Proteomic analysis of lithium orotate-treated neuronal cells
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Neuroproteomics Background
Lithium orotate, a compound consisting of lithium and orotic acid, has gained significant attention in neuroscience research due to its potential neuroprotective properties. This organic lithium salt differs from the more commonly used lithium carbonate in its bioavailability and ability to cross the blood-brain barrier more efficiently. The study of lithium orotate's effects on neuronal cells through proteomic analysis represents a cutting-edge approach to understanding its molecular mechanisms and potential therapeutic applications.
Proteomics, the large-scale study of proteins, has emerged as a powerful tool in neuroscience research. It allows for the comprehensive analysis of protein expression, modifications, and interactions within neuronal cells. By applying proteomic techniques to lithium orotate-treated neuronal cells, researchers aim to uncover the complex cellular responses and signaling pathways affected by this compound.
The background of this research is rooted in the long-standing use of lithium in psychiatry, particularly in the treatment of bipolar disorder. However, traditional lithium treatments often come with side effects and narrow therapeutic windows. Lithium orotate, with its potentially enhanced bioavailability and reduced toxicity, presents an opportunity to overcome these limitations and expand the therapeutic potential of lithium-based treatments.
Neuronal cells, the primary targets of lithium orotate in this context, are specialized cells of the nervous system responsible for processing and transmitting information. These cells are particularly vulnerable to various stressors and play crucial roles in neurodegenerative disorders and psychiatric conditions. Understanding how lithium orotate affects the proteome of these cells can provide insights into its neuroprotective mechanisms and potential applications in treating neurological disorders.
The proteomic analysis of lithium orotate-treated neuronal cells involves several key steps. First, neuronal cell cultures are treated with lithium orotate under controlled conditions. The cells are then lysed, and their proteins are extracted and separated using techniques such as two-dimensional gel electrophoresis or liquid chromatography. The separated proteins are subsequently identified and quantified using mass spectrometry, allowing for a comprehensive comparison between treated and untreated cells.
This research approach aims to identify changes in protein expression, post-translational modifications, and protein-protein interactions induced by lithium orotate treatment. Such changes can reveal the molecular pathways and cellular processes affected by the compound, potentially uncovering new therapeutic targets or mechanisms of action.
Proteomics, the large-scale study of proteins, has emerged as a powerful tool in neuroscience research. It allows for the comprehensive analysis of protein expression, modifications, and interactions within neuronal cells. By applying proteomic techniques to lithium orotate-treated neuronal cells, researchers aim to uncover the complex cellular responses and signaling pathways affected by this compound.
The background of this research is rooted in the long-standing use of lithium in psychiatry, particularly in the treatment of bipolar disorder. However, traditional lithium treatments often come with side effects and narrow therapeutic windows. Lithium orotate, with its potentially enhanced bioavailability and reduced toxicity, presents an opportunity to overcome these limitations and expand the therapeutic potential of lithium-based treatments.
Neuronal cells, the primary targets of lithium orotate in this context, are specialized cells of the nervous system responsible for processing and transmitting information. These cells are particularly vulnerable to various stressors and play crucial roles in neurodegenerative disorders and psychiatric conditions. Understanding how lithium orotate affects the proteome of these cells can provide insights into its neuroprotective mechanisms and potential applications in treating neurological disorders.
The proteomic analysis of lithium orotate-treated neuronal cells involves several key steps. First, neuronal cell cultures are treated with lithium orotate under controlled conditions. The cells are then lysed, and their proteins are extracted and separated using techniques such as two-dimensional gel electrophoresis or liquid chromatography. The separated proteins are subsequently identified and quantified using mass spectrometry, allowing for a comprehensive comparison between treated and untreated cells.
This research approach aims to identify changes in protein expression, post-translational modifications, and protein-protein interactions induced by lithium orotate treatment. Such changes can reveal the molecular pathways and cellular processes affected by the compound, potentially uncovering new therapeutic targets or mechanisms of action.
Market Demand for Neuropsychiatric Treatments
The market demand for neuropsychiatric treatments has been steadily increasing in recent years, driven by a growing awareness of mental health issues and the rising prevalence of neurological disorders. This trend is particularly evident in the context of lithium-based treatments, which have shown promise in addressing various neuropsychiatric conditions.
Lithium has long been recognized as an effective treatment for bipolar disorder, but recent research into lithium orotate has expanded its potential applications. The proteomic analysis of lithium orotate-treated neuronal cells has opened new avenues for understanding its mechanisms of action and potential therapeutic benefits in a broader range of neuropsychiatric disorders.
The global market for neuropsychiatric treatments is substantial and continues to grow. Factors contributing to this growth include an aging population, increased stress levels in modern society, and improved diagnostic capabilities. The demand for novel and more effective treatments is particularly strong in developed countries, where mental health awareness is high and healthcare systems are more advanced.
Lithium orotate, as a potential alternative to traditional lithium carbonate, has garnered significant interest due to its reported lower side effect profile and potentially higher bioavailability. This has created a niche market within the broader neuropsychiatric treatment landscape, with patients and healthcare providers seeking safer and more tolerable options for long-term management of mood disorders and other neurological conditions.
The market demand is further fueled by the growing body of research suggesting lithium's neuroprotective properties. Studies indicating its potential in preventing cognitive decline and neurodegenerative diseases have expanded the target demographic beyond those with mood disorders. This has led to increased interest from both the medical community and potential patients in preventive treatments for conditions such as Alzheimer's disease and other forms of dementia.
Moreover, the ongoing COVID-19 pandemic has heightened awareness of mental health issues, leading to an increased demand for effective neuropsychiatric treatments. The stress, isolation, and uncertainty associated with the pandemic have exacerbated existing mental health conditions and triggered new cases, creating a surge in the need for innovative therapeutic approaches.
As research in proteomics and neuronal cell biology advances, there is a growing expectation for personalized treatment options in neuropsychiatry. This trend aligns well with the detailed proteomic analysis of lithium orotate's effects on neuronal cells, as it may lead to more targeted and effective treatment strategies tailored to individual patient profiles.
Lithium has long been recognized as an effective treatment for bipolar disorder, but recent research into lithium orotate has expanded its potential applications. The proteomic analysis of lithium orotate-treated neuronal cells has opened new avenues for understanding its mechanisms of action and potential therapeutic benefits in a broader range of neuropsychiatric disorders.
The global market for neuropsychiatric treatments is substantial and continues to grow. Factors contributing to this growth include an aging population, increased stress levels in modern society, and improved diagnostic capabilities. The demand for novel and more effective treatments is particularly strong in developed countries, where mental health awareness is high and healthcare systems are more advanced.
Lithium orotate, as a potential alternative to traditional lithium carbonate, has garnered significant interest due to its reported lower side effect profile and potentially higher bioavailability. This has created a niche market within the broader neuropsychiatric treatment landscape, with patients and healthcare providers seeking safer and more tolerable options for long-term management of mood disorders and other neurological conditions.
The market demand is further fueled by the growing body of research suggesting lithium's neuroprotective properties. Studies indicating its potential in preventing cognitive decline and neurodegenerative diseases have expanded the target demographic beyond those with mood disorders. This has led to increased interest from both the medical community and potential patients in preventive treatments for conditions such as Alzheimer's disease and other forms of dementia.
Moreover, the ongoing COVID-19 pandemic has heightened awareness of mental health issues, leading to an increased demand for effective neuropsychiatric treatments. The stress, isolation, and uncertainty associated with the pandemic have exacerbated existing mental health conditions and triggered new cases, creating a surge in the need for innovative therapeutic approaches.
As research in proteomics and neuronal cell biology advances, there is a growing expectation for personalized treatment options in neuropsychiatry. This trend aligns well with the detailed proteomic analysis of lithium orotate's effects on neuronal cells, as it may lead to more targeted and effective treatment strategies tailored to individual patient profiles.
Current Challenges in Lithium Orotate Research
Despite the promising potential of lithium orotate in neuropsychiatric treatments, several challenges persist in its research and clinical application. One of the primary obstacles is the limited understanding of its precise mechanisms of action at the molecular level. While proteomic analysis offers valuable insights, the complexity of neuronal cell proteomes and the subtle changes induced by lithium orotate treatment make it challenging to identify and interpret relevant protein alterations.
The lack of standardized protocols for proteomic analysis of lithium orotate-treated neuronal cells poses another significant challenge. Variations in cell culture conditions, treatment durations, and analytical methods across different studies hinder the comparability and reproducibility of results. This inconsistency makes it difficult to draw definitive conclusions about the effects of lithium orotate on neuronal proteomes.
Another hurdle is the differentiation between the effects of lithium and orotate. As lithium orotate is a compound of lithium and orotic acid, distinguishing the individual contributions of each component to the observed proteomic changes remains complex. This complexity is further compounded by potential synergistic effects between lithium and orotate, which may lead to unique proteomic signatures distinct from those of lithium or orotate alone.
The dynamic nature of the neuronal proteome presents an additional challenge. Proteins undergo constant turnover and post-translational modifications, making it difficult to capture a comprehensive snapshot of the proteome at any given time point. This dynamism necessitates careful consideration of temporal factors in experimental design and data interpretation.
Furthermore, the heterogeneity of neuronal cell populations complicates proteomic analysis. Different neuronal subtypes may respond differently to lithium orotate treatment, potentially masking or diluting significant proteomic changes when analyzing mixed populations. Developing methods to isolate and analyze specific neuronal subtypes while maintaining physiological relevance remains a technical challenge.
Lastly, translating proteomic findings from in vitro neuronal cell models to in vivo systems and ultimately to clinical applications presents a significant hurdle. The complexity of the human brain and the potential differences in drug metabolism and distribution between cell cultures and living organisms necessitate careful validation and interpretation of proteomic data in the context of whole-organism physiology and pathology.
The lack of standardized protocols for proteomic analysis of lithium orotate-treated neuronal cells poses another significant challenge. Variations in cell culture conditions, treatment durations, and analytical methods across different studies hinder the comparability and reproducibility of results. This inconsistency makes it difficult to draw definitive conclusions about the effects of lithium orotate on neuronal proteomes.
Another hurdle is the differentiation between the effects of lithium and orotate. As lithium orotate is a compound of lithium and orotic acid, distinguishing the individual contributions of each component to the observed proteomic changes remains complex. This complexity is further compounded by potential synergistic effects between lithium and orotate, which may lead to unique proteomic signatures distinct from those of lithium or orotate alone.
The dynamic nature of the neuronal proteome presents an additional challenge. Proteins undergo constant turnover and post-translational modifications, making it difficult to capture a comprehensive snapshot of the proteome at any given time point. This dynamism necessitates careful consideration of temporal factors in experimental design and data interpretation.
Furthermore, the heterogeneity of neuronal cell populations complicates proteomic analysis. Different neuronal subtypes may respond differently to lithium orotate treatment, potentially masking or diluting significant proteomic changes when analyzing mixed populations. Developing methods to isolate and analyze specific neuronal subtypes while maintaining physiological relevance remains a technical challenge.
Lastly, translating proteomic findings from in vitro neuronal cell models to in vivo systems and ultimately to clinical applications presents a significant hurdle. The complexity of the human brain and the potential differences in drug metabolism and distribution between cell cultures and living organisms necessitate careful validation and interpretation of proteomic data in the context of whole-organism physiology and pathology.
Existing Proteomic Analysis Methods for Neuronal Cells
01 Proteomic analysis techniques for neuronal cells
Various techniques are employed for proteomic analysis of neuronal cells, including mass spectrometry, gel electrophoresis, and protein microarrays. These methods allow for the identification and quantification of proteins expressed in neuronal cells, providing insights into cellular functions, signaling pathways, and potential biomarkers for neurological disorders.- Proteomic analysis techniques for neuronal cells: Various techniques are employed for proteomic analysis of neuronal cells, including mass spectrometry, gel electrophoresis, and protein microarrays. These methods allow for the identification and quantification of proteins expressed in neuronal cells, providing insights into cellular functions, signaling pathways, and potential biomarkers for neurological disorders.
- Neuronal cell-specific protein markers: Identification and characterization of protein markers specific to neuronal cells are crucial for understanding neuronal development, function, and pathology. These markers can be used to distinguish different types of neurons, assess neuronal health, and track changes in protein expression during various physiological and pathological conditions.
- Proteomic profiling in neurodegenerative diseases: Proteomic profiling of neuronal cells is applied to study neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's disease. This approach helps identify alterations in protein expression, post-translational modifications, and protein-protein interactions associated with disease progression, potentially leading to new therapeutic targets and biomarkers.
- Neuronal proteomics in drug discovery and development: Proteomic profiling of neuronal cells plays a crucial role in drug discovery and development for neurological disorders. It aids in identifying drug targets, evaluating drug efficacy and toxicity at the protein level, and understanding the mechanisms of action of potential therapeutic compounds on neuronal cells.
- Integration of neuronal proteomics with other omics data: Integrating proteomic data from neuronal cells with other omics data, such as genomics, transcriptomics, and metabolomics, provides a comprehensive understanding of neuronal biology. This multi-omics approach enables the creation of more accurate models of neuronal function and disease, leading to improved diagnostic and therapeutic strategies for neurological disorders.
02 Neuronal cell culture and differentiation for proteomic studies
Protocols for culturing and differentiating neuronal cells are crucial for proteomic profiling. This includes methods for maintaining primary neuronal cultures, differentiating stem cells into neurons, and creating in vitro models of neuronal networks. These techniques enable the study of protein expression patterns during neuronal development and in response to various stimuli.Expand Specific Solutions03 Biomarker discovery for neurological disorders
Proteomic profiling of neuronal cells is used to identify potential biomarkers for neurological disorders such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis. This involves comparing protein expression patterns between healthy and diseased neuronal cells to identify proteins that may serve as diagnostic or prognostic indicators.Expand Specific Solutions04 Protein-protein interaction networks in neuronal cells
Studying protein-protein interaction networks in neuronal cells is essential for understanding cellular signaling pathways and molecular mechanisms underlying neuronal function. Techniques such as yeast two-hybrid screening, co-immunoprecipitation, and proximity ligation assays are used to map these interactions and construct comprehensive protein interaction networks.Expand Specific Solutions05 Post-translational modifications in neuronal proteomics
Analysis of post-translational modifications (PTMs) in neuronal proteins is crucial for understanding protein function and regulation. Techniques for identifying and quantifying PTMs such as phosphorylation, glycosylation, and ubiquitination in neuronal cells are employed to elucidate their roles in neuronal signaling, plasticity, and disease processes.Expand Specific Solutions
Key Players in Neuroproteomics and Lithium Research
The proteomic analysis of lithium orotate-treated neuronal cells represents an emerging field at the intersection of neuroscience and pharmacology. The competitive landscape is characterized by early-stage research, with a growing market potential as mental health treatments gain prominence. While the market size is still developing, it shows promise due to the increasing prevalence of neurological disorders. The technology is in its nascent stages, with key players like The Scripps Research Institute, California Institute of Technology, and Asterias Biotherapeutics leading research efforts. Other institutions such as Washington University in St. Louis and Duke University are also contributing to the field's advancement, indicating a collaborative yet competitive environment in this specialized area of neuroscience.
The Scripps Research Institute
Technical Solution: The Scripps Research Institute has developed a comprehensive proteomic analysis approach for studying the effects of lithium orotate on neuronal cells. Their method combines high-resolution mass spectrometry with advanced bioinformatics tools to identify and quantify protein changes in response to lithium orotate treatment. This approach allows for the detection of subtle alterations in protein expression, post-translational modifications, and signaling pathways affected by lithium orotate[1]. The institute has also implemented stable isotope labeling by amino acids in cell culture (SILAC) to enhance the accuracy of quantitative proteomics, enabling the comparison of treated and untreated neuronal cells with high precision[2].
Strengths: Cutting-edge mass spectrometry technology and bioinformatics expertise. Weaknesses: Resource-intensive approach that may be challenging to scale up for large-scale studies.
California Institute of Technology
Technical Solution: Caltech has developed a novel approach to proteomic analysis of lithium orotate-treated neuronal cells using advanced microfluidic devices. Their technology allows for single-cell proteomic analysis, providing unprecedented resolution in studying the effects of lithium orotate on individual neurons. The microfluidic platform integrates cell isolation, lysis, protein separation, and mass spectrometry analysis in a single chip[3]. This method enables the detection of cell-to-cell variability in protein expression and modification patterns in response to lithium orotate treatment. Additionally, Caltech researchers have implemented machine learning algorithms to analyze the complex datasets generated by their single-cell proteomic platform, allowing for the identification of subtle patterns and subpopulations of neurons with distinct responses to lithium orotate[4].
Strengths: Unparalleled single-cell resolution and integration of cutting-edge microfluidics with proteomics. Weaknesses: Potential limitations in throughput and the need for specialized equipment and expertise.
Core Innovations in Lithium Orotate Proteomics
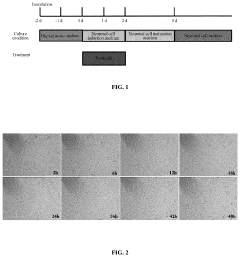
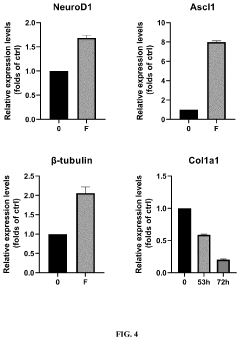
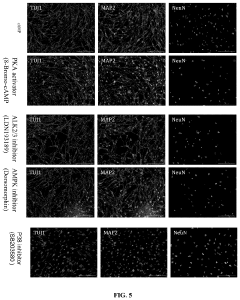
Method for efficiently inducing reprogramming of human cell into neuronal cell
PatentInactiveUS20240034991A1
Innovation
- A method using a single small molecule compound or gene to regulate key signaling pathways such as cAMP-PKA-CREB-JNK, specifically inhibiting or activating proteins like AMPK, ALK2, ALK3, P38, and JNK, to efficiently induce reprogramming of human cells into neuronal cells within two days with an 80% TUJ1-positive rate.
Pharmaceutical compositions for the treatment of neuropathies containing a lipid-soluble thiamine and a magnesium compound
PatentInactiveEP0820771A2
Innovation
- The combination of benfotiamine, a low-toxicity lipid-soluble thiamine derivative that can cross the blood-brain barrier, with magnesium orotate, which acts as a magnesium fixator in cells, is used to enhance treatment efficacy.
Regulatory Landscape for Psychiatric Medications
The regulatory landscape for psychiatric medications, including lithium-based treatments, is complex and multifaceted. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the approval and regulation of psychiatric drugs. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating new drug applications and ensuring the safety and efficacy of psychiatric medications before they reach the market.
For lithium-based treatments, such as lithium orotate, the regulatory framework is particularly stringent due to the narrow therapeutic index of lithium and its potential for toxicity. The FDA has approved lithium carbonate and lithium citrate for the treatment of bipolar disorder, but other forms of lithium, including lithium orotate, are not FDA-approved for psychiatric use. This creates a regulatory gray area for compounds like lithium orotate, which may be marketed as dietary supplements but cannot make claims about treating psychiatric conditions.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Therapeutic Goods Administration (TGA) in Australia have their own guidelines for psychiatric medications. These agencies often collaborate with the FDA but may have different approval processes and requirements. For instance, the EMA's Committee for Medicinal Products for Human Use (CHMP) provides scientific opinions on marketing authorization applications for psychiatric drugs in the European Union.
The regulatory landscape also encompasses post-market surveillance and pharmacovigilance. Regulatory agencies require pharmaceutical companies to conduct ongoing safety monitoring and report adverse events associated with psychiatric medications. This is particularly important for lithium-based treatments, given their potential for side effects and the need for regular blood level monitoring.
In recent years, there has been increased regulatory focus on personalized medicine in psychiatry. This includes the development of pharmacogenomic tests to guide medication selection and dosing. The FDA has issued guidance on the development and use of such tests, which could potentially impact the regulation of lithium and other psychiatric medications in the future.
Regulatory bodies are also addressing the challenges of off-label prescribing in psychiatry. While physicians may prescribe approved medications for unapproved uses based on their clinical judgment, regulatory agencies are working to balance this practice with the need for evidence-based medicine and patient safety. This is particularly relevant for compounds like lithium orotate, which may be used off-label despite lacking FDA approval for psychiatric indications.
For lithium-based treatments, such as lithium orotate, the regulatory framework is particularly stringent due to the narrow therapeutic index of lithium and its potential for toxicity. The FDA has approved lithium carbonate and lithium citrate for the treatment of bipolar disorder, but other forms of lithium, including lithium orotate, are not FDA-approved for psychiatric use. This creates a regulatory gray area for compounds like lithium orotate, which may be marketed as dietary supplements but cannot make claims about treating psychiatric conditions.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Therapeutic Goods Administration (TGA) in Australia have their own guidelines for psychiatric medications. These agencies often collaborate with the FDA but may have different approval processes and requirements. For instance, the EMA's Committee for Medicinal Products for Human Use (CHMP) provides scientific opinions on marketing authorization applications for psychiatric drugs in the European Union.
The regulatory landscape also encompasses post-market surveillance and pharmacovigilance. Regulatory agencies require pharmaceutical companies to conduct ongoing safety monitoring and report adverse events associated with psychiatric medications. This is particularly important for lithium-based treatments, given their potential for side effects and the need for regular blood level monitoring.
In recent years, there has been increased regulatory focus on personalized medicine in psychiatry. This includes the development of pharmacogenomic tests to guide medication selection and dosing. The FDA has issued guidance on the development and use of such tests, which could potentially impact the regulation of lithium and other psychiatric medications in the future.
Regulatory bodies are also addressing the challenges of off-label prescribing in psychiatry. While physicians may prescribe approved medications for unapproved uses based on their clinical judgment, regulatory agencies are working to balance this practice with the need for evidence-based medicine and patient safety. This is particularly relevant for compounds like lithium orotate, which may be used off-label despite lacking FDA approval for psychiatric indications.
Ethical Considerations in Neuropharmacology Research
Ethical considerations in neuropharmacology research, particularly in the context of proteomic analysis of lithium orotate-treated neuronal cells, are of paramount importance. The use of human or animal subjects in such studies necessitates a careful balance between scientific progress and the protection of research participants.
One primary ethical concern is the potential long-term effects of lithium orotate on neuronal cells. While the study aims to understand the proteomic changes, researchers must consider the possibility of unforeseen consequences on brain function and overall health. This requires thorough pre-clinical testing and a gradual approach to human trials, if applicable.
Informed consent is another crucial aspect of ethical research in this field. Participants, whether human subjects or donors of neuronal tissue, must be fully aware of the study's purpose, potential risks, and expected outcomes. Researchers have a responsibility to communicate complex scientific concepts in an accessible manner, ensuring that consent is truly informed.
The use of animal models in neuropharmacology research also raises ethical questions. While animal studies can provide valuable insights into the effects of lithium orotate on neuronal cells, researchers must adhere to the principles of the 3Rs: Replacement, Reduction, and Refinement. This involves exploring alternative methods where possible, minimizing the number of animals used, and refining protocols to reduce suffering.
Data privacy and confidentiality are paramount in proteomic analysis studies. The genetic and proteomic information obtained from neuronal cells can be highly sensitive. Researchers must implement robust data protection measures to safeguard participants' privacy and prevent unauthorized access or misuse of this information.
The potential for dual use of research findings in neuropharmacology also presents an ethical dilemma. While the primary aim may be therapeutic, there is a risk that knowledge gained from proteomic analysis could be misused for non-medical purposes. Researchers and institutions must be vigilant in monitoring and controlling the dissemination of sensitive information.
Equity in research participation and benefit sharing is another important consideration. Efforts should be made to ensure diverse representation in study populations, considering factors such as age, gender, and ethnicity. Additionally, if the research leads to therapeutic advancements, there is an ethical obligation to ensure fair access to these benefits across different socioeconomic groups.
Lastly, transparency in reporting research methods and results is crucial for maintaining ethical standards. This includes publishing both positive and negative findings to prevent bias in the scientific literature and to provide a complete picture of the effects of lithium orotate on neuronal cells.
One primary ethical concern is the potential long-term effects of lithium orotate on neuronal cells. While the study aims to understand the proteomic changes, researchers must consider the possibility of unforeseen consequences on brain function and overall health. This requires thorough pre-clinical testing and a gradual approach to human trials, if applicable.
Informed consent is another crucial aspect of ethical research in this field. Participants, whether human subjects or donors of neuronal tissue, must be fully aware of the study's purpose, potential risks, and expected outcomes. Researchers have a responsibility to communicate complex scientific concepts in an accessible manner, ensuring that consent is truly informed.
The use of animal models in neuropharmacology research also raises ethical questions. While animal studies can provide valuable insights into the effects of lithium orotate on neuronal cells, researchers must adhere to the principles of the 3Rs: Replacement, Reduction, and Refinement. This involves exploring alternative methods where possible, minimizing the number of animals used, and refining protocols to reduce suffering.
Data privacy and confidentiality are paramount in proteomic analysis studies. The genetic and proteomic information obtained from neuronal cells can be highly sensitive. Researchers must implement robust data protection measures to safeguard participants' privacy and prevent unauthorized access or misuse of this information.
The potential for dual use of research findings in neuropharmacology also presents an ethical dilemma. While the primary aim may be therapeutic, there is a risk that knowledge gained from proteomic analysis could be misused for non-medical purposes. Researchers and institutions must be vigilant in monitoring and controlling the dissemination of sensitive information.
Equity in research participation and benefit sharing is another important consideration. Efforts should be made to ensure diverse representation in study populations, considering factors such as age, gender, and ethnicity. Additionally, if the research leads to therapeutic advancements, there is an ethical obligation to ensure fair access to these benefits across different socioeconomic groups.
Lastly, transparency in reporting research methods and results is crucial for maintaining ethical standards. This includes publishing both positive and negative findings to prevent bias in the scientific literature and to provide a complete picture of the effects of lithium orotate on neuronal cells.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!