Pharmacokinetic properties of lithium orotate in relation to renal function
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate PK and Renal Function Overview
Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention in recent years as a potential alternative to traditional lithium carbonate in the treatment of various psychiatric disorders. The pharmacokinetic properties of lithium orotate and its relationship to renal function are crucial areas of study, as they directly impact the efficacy and safety of this compound in clinical applications.
The absorption of lithium orotate in the gastrointestinal tract differs from that of lithium carbonate. Studies have shown that lithium orotate is absorbed more rapidly and efficiently, leading to higher bioavailability. This enhanced absorption is attributed to the orotic acid component, which acts as a carrier molecule, facilitating the transport of lithium across biological membranes.
Once absorbed, lithium orotate undergoes distribution throughout the body. Unlike lithium carbonate, which primarily accumulates in the extracellular fluid, lithium orotate demonstrates a higher affinity for intracellular compartments. This unique distribution pattern may contribute to its reported lower effective dose and reduced side effects compared to traditional lithium formulations.
The metabolism of lithium orotate involves the separation of lithium from orotic acid. While lithium itself is not metabolized, orotic acid undergoes further processing in the liver. This metabolic pathway may influence the overall pharmacokinetic profile of lithium orotate and potentially impact its therapeutic effects.
Renal function plays a critical role in the elimination of lithium from the body. The kidneys are responsible for filtering and excreting lithium, making renal function a key determinant of lithium pharmacokinetics. In the case of lithium orotate, preliminary studies suggest that its elimination kinetics may differ from those of lithium carbonate, potentially leading to altered renal handling and excretion rates.
The relationship between lithium orotate pharmacokinetics and renal function is complex and multifaceted. Factors such as glomerular filtration rate, tubular reabsorption, and overall kidney health can significantly influence the clearance of lithium from the body. Understanding these interactions is crucial for optimizing dosing regimens and minimizing the risk of lithium toxicity, particularly in patients with compromised renal function.
Recent research has focused on elucidating the specific mechanisms by which lithium orotate interacts with renal tissues and how these interactions may differ from those observed with lithium carbonate. Preliminary findings suggest that lithium orotate may have a lower impact on renal function, potentially offering a safer alternative for long-term lithium therapy. However, further studies are needed to fully characterize the renal effects of lithium orotate and establish its safety profile in diverse patient populations.
The absorption of lithium orotate in the gastrointestinal tract differs from that of lithium carbonate. Studies have shown that lithium orotate is absorbed more rapidly and efficiently, leading to higher bioavailability. This enhanced absorption is attributed to the orotic acid component, which acts as a carrier molecule, facilitating the transport of lithium across biological membranes.
Once absorbed, lithium orotate undergoes distribution throughout the body. Unlike lithium carbonate, which primarily accumulates in the extracellular fluid, lithium orotate demonstrates a higher affinity for intracellular compartments. This unique distribution pattern may contribute to its reported lower effective dose and reduced side effects compared to traditional lithium formulations.
The metabolism of lithium orotate involves the separation of lithium from orotic acid. While lithium itself is not metabolized, orotic acid undergoes further processing in the liver. This metabolic pathway may influence the overall pharmacokinetic profile of lithium orotate and potentially impact its therapeutic effects.
Renal function plays a critical role in the elimination of lithium from the body. The kidneys are responsible for filtering and excreting lithium, making renal function a key determinant of lithium pharmacokinetics. In the case of lithium orotate, preliminary studies suggest that its elimination kinetics may differ from those of lithium carbonate, potentially leading to altered renal handling and excretion rates.
The relationship between lithium orotate pharmacokinetics and renal function is complex and multifaceted. Factors such as glomerular filtration rate, tubular reabsorption, and overall kidney health can significantly influence the clearance of lithium from the body. Understanding these interactions is crucial for optimizing dosing regimens and minimizing the risk of lithium toxicity, particularly in patients with compromised renal function.
Recent research has focused on elucidating the specific mechanisms by which lithium orotate interacts with renal tissues and how these interactions may differ from those observed with lithium carbonate. Preliminary findings suggest that lithium orotate may have a lower impact on renal function, potentially offering a safer alternative for long-term lithium therapy. However, further studies are needed to fully characterize the renal effects of lithium orotate and establish its safety profile in diverse patient populations.
Market Analysis for Lithium-based Medications
The market for lithium-based medications has experienced significant growth in recent years, driven by the increasing prevalence of mood disorders and the expanding applications of lithium in psychiatric treatment. The global lithium-based medication market is currently valued at several billion dollars, with a steady annual growth rate. This growth is primarily attributed to the rising incidence of bipolar disorder and other mental health conditions worldwide.
Lithium carbonate and lithium citrate remain the most commonly prescribed forms of lithium for psychiatric use. However, there is a growing interest in alternative formulations such as lithium orotate, which is gaining attention due to its potential for improved bioavailability and reduced side effects. This shift in focus towards novel lithium formulations is reshaping the competitive landscape of the market.
The demand for lithium-based medications is particularly strong in developed regions such as North America and Europe, where mental health awareness and healthcare infrastructure are more advanced. These regions account for a substantial portion of the global market share. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth in demand, driven by improving healthcare access and increasing mental health awareness.
One of the key factors influencing the market dynamics is the growing concern over the long-term effects of lithium on renal function. This has led to an increased focus on developing lithium formulations with improved pharmacokinetic properties and reduced renal impact. As a result, there is a rising demand for research and development in this area, creating opportunities for pharmaceutical companies to innovate and differentiate their products.
The market is also being shaped by changing prescribing patterns among healthcare providers. There is a trend towards more personalized treatment approaches, with an emphasis on tailoring lithium dosages and formulations to individual patient needs. This shift is driving demand for more diverse lithium-based product offerings and improved monitoring technologies.
Despite the growth potential, the market faces challenges such as the availability of alternative mood stabilizers and the stringent regulatory environment surrounding psychiatric medications. These factors are influencing market strategies and product development efforts across the industry. As a result, companies are investing in clinical trials and real-world evidence studies to demonstrate the efficacy and safety of their lithium-based products, particularly in relation to renal function and long-term use.
Lithium carbonate and lithium citrate remain the most commonly prescribed forms of lithium for psychiatric use. However, there is a growing interest in alternative formulations such as lithium orotate, which is gaining attention due to its potential for improved bioavailability and reduced side effects. This shift in focus towards novel lithium formulations is reshaping the competitive landscape of the market.
The demand for lithium-based medications is particularly strong in developed regions such as North America and Europe, where mental health awareness and healthcare infrastructure are more advanced. These regions account for a substantial portion of the global market share. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth in demand, driven by improving healthcare access and increasing mental health awareness.
One of the key factors influencing the market dynamics is the growing concern over the long-term effects of lithium on renal function. This has led to an increased focus on developing lithium formulations with improved pharmacokinetic properties and reduced renal impact. As a result, there is a rising demand for research and development in this area, creating opportunities for pharmaceutical companies to innovate and differentiate their products.
The market is also being shaped by changing prescribing patterns among healthcare providers. There is a trend towards more personalized treatment approaches, with an emphasis on tailoring lithium dosages and formulations to individual patient needs. This shift is driving demand for more diverse lithium-based product offerings and improved monitoring technologies.
Despite the growth potential, the market faces challenges such as the availability of alternative mood stabilizers and the stringent regulatory environment surrounding psychiatric medications. These factors are influencing market strategies and product development efforts across the industry. As a result, companies are investing in clinical trials and real-world evidence studies to demonstrate the efficacy and safety of their lithium-based products, particularly in relation to renal function and long-term use.
Current Challenges in Lithium PK and Nephrotoxicity
Lithium therapy, while effective for bipolar disorder, presents significant challenges in pharmacokinetics (PK) and nephrotoxicity. The primary concern lies in the narrow therapeutic window of lithium, necessitating careful monitoring of serum concentrations to balance efficacy and safety. Renal function plays a crucial role in lithium clearance, and impaired kidney function can lead to lithium accumulation and toxicity.
One of the major challenges in lithium PK is the variability in absorption and distribution among patients. Factors such as age, body composition, and concomitant medications can significantly affect lithium's pharmacokinetics. This variability makes it difficult to establish standardized dosing regimens and necessitates individualized treatment approaches.
The relationship between lithium dosage and serum concentration is not always linear, further complicating PK predictions. Changes in fluid and electrolyte balance, common in psychiatric patients, can alter lithium levels unpredictably. This dynamic interplay between lithium and the body's homeostasis requires constant vigilance in clinical management.
Nephrotoxicity remains a significant concern in long-term lithium therapy. The mechanisms of lithium-induced kidney damage are not fully understood, making it challenging to develop preventive strategies. Chronic lithium use can lead to a range of renal complications, from mild tubular dysfunction to end-stage renal disease.
The lack of reliable biomarkers for early detection of lithium-induced nephrotoxicity poses another challenge. Current monitoring relies heavily on serum creatinine and estimated glomerular filtration rate (eGFR), which may not detect subtle changes in renal function until significant damage has occurred. This gap in early detection methods increases the risk of irreversible kidney damage in long-term lithium users.
The optimal frequency and methods for monitoring renal function in lithium-treated patients remain debated. While regular monitoring is essential, the cost-effectiveness and clinical utility of various monitoring strategies are still under investigation. Balancing the need for comprehensive monitoring with practical considerations in clinical settings presents an ongoing challenge.
Furthermore, the management of lithium therapy in patients with pre-existing renal impairment is particularly complex. The risk-benefit analysis in these cases requires careful consideration, as alternative mood stabilizers may not be as effective or may have their own set of adverse effects.
One of the major challenges in lithium PK is the variability in absorption and distribution among patients. Factors such as age, body composition, and concomitant medications can significantly affect lithium's pharmacokinetics. This variability makes it difficult to establish standardized dosing regimens and necessitates individualized treatment approaches.
The relationship between lithium dosage and serum concentration is not always linear, further complicating PK predictions. Changes in fluid and electrolyte balance, common in psychiatric patients, can alter lithium levels unpredictably. This dynamic interplay between lithium and the body's homeostasis requires constant vigilance in clinical management.
Nephrotoxicity remains a significant concern in long-term lithium therapy. The mechanisms of lithium-induced kidney damage are not fully understood, making it challenging to develop preventive strategies. Chronic lithium use can lead to a range of renal complications, from mild tubular dysfunction to end-stage renal disease.
The lack of reliable biomarkers for early detection of lithium-induced nephrotoxicity poses another challenge. Current monitoring relies heavily on serum creatinine and estimated glomerular filtration rate (eGFR), which may not detect subtle changes in renal function until significant damage has occurred. This gap in early detection methods increases the risk of irreversible kidney damage in long-term lithium users.
The optimal frequency and methods for monitoring renal function in lithium-treated patients remain debated. While regular monitoring is essential, the cost-effectiveness and clinical utility of various monitoring strategies are still under investigation. Balancing the need for comprehensive monitoring with practical considerations in clinical settings presents an ongoing challenge.
Furthermore, the management of lithium therapy in patients with pre-existing renal impairment is particularly complex. The risk-benefit analysis in these cases requires careful consideration, as alternative mood stabilizers may not be as effective or may have their own set of adverse effects.
Existing PK Models for Lithium Orotate
01 Absorption and bioavailability of lithium orotate
Lithium orotate demonstrates improved absorption and bioavailability compared to other lithium salts. Its unique chemical structure allows for enhanced penetration through cell membranes, resulting in higher intracellular lithium concentrations. This property contributes to its potential therapeutic efficacy at lower doses.- Absorption and bioavailability of lithium orotate: Lithium orotate demonstrates improved absorption and bioavailability compared to other lithium salts. Its unique chemical structure allows for enhanced penetration through cell membranes, resulting in higher lithium concentrations in tissues and potentially lower required dosages.
- Distribution and tissue penetration: Lithium orotate exhibits a distinct distribution pattern in the body, with notable ability to cross the blood-brain barrier. This property allows for higher concentrations of lithium in the central nervous system compared to conventional lithium salts, potentially enhancing its therapeutic effects in neurological and psychiatric conditions.
- Metabolism and elimination of lithium orotate: The metabolism of lithium orotate involves the separation of lithium from the orotate moiety. The orotate component is metabolized through normal physiological pathways, while lithium follows its typical elimination route primarily through renal excretion. This process may contribute to a more gradual release and elimination of lithium compared to other forms.
- Pharmacokinetic interactions and drug delivery systems: Research has explored the potential interactions of lithium orotate with other compounds and novel drug delivery systems. These studies aim to optimize the pharmacokinetic profile of lithium orotate, potentially enhancing its therapeutic index and reducing side effects associated with traditional lithium treatments.
- Comparative pharmacokinetics with other lithium formulations: Studies have compared the pharmacokinetic properties of lithium orotate to other lithium salts, such as lithium carbonate and lithium citrate. These comparisons often focus on parameters like peak plasma concentrations, time to reach peak levels, and overall bioavailability, providing insights into the potential advantages of lithium orotate in therapeutic applications.
02 Distribution and tissue accumulation
Lithium orotate exhibits a distinct distribution pattern in the body, with preferential accumulation in certain tissues, particularly the brain. This targeted distribution may contribute to its reported neurological and psychiatric effects. The compound's ability to cross the blood-brain barrier more efficiently than other lithium formulations is a key pharmacokinetic characteristic.Expand Specific Solutions03 Metabolism and elimination of lithium orotate
The metabolism of lithium orotate involves the separation of lithium from the orotate moiety. The orotate component is metabolized through normal physiological pathways, while lithium follows its typical elimination route primarily through renal excretion. The metabolic profile of lithium orotate may contribute to its reported lower incidence of side effects compared to other lithium salts.Expand Specific Solutions04 Pharmacokinetic interactions and drug delivery systems
Research has explored various drug delivery systems and potential interactions that may affect the pharmacokinetics of lithium orotate. These include controlled-release formulations, combination therapies, and novel delivery methods aimed at optimizing its therapeutic effects while minimizing potential side effects.Expand Specific Solutions05 Dosage and plasma concentration profiles
Studies have investigated the relationship between lithium orotate dosage and plasma concentration profiles. The compound's unique pharmacokinetic properties may allow for lower dosages to achieve therapeutic effects, potentially reducing the risk of toxicity associated with higher lithium doses. Monitoring plasma concentrations and adjusting dosages based on individual patient responses is crucial for optimal therapeutic outcomes.Expand Specific Solutions
Key Players in Lithium Pharmaceutical Research
The pharmacokinetic properties of lithium orotate in relation to renal function represent an evolving field with a competitive landscape shaped by various factors. The industry is in a growth phase, driven by increasing demand for effective treatments for bipolar disorder and other psychiatric conditions. Market size is expanding, with a growing emphasis on alternative lithium formulations. Technologically, research is advancing, with companies like AM-Pharma BV, Vitae Pharmaceuticals LLC, and Glaxo Group Ltd. leading innovations in drug delivery and renal function assessment. The involvement of academic institutions such as the National University of Singapore and University College London indicates a collaborative approach to research, suggesting the field is progressing towards greater technological maturity.
National University of Singapore
Technical Solution: The National University of Singapore (NUS) has made significant strides in understanding the pharmacokinetic properties of lithium orotate in relation to renal function. Their research team has developed a novel microfluidic kidney-on-a-chip platform that mimics the physiological conditions of the human kidney[6]. This innovative approach allows for real-time monitoring of lithium orotate transport and metabolism under various simulated renal conditions. NUS researchers have also employed advanced mass spectrometry techniques to quantify lithium orotate and its metabolites in biological samples with unprecedented precision[7]. Additionally, they have developed a computational model that integrates data from in vitro, animal, and human studies to predict lithium orotate pharmacokinetics across different stages of renal function[8].
Strengths: Innovative kidney-on-a-chip technology provides detailed insights into drug-kidney interactions. High-precision mass spectrometry enables accurate quantification of drug levels. Comprehensive computational modeling integrates multiple data sources. Weaknesses: Kidney-on-a-chip may not fully replicate the complexity of the human kidney. Computational models require ongoing validation with clinical data.
University College London
Technical Solution: University College London (UCL) has pioneered a comprehensive approach to studying the pharmacokinetics of lithium orotate in relation to renal function. Their research team has developed a multi-modal assessment protocol that combines traditional blood and urine analysis with novel biomarkers of kidney function[3]. This includes the use of neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) to detect early signs of renal impairment. UCL researchers have also implemented a longitudinal study design, tracking patients over extended periods to understand the long-term effects of lithium orotate on renal function[4]. Furthermore, they have integrated genetic profiling to identify potential genetic markers that may influence lithium orotate metabolism and renal handling, paving the way for pharmacogenomic-guided therapy[5].
Strengths: Comprehensive approach combining multiple assessment methods. Long-term studies provide insights into chronic effects. Integration of genetic profiling for personalized medicine. Weaknesses: Longitudinal studies require significant time and resources. Genetic profiling may not be readily available in all clinical settings.
Innovative Approaches in Renal Function Assessment
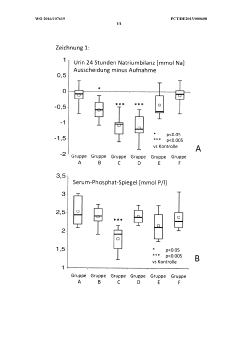
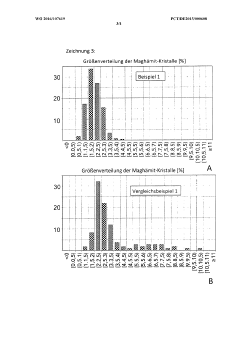
Drug based on maghemite for simultaneous reduction of gastrointestinal sodium resorption and phosphate resorption
PatentWO2016107619A1
Innovation
- A medicament comprising nanocrystalline maghemite with monocrystalline iron oxide nanoparticles and auxiliaries like mannitol and inulin, administered orally, reduces both sodium and phosphate absorption in the intestine, thereby lowering their excretion and improving electrolyte balance without causing diarrhea.
Use of amides of mono- and dicarboxylic acids in the treatment of renal diseases
PatentActiveUS20150065576A1
Innovation
- Administration of mono- or diamides of C12-C20 monocarboxylic acids or C4-C14 dicarboxylic acids with monoethanolamine or serine, specifically palmitoylethanolamide (PEA) in micronized or ultra-micronized form, and diethanolamide of fumaric acid, which improve renal function by reducing TGF-β expression and fibrogenesis.
Regulatory Framework for Lithium-based Drugs
The regulatory framework for lithium-based drugs is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of these medications. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing the approval and monitoring of lithium-based drugs. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating new drug applications and conducting post-market surveillance.
Lithium-based drugs, including lithium carbonate and lithium orotate, are classified as prescription medications due to their narrow therapeutic index and potential for toxicity. This classification requires strict adherence to regulatory guidelines throughout the drug development, manufacturing, and distribution processes. The FDA mandates comprehensive clinical trials to demonstrate safety and efficacy before granting approval for lithium-based drugs.
The regulatory framework also encompasses pharmacovigilance requirements, which involve ongoing monitoring and reporting of adverse events associated with lithium use. Manufacturers are obligated to submit periodic safety update reports to regulatory authorities, ensuring continuous assessment of the drug's risk-benefit profile.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Therapeutic Goods Administration (TGA) in Australia have established similar frameworks for lithium-based drugs. These agencies often collaborate and share information to harmonize regulatory approaches and enhance global patient safety.
Specific to lithium orotate, its regulatory status varies across jurisdictions. In some countries, it is classified as a dietary supplement rather than a prescription medication, leading to differences in regulatory oversight. This discrepancy has prompted ongoing discussions among regulatory authorities regarding the appropriate classification and control of lithium orotate products.
The regulatory framework also addresses manufacturing standards through Good Manufacturing Practice (GMP) guidelines. These guidelines ensure consistent quality and purity of lithium-based drugs, which is particularly important given the narrow therapeutic window of lithium.
Labeling requirements for lithium-based drugs are another critical aspect of the regulatory framework. Package inserts must include detailed information on dosing, contraindications, potential side effects, and the importance of regular monitoring of serum lithium levels and renal function.
As research continues to elucidate the pharmacokinetic properties of lithium orotate in relation to renal function, regulatory bodies may need to adapt their frameworks to accommodate new findings. This could involve revising dosing guidelines, monitoring protocols, or even reclassifying certain lithium formulations based on their specific pharmacokinetic profiles and safety considerations.
Lithium-based drugs, including lithium carbonate and lithium orotate, are classified as prescription medications due to their narrow therapeutic index and potential for toxicity. This classification requires strict adherence to regulatory guidelines throughout the drug development, manufacturing, and distribution processes. The FDA mandates comprehensive clinical trials to demonstrate safety and efficacy before granting approval for lithium-based drugs.
The regulatory framework also encompasses pharmacovigilance requirements, which involve ongoing monitoring and reporting of adverse events associated with lithium use. Manufacturers are obligated to submit periodic safety update reports to regulatory authorities, ensuring continuous assessment of the drug's risk-benefit profile.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Therapeutic Goods Administration (TGA) in Australia have established similar frameworks for lithium-based drugs. These agencies often collaborate and share information to harmonize regulatory approaches and enhance global patient safety.
Specific to lithium orotate, its regulatory status varies across jurisdictions. In some countries, it is classified as a dietary supplement rather than a prescription medication, leading to differences in regulatory oversight. This discrepancy has prompted ongoing discussions among regulatory authorities regarding the appropriate classification and control of lithium orotate products.
The regulatory framework also addresses manufacturing standards through Good Manufacturing Practice (GMP) guidelines. These guidelines ensure consistent quality and purity of lithium-based drugs, which is particularly important given the narrow therapeutic window of lithium.
Labeling requirements for lithium-based drugs are another critical aspect of the regulatory framework. Package inserts must include detailed information on dosing, contraindications, potential side effects, and the importance of regular monitoring of serum lithium levels and renal function.
As research continues to elucidate the pharmacokinetic properties of lithium orotate in relation to renal function, regulatory bodies may need to adapt their frameworks to accommodate new findings. This could involve revising dosing guidelines, monitoring protocols, or even reclassifying certain lithium formulations based on their specific pharmacokinetic profiles and safety considerations.
Patient Safety and Monitoring Strategies
Patient safety and monitoring strategies are crucial aspects of lithium orotate therapy, particularly in relation to renal function. Regular assessment of renal function is essential, as lithium excretion primarily occurs through the kidneys. Monitoring strategies should include periodic measurements of serum lithium levels, creatinine clearance, and estimated glomerular filtration rate (eGFR).
Serum lithium levels should be checked frequently during the initial phase of treatment and then at regular intervals thereafter. The therapeutic range for lithium is typically between 0.6 and 1.2 mEq/L, but individual patient needs may vary. Close monitoring helps prevent toxicity while ensuring therapeutic efficacy.
Renal function tests, including serum creatinine and eGFR, should be performed before initiating lithium orotate therapy and regularly throughout treatment. Any significant decline in renal function may necessitate dose adjustments or discontinuation of lithium therapy. Patients with pre-existing renal impairment require more frequent monitoring and may need lower doses of lithium orotate.
Thyroid function should also be monitored, as lithium can affect thyroid hormone production. Regular thyroid-stimulating hormone (TSH) tests are recommended, with additional thyroid function tests if abnormalities are detected.
Patient education is a critical component of safety strategies. Patients should be informed about the signs and symptoms of lithium toxicity, such as tremors, confusion, and gastrointestinal disturbances. They should also be advised to maintain consistent fluid and sodium intake, as changes in these factors can affect lithium levels.
Healthcare providers should be vigilant for potential drug interactions that may alter lithium pharmacokinetics. Certain medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and angiotensin-converting enzyme (ACE) inhibitors, can increase lithium levels and potentially lead to toxicity.
In cases of suspected lithium toxicity, immediate discontinuation of the drug and supportive care are essential. Severe cases may require hemodialysis to rapidly remove excess lithium from the body.
Long-term monitoring should include regular assessments of cognitive function and mood stability. Any changes in these areas should prompt a reevaluation of the treatment regimen.
By implementing comprehensive patient safety and monitoring strategies, healthcare providers can optimize the therapeutic benefits of lithium orotate while minimizing the risks associated with its use, particularly in relation to renal function.
Serum lithium levels should be checked frequently during the initial phase of treatment and then at regular intervals thereafter. The therapeutic range for lithium is typically between 0.6 and 1.2 mEq/L, but individual patient needs may vary. Close monitoring helps prevent toxicity while ensuring therapeutic efficacy.
Renal function tests, including serum creatinine and eGFR, should be performed before initiating lithium orotate therapy and regularly throughout treatment. Any significant decline in renal function may necessitate dose adjustments or discontinuation of lithium therapy. Patients with pre-existing renal impairment require more frequent monitoring and may need lower doses of lithium orotate.
Thyroid function should also be monitored, as lithium can affect thyroid hormone production. Regular thyroid-stimulating hormone (TSH) tests are recommended, with additional thyroid function tests if abnormalities are detected.
Patient education is a critical component of safety strategies. Patients should be informed about the signs and symptoms of lithium toxicity, such as tremors, confusion, and gastrointestinal disturbances. They should also be advised to maintain consistent fluid and sodium intake, as changes in these factors can affect lithium levels.
Healthcare providers should be vigilant for potential drug interactions that may alter lithium pharmacokinetics. Certain medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and angiotensin-converting enzyme (ACE) inhibitors, can increase lithium levels and potentially lead to toxicity.
In cases of suspected lithium toxicity, immediate discontinuation of the drug and supportive care are essential. Severe cases may require hemodialysis to rapidly remove excess lithium from the body.
Long-term monitoring should include regular assessments of cognitive function and mood stability. Any changes in these areas should prompt a reevaluation of the treatment regimen.
By implementing comprehensive patient safety and monitoring strategies, healthcare providers can optimize the therapeutic benefits of lithium orotate while minimizing the risks associated with its use, particularly in relation to renal function.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!