PVDF Applications in Healthcare: Revolutionizing Bone Implants
PVDF in Healthcare: Evolution and Objectives
Polyvinylidene fluoride (PVDF) has emerged as a revolutionary material in healthcare, particularly in the field of bone implants. The evolution of PVDF in medical applications can be traced back to the 1960s when its unique piezoelectric properties were first discovered. Since then, researchers and medical professionals have been exploring its potential to enhance bone regeneration and improve implant integration.
The primary objective of PVDF applications in healthcare is to develop advanced bone implants that can stimulate bone growth and accelerate healing processes. This goal aligns with the broader aim of improving patient outcomes and reducing recovery times in orthopedic surgeries. PVDF's ability to generate electrical charges in response to mechanical stress makes it an ideal candidate for creating "smart" implants that can actively promote bone formation.
Over the years, the focus of PVDF research in healthcare has shifted from basic material science to practical applications in bone tissue engineering. Early studies concentrated on understanding the fundamental properties of PVDF and its various forms, such as α, β, and γ phases. As research progressed, scientists began to explore methods to optimize PVDF for biomedical use, including surface modifications and the development of PVDF-based composites.
The technological evolution of PVDF in healthcare has been marked by several key milestones. These include the development of electrospun PVDF nanofibers for tissue scaffolds, the creation of PVDF-based piezoelectric sensors for monitoring bone healing, and the integration of PVDF into 3D-printed implants. Each of these advancements has contributed to the growing potential of PVDF in revolutionizing bone implant technology.
Current research objectives in PVDF applications for bone implants include enhancing biocompatibility, improving long-term stability in physiological environments, and developing methods to precisely control the piezoelectric response of PVDF-based implants. Additionally, there is a strong focus on translating laboratory findings into clinically viable products that can meet regulatory standards and improve patient care on a large scale.
Looking ahead, the future objectives for PVDF in healthcare extend beyond bone implants. Researchers are exploring its potential in other areas of regenerative medicine, such as nerve repair and cardiac tissue engineering. The versatility of PVDF opens up possibilities for creating multifunctional medical devices that can simultaneously promote tissue regeneration, deliver therapeutic agents, and provide real-time monitoring of healing processes.
Market Demand for Advanced Bone Implants
The global market for advanced bone implants has been experiencing significant growth, driven by an aging population, increasing prevalence of bone disorders, and rising demand for minimally invasive surgical procedures. As healthcare systems worldwide face the challenge of treating a growing number of patients with bone-related conditions, the need for innovative and effective bone implant solutions has become more pressing than ever.
PVDF (Polyvinylidene fluoride) has emerged as a promising material in the field of bone implants, offering unique properties that address many of the limitations of traditional implant materials. The market demand for PVDF-based bone implants is expected to surge in the coming years, as healthcare providers and patients alike seek more durable, biocompatible, and functionally superior alternatives to conventional implants.
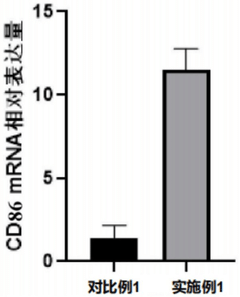
One of the key drivers of market demand for PVDF bone implants is their exceptional biocompatibility. Unlike some metallic implants that may cause allergic reactions or long-term complications, PVDF demonstrates excellent compatibility with human tissue, reducing the risk of rejection and improving patient outcomes. This factor alone has generated significant interest among orthopedic surgeons and patients, particularly those with sensitivities to traditional implant materials.
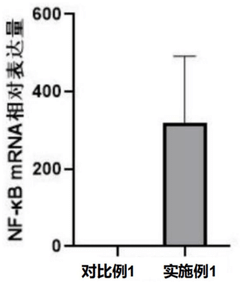
Another crucial aspect fueling the demand for PVDF bone implants is their piezoelectric properties. This unique characteristic allows PVDF implants to generate small electrical charges in response to mechanical stress, mimicking the natural bone's ability to stimulate growth and regeneration. As a result, PVDF implants have shown promising results in promoting faster healing and stronger bone integration, addressing a long-standing challenge in orthopedic medicine.
The market for PVDF bone implants is also being driven by the material's excellent mechanical properties. PVDF offers a favorable balance of strength, flexibility, and durability, making it suitable for a wide range of bone implant applications. From load-bearing implants in major joints to small-scale devices for facial reconstruction, PVDF's versatility is opening up new possibilities in orthopedic and maxillofacial surgery.
Furthermore, the growing trend towards personalized medicine has created a demand for customizable implant solutions. PVDF's processability and compatibility with advanced manufacturing techniques, such as 3D printing, position it as an ideal material for creating patient-specific implants. This capability is particularly valuable in complex cases where standard implants may not provide optimal results.
The market demand for PVDF bone implants is also influenced by the increasing focus on long-term implant performance and reduced revision surgery rates. The material's resistance to degradation and its ability to maintain its properties over time offer the potential for longer-lasting implants, which is a significant consideration for both healthcare providers and patients in terms of cost-effectiveness and quality of life.
As research continues to unveil new applications and benefits of PVDF in bone implants, the market demand is expected to expand further. Healthcare institutions and research facilities are investing in studies to explore the full potential of PVDF-based implants, which is likely to drive innovation and create new market opportunities in the coming years.
PVDF Technology: Current State and Challenges
Polyvinylidene fluoride (PVDF) has emerged as a promising material in healthcare applications, particularly in the field of bone implants. However, the current state of PVDF technology faces several challenges that need to be addressed for its widespread adoption in medical devices.
One of the primary challenges is the biocompatibility of PVDF. While the material has shown promising results in laboratory studies, long-term in vivo studies are still limited. Researchers are working to enhance the biocompatibility of PVDF through surface modifications and composite formulations to improve cell adhesion and proliferation on implant surfaces.
Another significant challenge is the mechanical properties of PVDF. Although it possesses excellent flexibility and durability, its strength and stiffness may not always match those of natural bone. This mismatch can lead to stress shielding and potential implant failure. Current research focuses on developing PVDF-based composites that can better mimic the mechanical properties of natural bone tissue.
The piezoelectric properties of PVDF, while advantageous for bone regeneration, present challenges in terms of consistent and controlled electrical stimulation. Researchers are exploring ways to optimize the piezoelectric response of PVDF in implant applications, including the development of novel poling techniques and the incorporation of piezoelectric nanoparticles.
Manufacturing scalability is another hurdle in PVDF technology for healthcare applications. Current production methods for PVDF-based implants are often complex and costly, limiting their widespread use. Efforts are underway to develop more efficient and cost-effective manufacturing processes, such as 3D printing and electrospinning techniques, to produce PVDF implants with precise geometries and properties.
The long-term stability and degradation behavior of PVDF in physiological environments remain areas of concern. While PVDF is generally considered biostable, its performance over extended periods in the human body needs further investigation. Researchers are studying the effects of various physiological conditions on PVDF properties and developing strategies to enhance its long-term stability.
Regulatory approval presents another significant challenge for PVDF-based medical devices. The novel nature of PVDF applications in bone implants requires extensive safety and efficacy data to meet regulatory standards. Collaborative efforts between researchers, industry, and regulatory bodies are crucial to establish clear guidelines and streamline the approval process for PVDF-based implants.
Lastly, the integration of PVDF with other materials and technologies, such as drug delivery systems and smart sensing capabilities, poses both opportunities and challenges. Researchers are exploring ways to incorporate these functionalities into PVDF-based implants while maintaining their core properties and performance.
Current PVDF Solutions for Bone Implants
01 PVDF-based bone implant materials
PVDF is used as a base material for bone implants due to its biocompatibility, durability, and piezoelectric properties. These implants can be designed to mimic the natural bone structure and promote bone growth. The PVDF-based materials can be modified or combined with other substances to enhance their performance in bone regeneration and integration.- PVDF-based bone implant materials: PVDF (Polyvinylidene fluoride) is used as a base material for bone implants due to its biocompatibility, durability, and piezoelectric properties. These implants can be designed to mimic the natural bone structure and promote bone growth. The PVDF material can be modified or combined with other substances to enhance its performance in bone regeneration and integration.
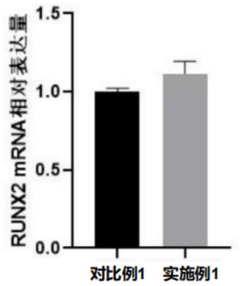
- Surface modification of PVDF bone implants: Various surface modification techniques are applied to PVDF bone implants to improve their bioactivity and osseointegration. These modifications can include plasma treatment, coating with bioactive materials, or creating specific surface topographies. Such treatments enhance cell adhesion, proliferation, and differentiation, leading to better bone-implant interfaces.
- Composite PVDF bone implants: PVDF is often combined with other materials to create composite bone implants with enhanced properties. These composites may include ceramic particles, hydroxyapatite, or other polymers. The resulting materials aim to improve mechanical strength, bioactivity, and osteoconductivity compared to pure PVDF implants.
- PVDF-based scaffolds for bone tissue engineering: PVDF is used to create porous scaffolds for bone tissue engineering applications. These scaffolds provide a three-dimensional structure that supports cell growth, differentiation, and new bone formation. Various techniques, such as electrospinning or 3D printing, are employed to fabricate these scaffolds with controlled porosity and mechanical properties.
- Drug-eluting PVDF bone implants: PVDF bone implants can be designed to incorporate and release therapeutic agents, such as antibiotics, growth factors, or anti-inflammatory drugs. This feature allows for localized drug delivery at the implant site, potentially reducing complications and promoting faster healing. The drug release kinetics can be controlled by modifying the PVDF structure or incorporating additional materials.
02 Surface modification of PVDF bone implants
Various surface modification techniques are applied to PVDF bone implants to improve their bioactivity and osseointegration. These modifications can include plasma treatment, coating with bioactive materials, or creating specific surface topographies. Such treatments enhance cell adhesion, proliferation, and differentiation on the implant surface.Expand Specific Solutions03 Composite PVDF materials for bone implants
PVDF is often combined with other materials to create composite bone implants with enhanced properties. These composites may include ceramic particles, hydroxyapatite, or other polymers. The resulting materials aim to improve mechanical strength, bioactivity, and bone-like characteristics of the implants.Expand Specific Solutions04 PVDF-based scaffolds for bone tissue engineering
PVDF is used to create porous scaffolds for bone tissue engineering applications. These scaffolds provide a three-dimensional structure for cell growth and bone formation. Various techniques, such as electrospinning or 3D printing, are employed to fabricate PVDF scaffolds with controlled porosity and mechanical properties.Expand Specific Solutions05 Functionalization of PVDF bone implants
PVDF bone implants are functionalized with bioactive molecules, growth factors, or drugs to enhance their therapeutic effects. This functionalization can promote bone regeneration, reduce inflammation, or prevent bacterial infections. Various methods are used to incorporate these functional elements into the PVDF matrix or onto its surface.Expand Specific Solutions
Key Players in PVDF-Based Medical Devices
The PVDF applications in healthcare, particularly in bone implants, are in a growth phase with increasing market potential. The global bone graft substitutes market, which includes PVDF implants, is expanding rapidly due to rising orthopedic procedures and an aging population. Technologically, PVDF is gaining traction for its biocompatibility and piezoelectric properties, attracting interest from both established medical device companies and research institutions. Key players like Warsaw Orthopedic, Stryker Corp., and Synthes GmbH are advancing PVDF technology in orthopedics, while universities such as Zhengzhou University and Wuhan University are contributing to fundamental research. The involvement of diverse entities, from large corporations to specialized firms like BioMimetic Therapeutics LLC and Nobil Bio Ricerche SRL, indicates a maturing but still evolving technological landscape with significant room for innovation and market growth.
Warsaw Orthopedic, Inc.
Genentech, Inc.
Innovative PVDF Technologies for Implants
- Zirconium and hydroxyapatite are deposited on the surface of PVDF films through magnetron sputtering technology to form zirconium and hydroxyapatite-doped PVDF films, improving its biocompatibility, antibacterial properties and osteogenic activity.
- A multifunctional biomagnetoactive composite (PVDF-Bio-Magnetic) with piezoelectric and magnetic properties, which can be externally stimulated to enhance bioactivity, is used to accelerate bone healing by applying magnetic fields, allowing 3D printing of customized implants and scaffolds.
Regulatory Framework for PVDF Medical Devices
The regulatory framework for PVDF medical devices, particularly in the context of bone implants, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the approval and regulation of medical devices containing PVDF. These devices typically fall under Class II or Class III classifications, depending on their intended use and potential risks.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their PVDF-based implant is substantially equivalent to a legally marketed predicate device. This process involves providing comprehensive data on the device's safety and effectiveness, including biocompatibility testing, mechanical properties, and clinical performance.
Class III devices, which may include certain novel PVDF bone implants, require a more rigorous premarket approval (PMA) process. This involves submitting extensive clinical trial data to prove the device's safety and efficacy. The FDA also mandates ongoing post-market surveillance to monitor long-term performance and identify any potential adverse events.
In the European Union, PVDF medical devices must comply with the Medical Device Regulation (MDR) 2017/745. This regulation emphasizes a life-cycle approach to device safety, requiring manufacturers to implement robust quality management systems and conduct thorough risk assessments. PVDF bone implants are typically classified as Class IIb or Class III devices under the MDR, necessitating conformity assessment by a notified body.
The regulatory framework also addresses the unique properties of PVDF in medical applications. Manufacturers must demonstrate that their PVDF-based implants meet specific requirements for biocompatibility, as outlined in ISO 10993. This includes evaluating potential cytotoxicity, sensitization, and local tissue effects.
Additionally, regulatory bodies are increasingly focusing on the long-term stability and degradation characteristics of PVDF in the physiological environment. Manufacturers must provide data on the material's resistance to hydrolysis, oxidation, and other potential degradation mechanisms that could affect the implant's performance over time.
As the field of PVDF applications in healthcare continues to advance, regulatory frameworks are adapting to address emerging challenges. This includes considerations for 3D-printed PVDF implants, which may require specialized testing protocols to ensure consistent quality and performance. Regulatory agencies are also developing guidance on the use of PVDF in combination products, where the material may be used in conjunction with drugs or biologics.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the application of PVDF for bone implants. The material's interaction with the human body must be thoroughly evaluated to ensure long-term safety and efficacy. PVDF has demonstrated excellent biocompatibility in various medical applications, including sutures and vascular grafts, which provides a promising foundation for its use in bone implants.
One of the primary advantages of PVDF in bone implants is its low toxicity and resistance to degradation in the body. Unlike some other polymers, PVDF does not release harmful byproducts as it ages, maintaining its structural integrity over time. This characteristic is crucial for long-term implant stability and patient safety.
The surface properties of PVDF play a significant role in its biocompatibility. The material's hydrophobic nature can be modified through various surface treatments to enhance cell adhesion and proliferation. Techniques such as plasma treatment or chemical functionalization can create a more favorable environment for osteoblast attachment and bone tissue integration.
Inflammatory response is a critical factor in implant biocompatibility. Studies have shown that PVDF elicits minimal inflammatory reactions compared to other commonly used implant materials. This reduced inflammation contributes to faster healing and a lower risk of implant rejection, enhancing overall patient outcomes.
The piezoelectric properties of PVDF offer unique advantages in bone implant applications. The material's ability to generate small electrical charges in response to mechanical stress mimics the natural electrical properties of bone, potentially stimulating bone growth and remodeling. However, the long-term effects of this piezoelectric activity on surrounding tissues must be carefully evaluated to ensure safety.
Sterilization compatibility is another crucial safety consideration for PVDF bone implants. The material must maintain its structural and functional properties after sterilization processes such as gamma irradiation or ethylene oxide treatment. Research has shown that PVDF retains its mechanical and piezoelectric properties after standard sterilization procedures, supporting its suitability for medical implants.
While PVDF shows promise in bone implant applications, comprehensive long-term studies are necessary to fully assess its safety profile. Factors such as wear particle generation, potential for metal ion release (in cases where PVDF is used in combination with metallic components), and the material's behavior under various physiological conditions must be thoroughly investigated.