PVDF Benefits for Biocompatibility: Advancing Medical Devices
PVDF Biocompatibility Evolution
The evolution of PVDF biocompatibility in medical devices has been a significant journey, marked by continuous advancements and breakthroughs. Initially, PVDF was primarily used in industrial applications due to its excellent chemical resistance and mechanical properties. However, its potential for biomedical applications was recognized in the late 1970s, leading to extensive research into its biocompatibility.
In the 1980s, early studies focused on understanding PVDF's interaction with biological systems. Researchers discovered that PVDF's inert nature and low toxicity made it a promising candidate for implantable devices. This period saw the first attempts to use PVDF in sutures and vascular grafts, although with limited success due to concerns about long-term stability and integration with host tissues.
The 1990s marked a turning point in PVDF biocompatibility research. Scientists began exploring surface modification techniques to enhance PVDF's biocompatibility. Plasma treatment and chemical grafting methods were developed to alter the surface properties of PVDF, improving cell adhesion and reducing foreign body responses. These advancements led to the first successful applications of PVDF in temporary implants and drug delivery systems.
The early 2000s witnessed a surge in PVDF applications for permanent implants. Improved understanding of protein adsorption and cell-material interactions allowed for the development of PVDF-based materials with enhanced biocompatibility. This era saw the introduction of PVDF in orthopedic implants, cardiovascular devices, and neural interfaces. Researchers also began exploring the piezoelectric properties of PVDF for tissue engineering applications, opening new avenues for bioactive implants.
In the past decade, nanotechnology has played a crucial role in further advancing PVDF biocompatibility. The development of PVDF nanofibers and nanocomposites has led to materials with superior mechanical properties and improved cell interactions. These innovations have expanded PVDF's use in regenerative medicine, particularly in scaffolds for tissue engineering and wound healing applications.
Recent years have seen a focus on tailoring PVDF properties for specific biomedical applications. Advanced surface functionalization techniques, such as biomolecule immobilization and controlled release systems, have been developed to enhance PVDF's performance in targeted therapies. Additionally, the integration of PVDF with other biocompatible materials has resulted in hybrid systems with synergistic properties, further expanding its potential in medical devices.
The ongoing research in PVDF biocompatibility continues to push the boundaries of its applications in medical devices. Current trends include the development of smart PVDF-based materials that can respond to biological stimuli, as well as the exploration of PVDF's potential in 3D-printed implants and personalized medicine. As our understanding of biocompatibility at the molecular level deepens, PVDF is poised to play an increasingly important role in advancing medical device technology.
Medical Device Market Trends
The medical device market is experiencing significant growth and transformation, driven by technological advancements, aging populations, and increasing healthcare expenditure worldwide. The global medical device market was valued at approximately $456 billion in 2021 and is projected to reach $658 billion by 2028, growing at a CAGR of 5.4% during the forecast period. This robust growth is attributed to several key trends shaping the industry.
One of the most prominent trends is the increasing demand for minimally invasive procedures and implantable devices. Patients and healthcare providers are seeking less invasive treatment options that offer faster recovery times and reduced complications. This has led to a surge in the development of advanced surgical instruments, endoscopes, and implantable devices, many of which utilize biocompatible materials like PVDF.
Another significant trend is the rise of wearable medical devices and remote patient monitoring systems. The global wearable medical device market is expected to grow at a CAGR of 26.8% from 2021 to 2028, driven by the increasing prevalence of chronic diseases and the need for continuous health monitoring. These devices often require materials with excellent biocompatibility and durability, making PVDF an attractive option for manufacturers.
The integration of artificial intelligence (AI) and machine learning in medical devices is also gaining traction. AI-powered diagnostic tools, surgical robots, and personalized treatment planning systems are revolutionizing healthcare delivery. This trend is creating new opportunities for advanced materials that can withstand complex manufacturing processes and maintain biocompatibility in diverse applications.
Personalized medicine is another key trend influencing the medical device market. The growing understanding of genetic factors in disease progression has led to the development of tailored medical devices and therapies. This trend is driving demand for materials that can be customized to individual patient needs while maintaining biocompatibility and performance.
Lastly, there is an increasing focus on sustainability and eco-friendly medical devices. Healthcare providers and patients are becoming more environmentally conscious, leading to a growing demand for recyclable and biodegradable materials in medical device manufacturing. This trend presents both challenges and opportunities for materials like PVDF, which offers excellent durability but may require innovative recycling solutions.
These market trends underscore the importance of advanced, biocompatible materials like PVDF in the medical device industry. As the sector continues to evolve, materials that can meet the demands of innovative technologies while ensuring patient safety will play a crucial role in shaping the future of healthcare.
PVDF Challenges in Medicine
While PVDF (Polyvinylidene fluoride) has shown great promise in advancing medical devices due to its biocompatibility, several challenges persist in its widespread adoption in medicine. One of the primary obstacles is the complex processing requirements for PVDF. The material's high melting point and tendency to degrade at elevated temperatures make it difficult to mold and shape into intricate medical device components. This complexity often leads to increased manufacturing costs and potential quality control issues.
Another significant challenge is the limited mechanical properties of PVDF compared to some traditional medical-grade materials. Although PVDF exhibits good chemical resistance and biocompatibility, its strength and durability may not meet the demanding requirements of certain medical applications, particularly those involving load-bearing or high-stress environments. This limitation can restrict its use in devices that require robust mechanical performance.
The long-term stability of PVDF in biological environments also presents a concern. While generally considered biocompatible, there is ongoing research to fully understand the material's behavior over extended periods of implantation. Questions remain about potential degradation products and their effects on surrounding tissues, especially in applications requiring long-term implantation.
PVDF's piezoelectric properties, while beneficial in some applications, can pose challenges in others. The material's ability to generate electrical charges in response to mechanical stress may interfere with certain medical procedures or diagnostic equipment. This characteristic necessitates careful consideration and potentially additional shielding in some medical device designs.
Furthermore, the regulatory landscape for PVDF in medical applications is still evolving. As a relatively new material in the medical field, PVDF faces rigorous scrutiny from regulatory bodies. Manufacturers must navigate complex approval processes and provide extensive documentation to demonstrate the safety and efficacy of PVDF-based medical devices. This regulatory hurdle can slow down innovation and increase time-to-market for new PVDF applications.
Lastly, the cost factor remains a significant challenge. While PVDF offers unique properties, its production and processing costs are generally higher than those of more conventional medical-grade polymers. This cost differential can be a barrier to adoption, particularly in price-sensitive markets or for high-volume medical devices. Balancing the material's benefits against its economic impact continues to be a key consideration for medical device manufacturers exploring PVDF applications.
Current PVDF Applications
01 Biocompatibility of PVDF in medical implants
PVDF demonstrates excellent biocompatibility for use in medical implants. Its non-toxic nature and resistance to degradation in biological environments make it suitable for long-term implantation. PVDF-based materials are used in various medical applications, including vascular grafts, sutures, and tissue engineering scaffolds.- Biocompatible PVDF membranes for medical applications: PVDF membranes are developed with enhanced biocompatibility for use in various medical applications. These membranes are designed to be non-toxic, non-irritating, and compatible with living tissues, making them suitable for implants, drug delivery systems, and tissue engineering scaffolds.
- Surface modification of PVDF to improve biocompatibility: Various surface modification techniques are employed to enhance the biocompatibility of PVDF. These methods include plasma treatment, grafting of biocompatible polymers, and incorporation of bioactive molecules to improve cell adhesion, proliferation, and reduce foreign body reactions.
- PVDF-based composites for biomedical applications: PVDF is combined with other biocompatible materials to create composite materials with improved properties. These composites often exhibit enhanced mechanical strength, biodegradability, or specific biological functions, making them suitable for a wide range of biomedical applications.
- Biocompatible PVDF coatings for medical devices: PVDF-based coatings are developed to improve the biocompatibility of medical devices and implants. These coatings provide a barrier between the device and the biological environment, reducing adverse reactions and improving the overall performance and longevity of the implanted devices.
- Evaluation and testing of PVDF biocompatibility: Various methods and protocols are developed to assess the biocompatibility of PVDF materials. These include in vitro cell culture studies, in vivo animal studies, and standardized biocompatibility tests to evaluate cytotoxicity, genotoxicity, and inflammatory responses to ensure the safety of PVDF-based medical products.
02 Surface modification of PVDF for enhanced biocompatibility
Various surface modification techniques are employed to improve the biocompatibility of PVDF. These include plasma treatment, chemical grafting, and coating with bioactive molecules. Such modifications can enhance cell adhesion, proliferation, and integration with surrounding tissues, making PVDF more suitable for specific biomedical applications.Expand Specific Solutions03 PVDF nanocomposites for biomedical applications
PVDF nanocomposites incorporating bioactive nanoparticles or other nanomaterials show improved biocompatibility and functionality. These nanocomposites can enhance antimicrobial properties, promote tissue regeneration, or provide additional mechanical strength, expanding the range of biomedical applications for PVDF-based materials.Expand Specific Solutions04 Biocompatibility assessment methods for PVDF
Various in vitro and in vivo methods are used to evaluate the biocompatibility of PVDF materials. These include cytotoxicity assays, cell adhesion studies, inflammatory response tests, and long-term implantation studies. Standardized testing protocols ensure consistent and reliable assessment of PVDF biocompatibility for different medical applications.Expand Specific Solutions05 PVDF in tissue engineering and regenerative medicine
PVDF's biocompatibility and piezoelectric properties make it an attractive material for tissue engineering and regenerative medicine applications. PVDF-based scaffolds and membranes are used to support cell growth, differentiation, and tissue regeneration in various contexts, including bone, nerve, and cardiac tissue engineering.Expand Specific Solutions
Key PVDF Manufacturers
The development of PVDF for biocompatibility in medical devices is in a growth phase, with increasing market size and technological advancements. The global market for biocompatible materials in medical devices is expanding rapidly, driven by the growing demand for minimally invasive procedures and implantable devices. Companies like Medtronic Vascular, Boston Scientific Scimed, and W. L. Gore & Associates are at the forefront of PVDF application in medical devices, demonstrating high technological maturity. Academic institutions such as Zhejiang University and Nanyang Technological University are contributing to research and innovation in this field. The collaboration between industry leaders and research institutions is accelerating the development of PVDF-based biocompatible solutions, positioning this technology as a key player in the future of medical device manufacturing.
Medtronic Vascular, Inc.
Boston Scientific Scimed, Inc.
PVDF Biocompatibility Research
- A PVDF-based antibacterial composition is developed by mixing poly(vinylidene fluoride) (PVDF) with pyridinium-modified poly(vinylidene fluoride) (Pyd-PVDF) in specific weight ratios, using a twin-screw extruder for solid-phase and liquid-phase mixing, which enhances antibacterial properties while maintaining mechanical strength and reducing crystallinity.
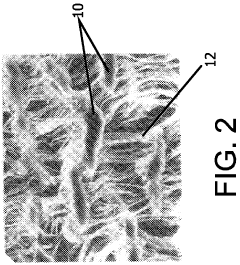
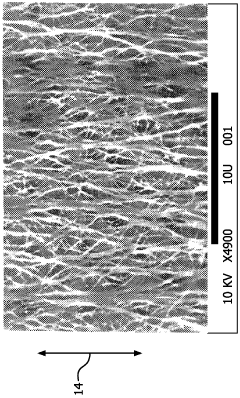
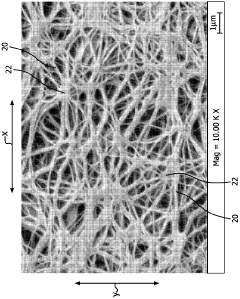
- A biomaterial with a unique node and fibril microstructure characterized by a mean internodal distance of 5 microns or less, providing a balanced distribution in both x and y directions, and optionally incorporating bioactive coatings, which allows for the creation of thin, conformable devices that minimize trauma and promote better blood-vessel interaction.
Regulatory Framework
The regulatory framework surrounding the use of PVDF (Polyvinylidene Fluoride) in medical devices is complex and multifaceted, reflecting the critical importance of ensuring patient safety and product efficacy. At the forefront of this framework are the guidelines set by major regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
In the United States, the FDA classifies medical devices into three categories based on their risk level and intended use. PVDF-based devices typically fall under Class II or III, requiring either a 510(k) premarket notification or a more rigorous Premarket Approval (PMA) process. The FDA's biocompatibility guidance, based on ISO 10993 standards, outlines specific testing requirements for materials like PVDF to ensure their safety in various medical applications.
The European Union, under the Medical Device Regulation (MDR), implements a similar risk-based classification system. PVDF devices must comply with the Essential Requirements outlined in the MDR, which includes demonstrating biocompatibility and meeting specific performance criteria. The CE marking process involves a thorough assessment of the device's safety and performance, often requiring clinical data to support claims.
Globally, the International Organization for Standardization (ISO) plays a crucial role in setting standards for medical device materials. ISO 10993 series, particularly ISO 10993-1, provides a framework for evaluating the biocompatibility of medical devices. This standard is widely recognized and adopted by regulatory agencies worldwide, serving as a cornerstone for assessing PVDF's suitability in various medical applications.
Regulatory bodies also focus on the manufacturing processes of PVDF-based devices. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of the regulatory framework. Manufacturers must demonstrate compliance with these standards to ensure consistent product quality and safety.
As the field of medical devices advances, regulatory frameworks continue to evolve. Recent trends include increased emphasis on post-market surveillance and the integration of real-world evidence in regulatory decision-making. For PVDF-based devices, this may involve long-term monitoring of device performance and patient outcomes to ensure ongoing safety and efficacy.
The regulatory landscape also addresses specific applications of PVDF in medical devices. For instance, its use in implantable devices may require additional long-term biocompatibility studies and more stringent approval processes. Conversely, its application in external medical devices might follow a less rigorous pathway, though still requiring thorough safety and performance evaluations.
PVDF Sustainability Impact
The sustainability impact of Polyvinylidene Fluoride (PVDF) in medical devices is a critical consideration as the healthcare industry strives for more environmentally friendly practices. PVDF, known for its biocompatibility and durability, offers several advantages in terms of sustainability compared to traditional materials used in medical devices.
One of the primary sustainability benefits of PVDF is its long lifespan. Medical devices made with PVDF tend to have extended durability, reducing the need for frequent replacements. This longevity translates to fewer resources consumed in manufacturing and less medical waste generated over time. The material's resistance to degradation also means that PVDF-based devices maintain their performance characteristics for longer periods, ensuring consistent quality of care while minimizing environmental impact.
PVDF's chemical stability contributes to its sustainability profile. Unlike some other polymers, PVDF does not leach harmful chemicals or degrade into potentially toxic substances when exposed to bodily fluids or sterilization processes. This stability not only enhances patient safety but also reduces the environmental burden associated with the disposal of medical devices.
The material's versatility in processing methods allows for more efficient manufacturing processes. PVDF can be molded, extruded, or 3D printed, enabling the production of complex medical device components with minimal waste. This flexibility in manufacturing also supports the trend towards personalized medicine, where custom-made devices can be produced on-demand, potentially reducing overproduction and associated waste.
From an energy perspective, PVDF's low processing temperature compared to some other high-performance polymers results in reduced energy consumption during manufacturing. This lower energy requirement contributes to a smaller carbon footprint in the production phase of medical devices.
PVDF's recyclability is another factor contributing to its sustainability. While recycling medical devices poses challenges due to contamination concerns, the potential for recycling PVDF at the end of a device's life cycle exists. Research is ongoing to develop safe and effective methods for recycling PVDF from medical applications, which could significantly reduce the environmental impact of these devices.
The material's resistance to microbial growth also plays a role in sustainability. By inhibiting bacterial colonization, PVDF-based devices may require less frequent replacement due to infection-related issues, further reducing waste and resource consumption in healthcare settings.
However, it's important to note that the production of PVDF does involve fluorine-based chemistry, which has its own environmental considerations. Ongoing research aims to develop more sustainable production methods for PVDF, focusing on reducing the use of harmful precursors and improving the overall environmental footprint of the manufacturing process.