability to selectively remove heavy metals from complex aqueous solutions makes it particularly attractive for industrial wastewater treatment applications.
Key market drivers include the rising global population, increasing water scarcity, and the growing emphasis on circular economy principles in industrial processes. These factors are pushing industries to adopt more sustainable water management practices, including the recovery and reuse of valuable metals from wastewater streams.
However, the market also faces challenges, such as high initial investment costs for advanced treatment technologies and the need for specialized expertise in implementing and operating these systems. Additionally, the variability in regulatory standards across different regions can impact the adoption rates of new technologies like MSH-assisted extraction.
Emerging trends in the heavy metal removal market include the integration of nanotechnology, the development of bio-based adsorbents, and the application of artificial intelligence for process optimization. These innovations are expected to further enhance the efficiency and cost-effectiveness of heavy metal extraction processes, potentially accelerating market growth in the coming years.
Current Challenges in Heavy Metal Extraction
The extraction of heavy metals from aqueous solutions remains a significant challenge in environmental remediation and industrial processes. Despite advancements in extraction technologies, several obstacles persist in achieving efficient and cost-effective removal of heavy metals from water sources.
One of the primary challenges is the selectivity of extraction methods. Many current techniques struggle to differentiate between various heavy metal ions, leading to inefficient removal of target pollutants. This lack of selectivity can result in the unnecessary extraction of non-toxic elements or the incomplete removal of harmful ones, compromising the overall effectiveness of the treatment process.
Another major hurdle is the limited capacity and saturation of extraction materials. Traditional adsorbents and ion exchange resins often exhibit rapid saturation, necessitating frequent regeneration or replacement. This not only increases operational costs but also generates secondary waste streams that require further treatment or disposal.
The pH sensitivity of many extraction processes poses an additional challenge. Most heavy metal extraction methods are highly dependent on the pH of the aqueous solution, with optimal performance occurring within narrow pH ranges. This sensitivity complicates the treatment of complex wastewater streams with varying pH levels, often requiring pre-treatment steps or continuous pH adjustment during the extraction process.
Furthermore, the presence of competing ions and organic matter in real-world water samples can significantly interfere with heavy metal extraction. These matrix effects can reduce the efficiency of extraction methods by blocking active sites on adsorbents or forming complexes with target metal ions, making them less accessible for removal.
The issue of metal speciation also presents a considerable challenge. Heavy metals can exist in various chemical forms or oxidation states in aqueous solutions, each with different mobility and toxicity profiles. Current extraction methods often struggle to address all these species effectively, potentially leaving behind toxic forms of the metals.
Lastly, the scalability and economic viability of heavy metal extraction technologies remain significant hurdles. Many promising techniques demonstrated at laboratory scales face difficulties in scaling up to industrial levels. Factors such as high energy consumption, expensive materials, and complex operational requirements often limit the practical implementation of novel extraction methods in large-scale water treatment facilities.
One of the primary challenges is the selectivity of extraction methods. Many current techniques struggle to differentiate between various heavy metal ions, leading to inefficient removal of target pollutants. This lack of selectivity can result in the unnecessary extraction of non-toxic elements or the incomplete removal of harmful ones, compromising the overall effectiveness of the treatment process.
Another major hurdle is the limited capacity and saturation of extraction materials. Traditional adsorbents and ion exchange resins often exhibit rapid saturation, necessitating frequent regeneration or replacement. This not only increases operational costs but also generates secondary waste streams that require further treatment or disposal.
The pH sensitivity of many extraction processes poses an additional challenge. Most heavy metal extraction methods are highly dependent on the pH of the aqueous solution, with optimal performance occurring within narrow pH ranges. This sensitivity complicates the treatment of complex wastewater streams with varying pH levels, often requiring pre-treatment steps or continuous pH adjustment during the extraction process.
Furthermore, the presence of competing ions and organic matter in real-world water samples can significantly interfere with heavy metal extraction. These matrix effects can reduce the efficiency of extraction methods by blocking active sites on adsorbents or forming complexes with target metal ions, making them less accessible for removal.
The issue of metal speciation also presents a considerable challenge. Heavy metals can exist in various chemical forms or oxidation states in aqueous solutions, each with different mobility and toxicity profiles. Current extraction methods often struggle to address all these species effectively, potentially leaving behind toxic forms of the metals.
Lastly, the scalability and economic viability of heavy metal extraction technologies remain significant hurdles. Many promising techniques demonstrated at laboratory scales face difficulties in scaling up to industrial levels. Factors such as high energy consumption, expensive materials, and complex operational requirements often limit the practical implementation of novel extraction methods in large-scale water treatment facilities.
Existing MSH-assisted Extraction Methodologies
01 Synthesis of mesoporous silica hybrid materials for heavy metal extraction
Mesoporous silica hybrid (MSH) materials are synthesized using various methods to create structures with high surface area and porosity. These materials are functionalized with organic groups to enhance their ability to adsorb and extract heavy metals from aqueous solutions. The synthesis process often involves sol-gel methods and the use of structure-directing agents to control pore size and distribution.- Synthesis of mesoporous silica hybrid materials for heavy metal extraction: Mesoporous silica hybrid (MSH) materials are synthesized using various methods to create structures with high surface area and porosity. These materials are functionalized with organic groups to enhance their ability to extract heavy metals from aqueous solutions. The synthesis process often involves sol-gel methods and the use of structure-directing agents to control pore size and distribution.
- Surface modification of MSH for improved heavy metal adsorption: The surface of mesoporous silica hybrid materials is modified with various functional groups to enhance their affinity for heavy metal ions. Common modifications include grafting of amine, thiol, or carboxyl groups onto the silica surface. These modifications increase the selectivity and capacity of the material for specific heavy metal ions, improving the overall extraction efficiency.
- Application of MSH in environmental remediation: Mesoporous silica hybrid materials are applied in various environmental remediation processes, particularly for the removal of heavy metals from contaminated water and soil. These materials are used in batch adsorption processes, column filtration systems, and in-situ remediation techniques. Their high adsorption capacity and regenerability make them effective for long-term environmental clean-up projects.
- Regeneration and reusability of MSH adsorbents: Methods for regenerating and reusing mesoporous silica hybrid adsorbents after heavy metal extraction are developed to improve their cost-effectiveness and sustainability. These techniques often involve chemical treatments to desorb the captured heavy metals without damaging the adsorbent structure. The regenerated materials maintain their adsorption capacity over multiple cycles, making them suitable for long-term use in industrial applications.
- Incorporation of magnetic properties in MSH for easier separation: Magnetic nanoparticles are incorporated into mesoporous silica hybrid materials to create magnetically separable adsorbents for heavy metal extraction. This modification allows for easier separation of the adsorbent from the treated solution using an external magnetic field, simplifying the recovery process and improving the overall efficiency of the extraction system.
02 Surface modification of MSH for improved heavy metal adsorption
The surface of mesoporous silica hybrid materials is modified with various functional groups to increase their affinity for heavy metal ions. Common modifications include grafting of amine, thiol, or carboxyl groups onto the silica surface. These functionalized MSH materials show enhanced selectivity and capacity for heavy metal extraction from contaminated water or industrial effluents.Expand Specific Solutions03 Application of MSH in environmental remediation
Mesoporous silica hybrid materials are applied in various environmental remediation processes, particularly for the removal of heavy metals from water and soil. These materials are used in batch adsorption systems, column filtration setups, or incorporated into membranes for continuous treatment of contaminated water sources. The high adsorption capacity and reusability of MSH make them effective for large-scale environmental cleanup operations.Expand Specific Solutions04 Regeneration and reusability of MSH adsorbents
Methods for regenerating and reusing mesoporous silica hybrid adsorbents after heavy metal extraction are developed to improve their economic viability. Techniques such as acid washing, thermal treatment, or electrochemical desorption are employed to remove adsorbed heavy metals and restore the adsorption capacity of the MSH materials. This allows for multiple cycles of use, reducing the overall cost of the heavy metal extraction process.Expand Specific Solutions05 Composite materials incorporating MSH for enhanced performance
Composite materials that incorporate mesoporous silica hybrids are developed to enhance heavy metal extraction performance. These composites may combine MSH with other adsorbents, such as activated carbon, magnetic nanoparticles, or polymeric materials. The resulting hybrid materials often exhibit synergistic effects, leading to improved adsorption capacity, selectivity, and ease of separation in heavy metal removal applications.Expand Specific Solutions
Key Players in MSH and Heavy Metal Removal Industry
The field of MSH-assisted heavy metal extraction from aqueous solutions is in a developing stage, with growing market potential due to increasing environmental concerns and industrial wastewater treatment needs. The global market for water treatment technologies is expanding, driven by stringent regulations and the need for sustainable solutions. While the technology is advancing, it is not yet fully mature, with ongoing research and development efforts. Key players in this field include established chemical companies like BASF Corp. and Cytec Industries, Inc., as well as research institutions such as King Fahd University of Petroleum & Minerals and the Commonwealth Scientific & Industrial Research Organisation. These organizations are actively contributing to the development and refinement of MSH-assisted heavy metal extraction techniques, indicating a competitive landscape with both industrial and academic involvement.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative MSH-assisted heavy metal extraction process for aqueous solutions. Their approach utilizes specially designed mesoporous silica hybrid (MSH) materials with tailored surface functionalities to selectively adsorb and extract heavy metal ions from water. The MSH materials are synthesized through a controlled sol-gel process, resulting in high surface area and tunable pore structures[1]. BASF's technology incorporates organic functional groups, such as thiol or amine moieties, onto the silica surface to enhance metal binding affinity and selectivity[3]. The extraction process involves passing the contaminated aqueous solution through a column packed with the MSH adsorbent, followed by a regeneration step using mild acid treatment to recover the metals and reuse the adsorbent[5].
Strengths: High selectivity for specific heavy metals, large adsorption capacity, and reusability of the adsorbent. Weaknesses: Potential for fouling in complex water matrices and relatively high production costs of specialized MSH materials.
Cytec Technology Corp.
Technical Solution: Cytec Technology Corp. has developed a proprietary MSH-assisted heavy metal extraction technology for aqueous solutions. Their approach utilizes advanced mesoporous silica hybrid (MSH) materials functionalized with specific chelating agents to selectively capture and remove heavy metal ions from water. The MSH materials are synthesized using a templated sol-gel process, resulting in highly ordered porous structures with large surface areas[2]. Cytec's technology incorporates custom-designed organic ligands, such as phosphonic acid or iminodiacetic acid derivatives, covalently attached to the silica framework to enhance metal binding efficiency and selectivity[4]. The extraction process involves a continuous flow system where the contaminated aqueous solution is passed through a fixed bed of the MSH adsorbent, followed by a regeneration step using a proprietary elution solution to recover the metals and restore the adsorbent's capacity[6].
Strengths: High metal removal efficiency, excellent selectivity for target metals, and good regeneration capabilities. Weaknesses: Complexity of the synthesis process and potential for reduced performance in the presence of competing ions.
Core Innovations in MSH-assisted Extraction
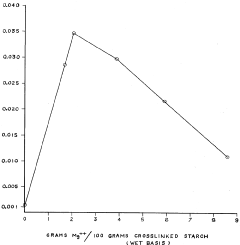
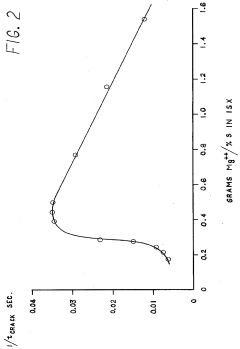
Removal of heavy metal ions from aqueous solutions with insoluble crosslinked-starch-xanthates
PatentInactiveUS4051316A
Innovation
- The development of insoluble crosslinked-starch-xanthate compositions with a specific degree of crosslinking that allows for effective heavy metal ion removal, achieved by crosslinking starch with agents like epichlorohydrin and xanthating in the presence of alkali metals, resulting in products that are insoluble and capable of efficiently removing heavy metals from aqueous solutions.
Removal of heavy metals from aqueous solutions
PatentInactiveUS3859210A
Innovation
- Contacting the aqueous stream with cationic, anionic, or nonionic polyelectrolytes in the presence of particulate matter, allowing the heavy metals to bind to the particulate matter, and then separating the particulate matter from the stream, using polyelectrolytes like polyalkylene polyamines and particulate matter such as sand or clay to effectively remove heavy metals like iron, nickel, chromium, and mercury.
Environmental Impact Assessment
The environmental impact assessment of MSH-assisted heavy metal extraction from aqueous solutions reveals both positive and negative implications. On the positive side, this technology offers a promising solution for removing toxic heavy metals from contaminated water sources, potentially improving water quality and reducing environmental pollution. The use of MSH (Mesoporous Silica Hybrid) materials as adsorbents provides a more efficient and selective extraction process compared to traditional methods, potentially reducing the overall environmental footprint of heavy metal remediation efforts.
However, the production and disposal of MSH materials themselves may pose environmental concerns. The synthesis of these materials often involves the use of chemicals and energy-intensive processes, which could contribute to greenhouse gas emissions and resource depletion if not managed sustainably. Additionally, the disposal or regeneration of spent MSH adsorbents after heavy metal extraction requires careful consideration to prevent secondary pollution.
The implementation of MSH-assisted heavy metal extraction may also impact local ecosystems. While the removal of heavy metals from aqueous solutions can benefit aquatic life and surrounding ecosystems, the introduction of MSH materials into water bodies, even in trace amounts, could have unforeseen consequences on microbial communities and aquatic organisms. Long-term studies are needed to assess the potential bioaccumulation and ecological effects of MSH particles in the environment.
From a broader perspective, the adoption of this technology could lead to changes in land use and water management practices. Treatment facilities employing MSH-assisted extraction may require dedicated infrastructure, potentially altering local landscapes. However, the improved efficiency of heavy metal removal could reduce the need for large-scale treatment plants, potentially minimizing land use impacts in the long term.
Energy consumption is another crucial factor to consider. While MSH-assisted extraction may be more energy-efficient than some conventional methods, the overall energy requirements for large-scale implementation need to be carefully evaluated. The environmental impact of energy production to power these systems must be weighed against the benefits of improved water quality.
Lastly, the life cycle assessment of MSH materials, from production to disposal or recycling, is essential for a comprehensive understanding of their environmental impact. This includes considering the sourcing of raw materials, manufacturing processes, transportation, and end-of-life management. Developing sustainable practices for the entire life cycle of MSH materials will be crucial for minimizing the overall environmental footprint of this heavy metal extraction technology.
However, the production and disposal of MSH materials themselves may pose environmental concerns. The synthesis of these materials often involves the use of chemicals and energy-intensive processes, which could contribute to greenhouse gas emissions and resource depletion if not managed sustainably. Additionally, the disposal or regeneration of spent MSH adsorbents after heavy metal extraction requires careful consideration to prevent secondary pollution.
The implementation of MSH-assisted heavy metal extraction may also impact local ecosystems. While the removal of heavy metals from aqueous solutions can benefit aquatic life and surrounding ecosystems, the introduction of MSH materials into water bodies, even in trace amounts, could have unforeseen consequences on microbial communities and aquatic organisms. Long-term studies are needed to assess the potential bioaccumulation and ecological effects of MSH particles in the environment.
From a broader perspective, the adoption of this technology could lead to changes in land use and water management practices. Treatment facilities employing MSH-assisted extraction may require dedicated infrastructure, potentially altering local landscapes. However, the improved efficiency of heavy metal removal could reduce the need for large-scale treatment plants, potentially minimizing land use impacts in the long term.
Energy consumption is another crucial factor to consider. While MSH-assisted extraction may be more energy-efficient than some conventional methods, the overall energy requirements for large-scale implementation need to be carefully evaluated. The environmental impact of energy production to power these systems must be weighed against the benefits of improved water quality.
Lastly, the life cycle assessment of MSH materials, from production to disposal or recycling, is essential for a comprehensive understanding of their environmental impact. This includes considering the sourcing of raw materials, manufacturing processes, transportation, and end-of-life management. Developing sustainable practices for the entire life cycle of MSH materials will be crucial for minimizing the overall environmental footprint of this heavy metal extraction technology.
Regulatory Framework for Heavy Metal Remediation
The regulatory framework for heavy metal remediation plays a crucial role in addressing the environmental and health concerns associated with heavy metal contamination in aqueous solutions. Governments and international organizations have established comprehensive guidelines and standards to ensure effective management and mitigation of heavy metal pollution.
At the global level, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework for promoting chemical safety around the world. This initiative includes specific provisions for heavy metal management and remediation, emphasizing the importance of international cooperation in addressing this global challenge.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations under the Clean Water Act and the Safe Drinking Water Act. These regulations set maximum contaminant levels (MCLs) for various heavy metals in drinking water and establish effluent limitations for industrial discharges. The EPA also enforces the Resource Conservation and Recovery Act (RCRA), which governs the management of hazardous waste containing heavy metals.
The European Union has adopted the Water Framework Directive (WFD) and the Groundwater Directive, which set environmental quality standards for priority substances, including heavy metals. These directives require member states to implement measures to achieve good ecological and chemical status of water bodies, including the reduction of heavy metal contamination.
In developing countries, regulatory frameworks for heavy metal remediation are often less comprehensive but are gradually improving. Many nations are adopting international standards and best practices, with support from organizations such as the World Health Organization (WHO) and the United Nations Development Programme (UNDP).
Specific to MSH-assisted heavy metal extraction, regulatory bodies are increasingly recognizing the potential of novel remediation technologies. However, the approval process for new techniques often requires extensive testing and validation to ensure their safety and efficacy. Regulatory agencies typically require detailed environmental impact assessments and risk analyses before permitting the use of innovative remediation methods.
As the field of heavy metal remediation continues to evolve, regulatory frameworks are adapting to incorporate emerging technologies and scientific understanding. This includes the development of risk-based approaches to remediation, which consider site-specific factors and potential exposure pathways. Additionally, there is a growing emphasis on sustainable remediation practices that minimize environmental impacts and promote resource recovery.
At the global level, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework for promoting chemical safety around the world. This initiative includes specific provisions for heavy metal management and remediation, emphasizing the importance of international cooperation in addressing this global challenge.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations under the Clean Water Act and the Safe Drinking Water Act. These regulations set maximum contaminant levels (MCLs) for various heavy metals in drinking water and establish effluent limitations for industrial discharges. The EPA also enforces the Resource Conservation and Recovery Act (RCRA), which governs the management of hazardous waste containing heavy metals.
The European Union has adopted the Water Framework Directive (WFD) and the Groundwater Directive, which set environmental quality standards for priority substances, including heavy metals. These directives require member states to implement measures to achieve good ecological and chemical status of water bodies, including the reduction of heavy metal contamination.
In developing countries, regulatory frameworks for heavy metal remediation are often less comprehensive but are gradually improving. Many nations are adopting international standards and best practices, with support from organizations such as the World Health Organization (WHO) and the United Nations Development Programme (UNDP).
Specific to MSH-assisted heavy metal extraction, regulatory bodies are increasingly recognizing the potential of novel remediation technologies. However, the approval process for new techniques often requires extensive testing and validation to ensure their safety and efficacy. Regulatory agencies typically require detailed environmental impact assessments and risk analyses before permitting the use of innovative remediation methods.
As the field of heavy metal remediation continues to evolve, regulatory frameworks are adapting to incorporate emerging technologies and scientific understanding. This includes the development of risk-based approaches to remediation, which consider site-specific factors and potential exposure pathways. Additionally, there is a growing emphasis on sustainable remediation practices that minimize environmental impacts and promote resource recovery.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!