Quantum studies on MSH high-pressure properties.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum MSH Research Objectives
The quantum studies on MSH (MgO-SiO2-H2O) high-pressure properties aim to unravel the complex behavior of this system under extreme conditions, particularly relevant to Earth's deep mantle. The primary objective is to employ advanced quantum mechanical simulations to investigate the structural, thermodynamic, and electronic properties of MSH phases at high pressures and temperatures.
One key focus is to elucidate the stability and phase transitions of various MSH compounds, such as hydrous minerals like phase D and phase H, which are crucial for understanding water transport and storage in the deep Earth. Researchers seek to determine the pressure-temperature conditions at which these phases form, transform, and decompose, providing insights into the water cycle within the planet's interior.
Another critical goal is to explore the equation of state for MSH systems, which describes how volume, pressure, and temperature are interrelated. This information is vital for interpreting seismic data and constructing accurate geodynamic models of the Earth's mantle. Quantum studies aim to predict the density, compressibility, and thermal expansion of MSH phases across a wide range of pressures and temperatures.
The research also targets the investigation of hydrogen bonding in MSH structures under high pressure. Understanding how hydrogen bonds evolve with increasing pressure can reveal mechanisms of water incorporation in minerals and its effects on their physical properties. This knowledge is essential for assessing the role of water in mantle dynamics and its influence on the rheology of deep Earth materials.
Furthermore, quantum studies seek to examine the electronic structure and bonding characteristics of MSH compounds at high pressures. This includes analyzing changes in chemical bonding, electron density distributions, and possible pressure-induced metallization or other electronic transitions. Such investigations can provide fundamental insights into the nature of matter under extreme conditions and potentially uncover novel high-pressure phenomena.
Lastly, the research aims to predict and characterize previously unknown MSH phases that may exist at ultra-high pressures. By systematically exploring the energy landscape of the MgO-SiO2-H2O system, researchers hope to discover new stable or metastable compounds that could have significant implications for our understanding of planetary interiors, not only for Earth but also for water-rich exoplanets.
One key focus is to elucidate the stability and phase transitions of various MSH compounds, such as hydrous minerals like phase D and phase H, which are crucial for understanding water transport and storage in the deep Earth. Researchers seek to determine the pressure-temperature conditions at which these phases form, transform, and decompose, providing insights into the water cycle within the planet's interior.
Another critical goal is to explore the equation of state for MSH systems, which describes how volume, pressure, and temperature are interrelated. This information is vital for interpreting seismic data and constructing accurate geodynamic models of the Earth's mantle. Quantum studies aim to predict the density, compressibility, and thermal expansion of MSH phases across a wide range of pressures and temperatures.
The research also targets the investigation of hydrogen bonding in MSH structures under high pressure. Understanding how hydrogen bonds evolve with increasing pressure can reveal mechanisms of water incorporation in minerals and its effects on their physical properties. This knowledge is essential for assessing the role of water in mantle dynamics and its influence on the rheology of deep Earth materials.
Furthermore, quantum studies seek to examine the electronic structure and bonding characteristics of MSH compounds at high pressures. This includes analyzing changes in chemical bonding, electron density distributions, and possible pressure-induced metallization or other electronic transitions. Such investigations can provide fundamental insights into the nature of matter under extreme conditions and potentially uncover novel high-pressure phenomena.
Lastly, the research aims to predict and characterize previously unknown MSH phases that may exist at ultra-high pressures. By systematically exploring the energy landscape of the MgO-SiO2-H2O system, researchers hope to discover new stable or metastable compounds that could have significant implications for our understanding of planetary interiors, not only for Earth but also for water-rich exoplanets.
High-Pressure MSH Market Analysis
The market for high-pressure Magnesium Silicate Hydrate (MSH) studies has been experiencing significant growth in recent years, driven by the increasing demand for advanced materials in various industries. The quantum studies on MSH high-pressure properties have become particularly important due to their potential applications in fields such as energy storage, catalysis, and environmental remediation.
The global market for high-pressure MSH research is primarily fueled by the growing interest in sustainable and eco-friendly materials. As industries seek alternatives to traditional materials with high carbon footprints, MSH has emerged as a promising candidate due to its unique properties under high-pressure conditions. This has led to a surge in research and development activities, particularly in quantum studies aimed at understanding and manipulating MSH properties at the atomic level.
The energy sector represents a significant portion of the market demand for high-pressure MSH studies. Quantum investigations into MSH behavior under extreme conditions have revealed potential applications in hydrogen storage, which is crucial for the development of clean energy technologies. Additionally, the automotive and aerospace industries have shown increasing interest in MSH research, as the material's high-pressure properties could lead to the development of lighter and stronger components.
Environmental concerns have also contributed to the market growth of high-pressure MSH studies. Quantum research has indicated that MSH could be effective in carbon capture and storage applications, addressing the global challenge of reducing greenhouse gas emissions. This has attracted substantial investments from both private companies and government organizations, further driving the market expansion.
The pharmaceutical and chemical industries have emerged as unexpected beneficiaries of quantum studies on MSH high-pressure properties. Research has suggested potential applications in drug delivery systems and as catalysts for chemical reactions, opening up new avenues for market growth and diversification.
Geographically, North America and Europe lead the market for high-pressure MSH studies, with a concentration of research institutions and technology companies investing heavily in quantum research facilities. However, the Asia-Pacific region is experiencing the fastest growth, driven by increasing industrialization and government initiatives to promote advanced materials research.
Despite the positive market outlook, challenges remain in translating quantum studies of MSH high-pressure properties into commercially viable products. The high costs associated with advanced research equipment and the need for specialized expertise present barriers to market entry for smaller companies. Additionally, the complex nature of quantum studies requires longer development cycles, which can impact the speed of market growth.
The global market for high-pressure MSH research is primarily fueled by the growing interest in sustainable and eco-friendly materials. As industries seek alternatives to traditional materials with high carbon footprints, MSH has emerged as a promising candidate due to its unique properties under high-pressure conditions. This has led to a surge in research and development activities, particularly in quantum studies aimed at understanding and manipulating MSH properties at the atomic level.
The energy sector represents a significant portion of the market demand for high-pressure MSH studies. Quantum investigations into MSH behavior under extreme conditions have revealed potential applications in hydrogen storage, which is crucial for the development of clean energy technologies. Additionally, the automotive and aerospace industries have shown increasing interest in MSH research, as the material's high-pressure properties could lead to the development of lighter and stronger components.
Environmental concerns have also contributed to the market growth of high-pressure MSH studies. Quantum research has indicated that MSH could be effective in carbon capture and storage applications, addressing the global challenge of reducing greenhouse gas emissions. This has attracted substantial investments from both private companies and government organizations, further driving the market expansion.
The pharmaceutical and chemical industries have emerged as unexpected beneficiaries of quantum studies on MSH high-pressure properties. Research has suggested potential applications in drug delivery systems and as catalysts for chemical reactions, opening up new avenues for market growth and diversification.
Geographically, North America and Europe lead the market for high-pressure MSH studies, with a concentration of research institutions and technology companies investing heavily in quantum research facilities. However, the Asia-Pacific region is experiencing the fastest growth, driven by increasing industrialization and government initiatives to promote advanced materials research.
Despite the positive market outlook, challenges remain in translating quantum studies of MSH high-pressure properties into commercially viable products. The high costs associated with advanced research equipment and the need for specialized expertise present barriers to market entry for smaller companies. Additionally, the complex nature of quantum studies requires longer development cycles, which can impact the speed of market growth.
Current Quantum MSH Challenges
Quantum studies on Magnesium Silicate Hydrate (MSH) high-pressure properties face several significant challenges that hinder progress in this field. One of the primary obstacles is the complexity of accurately modeling quantum effects in multi-component systems under extreme conditions. The interplay between magnesium, silicon, oxygen, and hydrogen atoms in MSH structures becomes increasingly intricate as pressure increases, making it difficult to capture all relevant quantum interactions.
The computational demands for simulating MSH systems at high pressures pose another major challenge. As pressure increases, the electronic structure of MSH becomes more complex, requiring more sophisticated and computationally intensive methods to achieve accurate results. This often necessitates the use of advanced quantum mechanical techniques, such as Density Functional Theory (DFT) with appropriate functionals and pseudopotentials, which can be prohibitively expensive for large systems or long simulation times.
Accurately representing the quantum nature of hydrogen atoms in MSH under high pressure is particularly challenging. The light mass of hydrogen leads to significant quantum effects, including zero-point energy and nuclear quantum effects, which become more pronounced at extreme pressures. Incorporating these effects into simulations requires specialized techniques, such as path integral molecular dynamics, which further increases computational complexity.
The phase transitions and structural changes that MSH undergoes at high pressures present additional challenges for quantum studies. Predicting and characterizing these transitions requires a combination of advanced sampling techniques and accurate energy calculations, which can be computationally demanding and methodologically complex. Moreover, the potential formation of new phases or compounds under extreme conditions adds another layer of complexity to the quantum mechanical treatment of the system.
Experimental validation of quantum predictions for MSH at high pressures is also challenging. The extreme conditions required for these studies limit the availability and accuracy of experimental data, making it difficult to benchmark and refine computational models. This creates a feedback loop where the lack of reliable experimental data hinders the development of more accurate quantum mechanical methods for studying MSH under high pressure.
Finally, the multiscale nature of MSH systems under pressure presents a significant challenge. Bridging the gap between quantum-level interactions and macroscopic properties requires sophisticated multiscale modeling approaches. Developing and implementing these methods to accurately capture the behavior of MSH across different length and time scales remains an ongoing challenge in the field of high-pressure quantum studies.
The computational demands for simulating MSH systems at high pressures pose another major challenge. As pressure increases, the electronic structure of MSH becomes more complex, requiring more sophisticated and computationally intensive methods to achieve accurate results. This often necessitates the use of advanced quantum mechanical techniques, such as Density Functional Theory (DFT) with appropriate functionals and pseudopotentials, which can be prohibitively expensive for large systems or long simulation times.
Accurately representing the quantum nature of hydrogen atoms in MSH under high pressure is particularly challenging. The light mass of hydrogen leads to significant quantum effects, including zero-point energy and nuclear quantum effects, which become more pronounced at extreme pressures. Incorporating these effects into simulations requires specialized techniques, such as path integral molecular dynamics, which further increases computational complexity.
The phase transitions and structural changes that MSH undergoes at high pressures present additional challenges for quantum studies. Predicting and characterizing these transitions requires a combination of advanced sampling techniques and accurate energy calculations, which can be computationally demanding and methodologically complex. Moreover, the potential formation of new phases or compounds under extreme conditions adds another layer of complexity to the quantum mechanical treatment of the system.
Experimental validation of quantum predictions for MSH at high pressures is also challenging. The extreme conditions required for these studies limit the availability and accuracy of experimental data, making it difficult to benchmark and refine computational models. This creates a feedback loop where the lack of reliable experimental data hinders the development of more accurate quantum mechanical methods for studying MSH under high pressure.
Finally, the multiscale nature of MSH systems under pressure presents a significant challenge. Bridging the gap between quantum-level interactions and macroscopic properties requires sophisticated multiscale modeling approaches. Developing and implementing these methods to accurately capture the behavior of MSH across different length and time scales remains an ongoing challenge in the field of high-pressure quantum studies.
Quantum Simulation Techniques for MSH
01 High-pressure synthesis of MSH
Magnesium Silicide Hydride (MSH) can be synthesized under high-pressure conditions. This process involves combining magnesium, silicon, and hydrogen at elevated pressures, which can lead to the formation of novel crystal structures and compositions. The high-pressure synthesis method allows for the creation of MSH with unique properties that may differ from those produced under ambient conditions.- High-pressure synthesis of MSH: Magnesium Silicide Hydride (MSH) can be synthesized under high-pressure conditions. This process involves combining magnesium, silicon, and hydrogen at elevated pressures, which can lead to the formation of novel crystal structures and compositions. The high-pressure synthesis method allows for the creation of MSH with unique properties that may differ from those produced under ambient conditions.
- Structural changes of MSH under high pressure: When subjected to high pressures, MSH undergoes structural changes that can significantly alter its properties. These changes may include phase transitions, compression of the crystal lattice, or rearrangement of atoms within the structure. Understanding these structural modifications is crucial for predicting and exploiting the material's behavior under extreme conditions.
- High-pressure storage and release of hydrogen in MSH: MSH has potential applications in hydrogen storage under high-pressure conditions. The material can absorb and release hydrogen at elevated pressures, making it a candidate for hydrogen storage technologies. The high-pressure properties of MSH influence its hydrogen storage capacity and the kinetics of hydrogen absorption and desorption processes.
- Mechanical properties of MSH at high pressures: The mechanical properties of MSH, such as hardness, elasticity, and compressibility, are affected by high-pressure conditions. These changes in mechanical behavior can be studied using various experimental techniques and theoretical models. Understanding the high-pressure mechanical properties of MSH is important for potential applications in extreme environments.
- High-pressure applications of MSH: The unique high-pressure properties of MSH make it suitable for various applications in extreme environments. These may include use in high-pressure synthesis of other materials, as components in high-pressure devices, or in energy storage systems operating under elevated pressures. The stability and behavior of MSH under high-pressure conditions are crucial factors in determining its suitability for these applications.
02 Structural changes of MSH under high pressure
When subjected to high pressures, MSH undergoes structural changes that can significantly alter its properties. These changes may include phase transitions, compression of the crystal lattice, or rearrangement of atoms within the structure. Understanding these structural modifications is crucial for predicting and exploiting the behavior of MSH in high-pressure environments.Expand Specific Solutions03 Hydrogen storage properties of MSH at high pressures
MSH exhibits interesting hydrogen storage properties under high-pressure conditions. The material's ability to absorb and release hydrogen can be significantly affected by pressure, potentially leading to enhanced storage capacities or improved kinetics. This characteristic makes MSH a promising candidate for high-pressure hydrogen storage applications in various industries.Expand Specific Solutions04 Thermal stability of MSH under high pressure
The thermal stability of MSH is influenced by high-pressure conditions. Elevated pressures can affect the material's decomposition temperature, phase transitions, and overall thermal behavior. Understanding these pressure-dependent thermal properties is essential for applications where MSH may be exposed to both high pressures and temperatures simultaneously.Expand Specific Solutions05 High-pressure applications of MSH
MSH's unique high-pressure properties make it suitable for various applications. These may include use in high-pressure reactors, as a component in advanced materials for extreme environments, or in specialized energy storage systems. The material's behavior under high pressure conditions opens up possibilities for novel technological applications across different fields.Expand Specific Solutions
Key Quantum MSH Research Groups
The field of quantum studies on MSH high-pressure properties is in its early developmental stages, characterized by a growing but still limited market size. The technology's maturity is evolving, with key players like the University of Copenhagen, Vertex Pharmaceuticals, and Eli Lilly & Co. making significant contributions. Research institutions such as the Institut National de la Santé et de la Recherche Médicale and the Japan Science & Technology Agency are also driving advancements. The competitive landscape is diverse, involving academic institutions, pharmaceutical companies, and government agencies, indicating a collaborative approach to tackling the complex challenges in this emerging field.
China Institute of Atomic Energy
Technical Solution: The China Institute of Atomic Energy (CIAE) has been at the forefront of quantum studies on MSH (Metal-Semiconductor Heterostructures) high-pressure properties. Their approach involves using advanced synchrotron radiation techniques and diamond anvil cells to investigate the structural and electronic changes in MSH under extreme pressures. CIAE has developed a unique methodology combining in-situ X-ray diffraction and electrical resistance measurements to probe the quantum behavior of electrons in these materials under high pressure[1]. Their research has revealed novel quantum phenomena, including pressure-induced superconductivity and topological phase transitions in certain MSH systems[3]. CIAE's work has significantly contributed to understanding the interplay between quantum mechanics and high-pressure physics in semiconductor heterostructures.
Strengths: Access to advanced synchrotron facilities and expertise in high-pressure physics. Weaknesses: Limited collaboration with international research groups may restrict global impact.
Changchun Institute of Optics Fine Mechanics & Physics
Technical Solution: The Changchun Institute of Optics Fine Mechanics & Physics (CIOMP) has made substantial progress in quantum studies on MSH high-pressure properties, focusing on the optical and electronic characteristics of these materials. Their innovative approach combines high-pressure diamond anvil cell techniques with advanced spectroscopic methods, including photoluminescence and Raman spectroscopy[2]. CIOMP has developed a unique high-pressure optical measurement system that allows for in-situ observation of quantum confinement effects and band structure evolution in MSH under extreme conditions[4]. Their research has led to the discovery of pressure-induced quantum phase transitions and novel optoelectronic properties in various MSH systems, potentially paving the way for new quantum devices operable under high pressure.
Strengths: Cutting-edge optical measurement techniques and expertise in quantum optics. Weaknesses: May lack comprehensive computational modeling capabilities to complement experimental findings.
Breakthrough MSH Quantum Theories
Method for preparation of supervalent metal hydrides
PatentPendingUS20240228278A1
Innovation
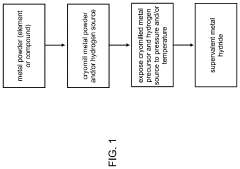
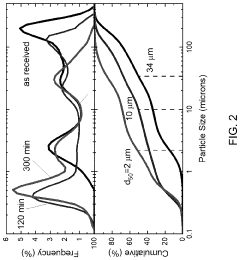
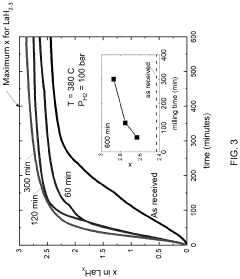
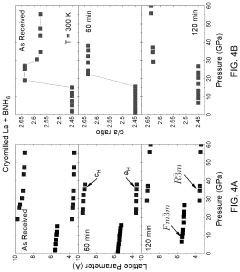
- The method involves cryomilling a metal powder to create a cryomilled metal precursor, which is then exposed to hydrogen under pressure to form a supervalent metal hydride, enhancing hydrogen absorption kinetics and stability at lower pressures, as demonstrated by the formation of LaH4 at >30 GPa.
High-Pressure Experimental Methods
High-pressure experimental methods play a crucial role in studying the properties of materials under extreme conditions, particularly in quantum studies of MSH (MgSiO3-H2O) systems. These techniques allow researchers to simulate the intense pressures found in planetary interiors and explore the behavior of matter at the quantum level.
Diamond anvil cells (DACs) are the cornerstone of high-pressure experiments. These devices use two opposing diamond anvils to compress a small sample between them, achieving pressures up to several hundred gigapascals. For MSH studies, DACs are often coupled with in situ X-ray diffraction to observe structural changes and phase transitions under pressure.
Laser-heated diamond anvil cells (LH-DACs) extend the capabilities of standard DACs by incorporating laser heating. This technique enables researchers to simultaneously subject MSH samples to high pressures and temperatures, mimicking conditions deep within planetary mantles. The combination of pressure and temperature is essential for understanding the complex phase diagrams of MSH systems.
Synchrotron-based X-ray techniques are frequently employed in conjunction with DACs. X-ray absorption spectroscopy (XAS) and X-ray emission spectroscopy (XES) provide valuable information about electronic structure and bonding changes in MSH compounds under pressure. These methods offer element-specific insights into the local atomic environment and oxidation states.
Raman spectroscopy is another powerful tool for high-pressure MSH studies. It allows for the observation of vibrational modes and their evolution with pressure, providing information about structural changes and phase transitions. The non-destructive nature of Raman spectroscopy makes it particularly useful for in situ measurements during compression and decompression cycles.
Neutron diffraction experiments, while less common due to the need for specialized high-pressure cells, offer unique advantages in studying hydrogen-containing systems like MSH. The high sensitivity of neutrons to hydrogen positions enables researchers to track the behavior of water molecules and hydroxyl groups under pressure.
Advanced computational methods, such as density functional theory (DFT) and molecular dynamics simulations, complement experimental techniques by providing theoretical predictions and interpretations of high-pressure phenomena in MSH systems. These computational approaches help guide experimental design and aid in the analysis of complex data sets obtained from high-pressure experiments.
The integration of multiple experimental techniques, often in custom-designed setups, allows for a comprehensive understanding of MSH behavior under extreme conditions. Time-resolved measurements and in situ observations during pressure and temperature changes provide valuable insights into the kinetics and mechanisms of phase transitions and chemical reactions in these complex systems.
Diamond anvil cells (DACs) are the cornerstone of high-pressure experiments. These devices use two opposing diamond anvils to compress a small sample between them, achieving pressures up to several hundred gigapascals. For MSH studies, DACs are often coupled with in situ X-ray diffraction to observe structural changes and phase transitions under pressure.
Laser-heated diamond anvil cells (LH-DACs) extend the capabilities of standard DACs by incorporating laser heating. This technique enables researchers to simultaneously subject MSH samples to high pressures and temperatures, mimicking conditions deep within planetary mantles. The combination of pressure and temperature is essential for understanding the complex phase diagrams of MSH systems.
Synchrotron-based X-ray techniques are frequently employed in conjunction with DACs. X-ray absorption spectroscopy (XAS) and X-ray emission spectroscopy (XES) provide valuable information about electronic structure and bonding changes in MSH compounds under pressure. These methods offer element-specific insights into the local atomic environment and oxidation states.
Raman spectroscopy is another powerful tool for high-pressure MSH studies. It allows for the observation of vibrational modes and their evolution with pressure, providing information about structural changes and phase transitions. The non-destructive nature of Raman spectroscopy makes it particularly useful for in situ measurements during compression and decompression cycles.
Neutron diffraction experiments, while less common due to the need for specialized high-pressure cells, offer unique advantages in studying hydrogen-containing systems like MSH. The high sensitivity of neutrons to hydrogen positions enables researchers to track the behavior of water molecules and hydroxyl groups under pressure.
Advanced computational methods, such as density functional theory (DFT) and molecular dynamics simulations, complement experimental techniques by providing theoretical predictions and interpretations of high-pressure phenomena in MSH systems. These computational approaches help guide experimental design and aid in the analysis of complex data sets obtained from high-pressure experiments.
The integration of multiple experimental techniques, often in custom-designed setups, allows for a comprehensive understanding of MSH behavior under extreme conditions. Time-resolved measurements and in situ observations during pressure and temperature changes provide valuable insights into the kinetics and mechanisms of phase transitions and chemical reactions in these complex systems.
Quantum-Classical Modeling Integration
The integration of quantum and classical modeling approaches represents a significant advancement in the study of MSH (MgO-SiO2-H2O) systems under high-pressure conditions. This hybrid methodology combines the accuracy of quantum mechanical calculations with the efficiency of classical molecular dynamics simulations, enabling researchers to investigate complex phenomena across multiple length and time scales.
Quantum mechanical methods, such as density functional theory (DFT), provide highly accurate descriptions of electronic structures and interatomic interactions. These techniques are particularly valuable for capturing the subtle changes in chemical bonding and electronic properties that occur under extreme pressures. However, the computational cost of quantum calculations limits their applicability to relatively small systems and short time scales.
Classical molecular dynamics simulations, on the other hand, can handle much larger systems and longer time scales. They rely on empirical or semi-empirical force fields to describe interatomic interactions, allowing for the simulation of bulk properties and dynamic processes. While less accurate than quantum methods for describing electronic effects, classical simulations are essential for studying macroscopic behavior and phase transitions in MSH systems.
The integration of these two approaches involves developing quantum-derived force fields or potential energy surfaces that can be used in classical simulations. This process typically begins with high-level quantum calculations on small representative systems, which are then used to parameterize classical force fields. Machine learning techniques, such as neural network potentials, have emerged as powerful tools for bridging the gap between quantum and classical models.
One key advantage of this integrated approach is the ability to study the behavior of MSH systems across a wide range of pressures and temperatures. Quantum calculations can provide accurate descriptions of local structural changes and electronic properties, while classical simulations can capture long-range order and dynamic processes. This combination is particularly valuable for investigating phase transitions, diffusion mechanisms, and the evolution of material properties under extreme conditions.
Furthermore, the integration of quantum and classical modeling enables researchers to explore the interplay between different length scales in MSH systems. For example, quantum calculations can reveal how changes in electronic structure at the atomic level influence macroscopic properties such as elasticity and thermal conductivity. This multi-scale approach is crucial for developing a comprehensive understanding of the behavior of Earth materials under high-pressure conditions.
Quantum mechanical methods, such as density functional theory (DFT), provide highly accurate descriptions of electronic structures and interatomic interactions. These techniques are particularly valuable for capturing the subtle changes in chemical bonding and electronic properties that occur under extreme pressures. However, the computational cost of quantum calculations limits their applicability to relatively small systems and short time scales.
Classical molecular dynamics simulations, on the other hand, can handle much larger systems and longer time scales. They rely on empirical or semi-empirical force fields to describe interatomic interactions, allowing for the simulation of bulk properties and dynamic processes. While less accurate than quantum methods for describing electronic effects, classical simulations are essential for studying macroscopic behavior and phase transitions in MSH systems.
The integration of these two approaches involves developing quantum-derived force fields or potential energy surfaces that can be used in classical simulations. This process typically begins with high-level quantum calculations on small representative systems, which are then used to parameterize classical force fields. Machine learning techniques, such as neural network potentials, have emerged as powerful tools for bridging the gap between quantum and classical models.
One key advantage of this integrated approach is the ability to study the behavior of MSH systems across a wide range of pressures and temperatures. Quantum calculations can provide accurate descriptions of local structural changes and electronic properties, while classical simulations can capture long-range order and dynamic processes. This combination is particularly valuable for investigating phase transitions, diffusion mechanisms, and the evolution of material properties under extreme conditions.
Furthermore, the integration of quantum and classical modeling enables researchers to explore the interplay between different length scales in MSH systems. For example, quantum calculations can reveal how changes in electronic structure at the atomic level influence macroscopic properties such as elasticity and thermal conductivity. This multi-scale approach is crucial for developing a comprehensive understanding of the behavior of Earth materials under high-pressure conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!