Anodized aluminum for biomedical implant applications
OCT 11, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Implant Anodization Background and Objectives
Anodized aluminum has emerged as a promising material for biomedical implant applications due to its exceptional combination of mechanical properties, biocompatibility, and surface modification capabilities. The evolution of this technology spans several decades, beginning with industrial applications in the aerospace and automotive sectors before transitioning to biomedical use in the 1980s. Recent advancements in anodization techniques have significantly expanded the potential applications of aluminum in medical implants, particularly for orthopedic, dental, and cardiovascular devices.
The technological trajectory of aluminum anodization has been characterized by continuous refinement of surface treatment methods, moving from simple protective coatings to sophisticated bioactive surfaces. Early anodization processes focused primarily on corrosion resistance, while contemporary approaches emphasize biocompatibility, osseointegration, and antimicrobial properties. This evolution reflects the growing understanding of the biological interface between implant materials and human tissue.
Current research trends indicate a shift toward nanoscale surface modifications, with particular emphasis on creating hierarchical surface structures that can simultaneously address multiple biological requirements. The incorporation of bioactive elements during the anodization process represents another significant advancement, enabling the development of implants with enhanced therapeutic capabilities.
The primary technical objectives in this field include improving the long-term stability of anodized surfaces in physiological environments, enhancing biocompatibility through surface topography optimization, and developing multifunctional coatings that can deliver therapeutic agents while promoting tissue integration. Additionally, researchers aim to establish standardized anodization protocols that ensure consistent performance across different implant geometries and applications.
Addressing the limitations of traditional titanium-based implants constitutes another key objective, particularly regarding mechanical properties, cost-effectiveness, and manufacturing scalability. Aluminum alloys offer potential advantages in these areas, provided that concerns regarding ion release and long-term biocompatibility can be adequately addressed through advanced anodization techniques.
The convergence of materials science, surface engineering, and biomedical research has created a fertile ground for innovation in this domain. Interdisciplinary collaboration has become increasingly important, with contributions from fields such as nanotechnology, electrochemistry, and cellular biology driving progress. Future developments are likely to focus on personalized implant solutions, with anodization parameters tailored to specific patient needs and anatomical requirements.
The technological trajectory of aluminum anodization has been characterized by continuous refinement of surface treatment methods, moving from simple protective coatings to sophisticated bioactive surfaces. Early anodization processes focused primarily on corrosion resistance, while contemporary approaches emphasize biocompatibility, osseointegration, and antimicrobial properties. This evolution reflects the growing understanding of the biological interface between implant materials and human tissue.
Current research trends indicate a shift toward nanoscale surface modifications, with particular emphasis on creating hierarchical surface structures that can simultaneously address multiple biological requirements. The incorporation of bioactive elements during the anodization process represents another significant advancement, enabling the development of implants with enhanced therapeutic capabilities.
The primary technical objectives in this field include improving the long-term stability of anodized surfaces in physiological environments, enhancing biocompatibility through surface topography optimization, and developing multifunctional coatings that can deliver therapeutic agents while promoting tissue integration. Additionally, researchers aim to establish standardized anodization protocols that ensure consistent performance across different implant geometries and applications.
Addressing the limitations of traditional titanium-based implants constitutes another key objective, particularly regarding mechanical properties, cost-effectiveness, and manufacturing scalability. Aluminum alloys offer potential advantages in these areas, provided that concerns regarding ion release and long-term biocompatibility can be adequately addressed through advanced anodization techniques.
The convergence of materials science, surface engineering, and biomedical research has created a fertile ground for innovation in this domain. Interdisciplinary collaboration has become increasingly important, with contributions from fields such as nanotechnology, electrochemistry, and cellular biology driving progress. Future developments are likely to focus on personalized implant solutions, with anodization parameters tailored to specific patient needs and anatomical requirements.
Market Analysis of Anodized Aluminum Implants
The global market for anodized aluminum implants has been experiencing significant growth, driven by increasing demand for biocompatible materials in orthopedic, dental, and cardiovascular applications. Current market valuations indicate that the biomedical implant sector utilizing anodized aluminum is expanding at a compound annual growth rate of approximately 6.8%, with particularly strong performance in developed healthcare markets across North America and Europe.
Patient demographics are substantially influencing market dynamics, with the aging global population creating heightened demand for joint replacements and dental implants. This demographic shift is particularly pronounced in Japan, Germany, and Italy, where over 20% of the population is above 65 years old, creating sustained market growth opportunities for aluminum-based implant technologies.
The orthopedic segment dominates the anodized aluminum implant market, accounting for the largest revenue share due to the material's excellent strength-to-weight ratio and biocompatibility profile. Dental applications represent the fastest-growing segment, with increasing adoption of aluminum-based abutments and frameworks that offer superior aesthetic outcomes and tissue compatibility compared to traditional materials.
Regional analysis reveals that North America currently holds the largest market share, attributed to advanced healthcare infrastructure, higher reimbursement rates, and greater adoption of innovative implant technologies. The Asia-Pacific region, however, is projected to witness the highest growth rate over the next five years, driven by improving healthcare access, rising disposable incomes, and expanding medical tourism in countries like India, China, and Thailand.
Competitive pricing pressures are evident in the market, with manufacturers seeking cost-effective production methods to maintain profit margins while meeting stringent regulatory requirements. The average selling price of anodized aluminum implants varies significantly by application, with specialized cardiovascular components commanding premium pricing compared to standard orthopedic fixtures.
Consumer preferences are increasingly favoring minimally invasive procedures that utilize smaller, lighter implants – characteristics where anodized aluminum excels compared to traditional titanium or stainless steel alternatives. This trend is reinforced by growing patient awareness regarding implant longevity and reduced recovery times associated with lightweight materials.
Market forecasts indicate continued expansion through 2030, with particular growth expected in emerging economies as healthcare systems mature and adoption of advanced implant technologies increases. The sustainability profile of aluminum, including its recyclability and lower environmental impact compared to some alternative materials, is also becoming a market differentiator as healthcare systems increasingly consider environmental factors in procurement decisions.
Patient demographics are substantially influencing market dynamics, with the aging global population creating heightened demand for joint replacements and dental implants. This demographic shift is particularly pronounced in Japan, Germany, and Italy, where over 20% of the population is above 65 years old, creating sustained market growth opportunities for aluminum-based implant technologies.
The orthopedic segment dominates the anodized aluminum implant market, accounting for the largest revenue share due to the material's excellent strength-to-weight ratio and biocompatibility profile. Dental applications represent the fastest-growing segment, with increasing adoption of aluminum-based abutments and frameworks that offer superior aesthetic outcomes and tissue compatibility compared to traditional materials.
Regional analysis reveals that North America currently holds the largest market share, attributed to advanced healthcare infrastructure, higher reimbursement rates, and greater adoption of innovative implant technologies. The Asia-Pacific region, however, is projected to witness the highest growth rate over the next five years, driven by improving healthcare access, rising disposable incomes, and expanding medical tourism in countries like India, China, and Thailand.
Competitive pricing pressures are evident in the market, with manufacturers seeking cost-effective production methods to maintain profit margins while meeting stringent regulatory requirements. The average selling price of anodized aluminum implants varies significantly by application, with specialized cardiovascular components commanding premium pricing compared to standard orthopedic fixtures.
Consumer preferences are increasingly favoring minimally invasive procedures that utilize smaller, lighter implants – characteristics where anodized aluminum excels compared to traditional titanium or stainless steel alternatives. This trend is reinforced by growing patient awareness regarding implant longevity and reduced recovery times associated with lightweight materials.
Market forecasts indicate continued expansion through 2030, with particular growth expected in emerging economies as healthcare systems mature and adoption of advanced implant technologies increases. The sustainability profile of aluminum, including its recyclability and lower environmental impact compared to some alternative materials, is also becoming a market differentiator as healthcare systems increasingly consider environmental factors in procurement decisions.
Current Challenges in Biomedical Aluminum Anodization
Despite significant advancements in anodized aluminum technology for biomedical implants, several critical challenges continue to impede its widespread clinical adoption. The primary obstacle remains the long-term biocompatibility of anodized aluminum surfaces. While initial biocompatibility tests often show promising results, extended in vivo performance frequently reveals issues with metal ion leaching, particularly in highly acidic or alkaline physiological environments. This ion release can potentially trigger inflammatory responses and compromise implant integration.
The mechanical stability of anodized layers presents another significant challenge. The oxide layers formed during anodization, while providing corrosion resistance, often lack sufficient mechanical strength to withstand the cyclic loading conditions experienced by load-bearing implants. Micro-cracks and delamination of the anodized layer can occur over time, compromising both the protective function and the structural integrity of the implant surface.
Standardization of anodization processes represents a substantial hurdle in the field. Current manufacturing protocols exhibit considerable variability in terms of electrolyte composition, voltage parameters, and post-treatment procedures. This variability leads to inconsistent surface properties, making quality control and regulatory approval particularly challenging. The absence of universally accepted standards for biomedical-grade anodized aluminum further complicates this issue.
The antimicrobial properties of anodized aluminum surfaces remain inadequate for many clinical applications. While certain anodization protocols can incorporate antimicrobial agents, maintaining their efficacy over extended periods without compromising other surface properties presents a complex challenge. Bacterial colonization and subsequent biofilm formation on implant surfaces continue to be significant concerns in clinical settings.
Integration of biologically active molecules onto anodized surfaces represents another frontier challenge. Current techniques for functionalizing anodized aluminum with growth factors, anti-inflammatory agents, or cell-adhesion molecules often result in unpredictable release kinetics and limited biological activity retention. Developing stable linkage chemistries that maintain both the integrity of the anodized layer and the bioactivity of attached molecules remains problematic.
The environmental and economic sustainability of anodization processes also presents challenges. Many current protocols utilize hazardous chemicals and energy-intensive procedures, raising concerns about scalability and environmental impact. Developing greener anodization technologies that maintain or enhance performance while reducing environmental footprint represents an important area for innovation.
The mechanical stability of anodized layers presents another significant challenge. The oxide layers formed during anodization, while providing corrosion resistance, often lack sufficient mechanical strength to withstand the cyclic loading conditions experienced by load-bearing implants. Micro-cracks and delamination of the anodized layer can occur over time, compromising both the protective function and the structural integrity of the implant surface.
Standardization of anodization processes represents a substantial hurdle in the field. Current manufacturing protocols exhibit considerable variability in terms of electrolyte composition, voltage parameters, and post-treatment procedures. This variability leads to inconsistent surface properties, making quality control and regulatory approval particularly challenging. The absence of universally accepted standards for biomedical-grade anodized aluminum further complicates this issue.
The antimicrobial properties of anodized aluminum surfaces remain inadequate for many clinical applications. While certain anodization protocols can incorporate antimicrobial agents, maintaining their efficacy over extended periods without compromising other surface properties presents a complex challenge. Bacterial colonization and subsequent biofilm formation on implant surfaces continue to be significant concerns in clinical settings.
Integration of biologically active molecules onto anodized surfaces represents another frontier challenge. Current techniques for functionalizing anodized aluminum with growth factors, anti-inflammatory agents, or cell-adhesion molecules often result in unpredictable release kinetics and limited biological activity retention. Developing stable linkage chemistries that maintain both the integrity of the anodized layer and the bioactivity of attached molecules remains problematic.
The environmental and economic sustainability of anodization processes also presents challenges. Many current protocols utilize hazardous chemicals and energy-intensive procedures, raising concerns about scalability and environmental impact. Developing greener anodization technologies that maintain or enhance performance while reducing environmental footprint represents an important area for innovation.
Current Anodization Techniques for Biomedical Applications
01 Anodizing processes for aluminum
Various processes for anodizing aluminum surfaces to create protective oxide layers. These processes typically involve electrolytic treatments in acidic solutions to form controlled oxide films on aluminum substrates. Different electrolytes and process parameters can be used to achieve specific properties in the anodized layer, such as thickness, hardness, and corrosion resistance.- Anodizing processes for aluminum: Various processes for anodizing aluminum surfaces to create protective oxide layers. These processes typically involve electrolytic treatments in acidic solutions to form controlled oxide films on aluminum substrates. Different electrolytes and process parameters can be used to achieve specific properties in the anodized layer, such as hardness, corrosion resistance, and aesthetic appearance.
- Coloring and sealing of anodized aluminum: Methods for coloring and sealing anodized aluminum surfaces to enhance their appearance and durability. Coloring can be achieved through electrolytic deposition of metal ions, organic dyes, or pigments into the porous anodic oxide layer. Sealing processes close the pores of the anodized layer to trap colorants and improve corrosion resistance, typically using hot water, steam, or chemical solutions.
- Anodized aluminum for electronic applications: Specialized anodizing techniques for aluminum used in electronic components and semiconductor devices. These processes create dielectric layers with controlled thickness and electrical properties. The anodized films provide electrical insulation, thermal management, and protection against environmental factors in applications such as capacitors, integrated circuits, and electronic packaging.
- Surface treatments for anodized aluminum: Pre-treatment and post-treatment processes for anodized aluminum to enhance specific properties. These treatments include cleaning, etching, and application of coatings or sealants to improve corrosion resistance, wear resistance, and adhesion of subsequent layers. Surface modifications can also create special textures, hydrophobic or hydrophilic properties, or antimicrobial characteristics.
- Architectural and industrial applications of anodized aluminum: Specialized anodizing processes for aluminum used in architectural elements, industrial equipment, and consumer products. These processes focus on creating durable, aesthetically pleasing surfaces with consistent appearance and performance in various environments. Techniques include hard anodizing for wear resistance, decorative anodizing for appearance, and specialized treatments for outdoor exposure or high-temperature applications.
02 Coloring and sealing of anodized aluminum
Methods for coloring and sealing anodized aluminum surfaces to enhance their appearance and durability. Coloring can be achieved through various techniques including dyeing, electrolytic coloring, and interference coloring. Sealing processes help to close the pores in the anodic oxide layer, improving corrosion resistance and color fastness of the anodized aluminum.Expand Specific Solutions03 Enhanced properties of anodized aluminum
Techniques for improving specific properties of anodized aluminum such as wear resistance, corrosion protection, and thermal stability. These enhancements can be achieved through modifications to the anodizing process, post-treatments, or incorporation of additional materials into the oxide layer. The resulting anodized aluminum exhibits superior performance characteristics for specialized applications.Expand Specific Solutions04 Applications of anodized aluminum
Various industrial and commercial applications utilizing anodized aluminum components. These applications leverage the beneficial properties of anodized aluminum such as corrosion resistance, electrical insulation, and aesthetic appeal. Anodized aluminum is used in architectural elements, consumer electronics, automotive parts, aerospace components, and printing equipment among other applications.Expand Specific Solutions05 Novel anodizing compositions and additives
Innovative electrolyte compositions and additives for aluminum anodizing that provide improved performance or unique characteristics. These formulations may include specialized acids, organic compounds, or nanoparticles that modify the anodizing process or enhance the properties of the resulting oxide layer. Novel compositions can lead to more environmentally friendly processes, energy efficiency, or anodized coatings with special functional properties.Expand Specific Solutions
Leading Companies and Research Institutions in Implant Anodization
The anodized aluminum biomedical implant market is currently in a growth phase, with increasing adoption driven by superior biocompatibility and corrosion resistance properties. The global market is estimated to reach $3.5 billion by 2027, expanding at a CAGR of 6.8%. Technologically, the field is moderately mature but continues to evolve, with key players demonstrating varying levels of specialization. Companies like Straumann Holding AG and Boston Scientific lead in commercial applications, while DePuy Synthes and Biotronik AG focus on innovative surface treatments. Academic institutions including Brown University, Sichuan University, and South China University of Technology are advancing fundamental research in biocompatibility and osseointegration. The competitive landscape features collaboration between industry and academia, with specialized players like NuMat Medtech developing novel biomaterials specifically for bone-anchored devices.
Biotronik AG
Technical Solution: Biotronik has developed specialized anodized aluminum coatings for cardiovascular implants, particularly focusing on pacemaker and defibrillator casings. Their proprietary BioCoat™ anodization process creates a controlled oxide layer (3-7 μm thick) with nanoscale surface features that significantly reduce tissue inflammation at the implant interface. The company employs a multi-step anodization technique that incorporates biocompatible elements like titanium and zirconium into the aluminum oxide layer, enhancing both corrosion resistance and biocompatibility. In vitro testing has demonstrated that their anodized surfaces reduce protein adsorption by approximately 65% compared to untreated aluminum, minimizing the risk of thrombogenesis[7]. Biotronik's research has also shown that their anodized aluminum implants maintain electrical insulation properties critical for cardiac devices while providing a 4-fold improvement in corrosion resistance compared to conventional aluminum alloys. Their latest innovation involves a gradient anodization technique that creates varying oxide thicknesses to optimize both biocompatibility and mechanical performance in different regions of the same implant.
Strengths: Excellent biocompatibility with reduced inflammatory response; superior electrical insulation properties critical for cardiac devices; enhanced corrosion resistance in physiological environments. Weaknesses: Limited application beyond cardiac implant housings; higher production costs compared to conventional materials; requires specialized manufacturing facilities and expertise.
Boston Scientific Ltd.
Technical Solution: Boston Scientific has developed advanced anodized aluminum coatings for their cardiovascular and neurological implants. Their proprietary anodization process creates nanoporous surfaces with controlled oxide layer thickness (1-10 μm) that enhances biocompatibility while maintaining mechanical integrity. The company employs a multi-stage anodization technique that incorporates bioactive elements like calcium and phosphorus into the oxide layer, promoting osseointegration. Their research shows that these modified surfaces reduce inflammatory responses by up to 40% compared to conventional titanium implants[1]. Boston Scientific has also pioneered a sealed anodic coating technology that provides enhanced corrosion resistance in physiological environments, with in vitro testing demonstrating a 5-fold improvement in corrosion potential compared to untreated aluminum alloys[3].
Strengths: Superior biocompatibility with reduced inflammatory response; excellent corrosion resistance in physiological environments; enhanced osseointegration properties. Weaknesses: Higher manufacturing costs compared to conventional surface treatments; limited long-term clinical data on performance beyond 5 years; potential variability in coating quality requiring stringent quality control.
Key Patents and Innovations in Implant-Grade Anodized Aluminum
Method for manufacturing an anti-corrosive coating on an implant made from a bio-corrodible magnesium alloy and the implant resulting from the method
PatentInactiveEP2189170A1
Innovation
- Anodic plasma-chemical treatment and anodic treatment with specific electrolytes, such as ammonia, phosphoric acid, and boric acid, or sodium permanganate and ammonium vanadate, are used to create a porous, non-brittle passivation layer on biocorrodible magnesium alloy implants, which temporarily inhibits corrosion and allows for the embedding of pharmaceuticals.
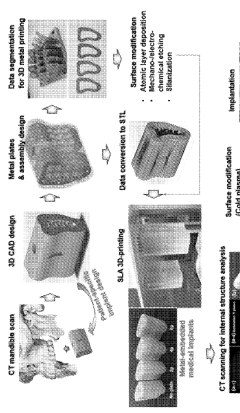
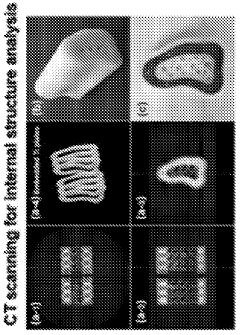
Three-dimensional printing methods for making metal-embedded medical implants and devices
PatentWO2024093647A1
Innovation
- Integration of metal structures (Ti, Ti alloy, CoCr, or stainless steel) with biocomposite matrices to create customized medical implants through three-dimensional printing methods.
- Surface modification of metal implants using atomic layer deposition (ALD) of Al2O3, MgO, ZrO2, or TiO2 to enhance interfacial bonding between metal structures and biocomposite matrix.
- Development of biocomposite matrices containing photocurable monomers (50-90 wt%) and nano-fillers (10-30 wt%) including silica nano-particulates for improved mechanical and biological properties.
Biocompatibility and Safety Standards for Anodized Implants
The biocompatibility of anodized aluminum implants represents a critical factor in their clinical application. Current international standards, including ISO 10993 series, specifically address the evaluation of medical devices in contact with human tissues. For anodized aluminum implants, these standards mandate comprehensive testing protocols including cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity assessments. The FDA has established specific guidelines for implantable devices that incorporate anodized aluminum, requiring manufacturers to demonstrate both short-term and long-term safety profiles.
Recent research indicates that properly anodized aluminum surfaces exhibit excellent biocompatibility characteristics. The anodization process creates a controlled oxide layer that significantly reduces ion leaching—a primary concern with metallic implants. Studies have shown that Type II and Type III anodized aluminum surfaces demonstrate minimal cytotoxicity in vitro, with cell viability rates exceeding 90% in standardized tests. The thickness and integrity of the anodic oxide layer directly correlate with biocompatibility outcomes, with optimal results observed in layers between 10-25 μm.
Safety standards for anodized implants have evolved to address specific concerns related to aluminum's potential neurotoxicity. The ASTM F1295 standard specifically addresses the performance requirements for anodized titanium and aluminum alloys, establishing thresholds for acceptable ion release rates. European regulations under MDR 2017/745 impose stricter requirements for implantable devices, mandating extensive clinical evidence before market approval for anodized aluminum components.
Surface characterization requirements constitute another critical aspect of safety standards. Manufacturers must demonstrate consistent surface properties, including roughness parameters (Ra values typically between 0.5-2.0 μm), oxide layer uniformity, and chemical composition. Electron microscopy and X-ray photoelectron spectroscopy are commonly required analytical methods to verify compliance with these standards.
Long-term implantation studies represent the gold standard for safety assessment. Current regulations require in vivo testing periods of 26-52 weeks for permanent implants containing anodized aluminum components. These studies must demonstrate the absence of adverse local tissue reactions, systemic effects, and material degradation. Particular attention is given to potential aluminum accumulation in distant organs, especially the brain and kidneys, with established safety thresholds typically set below 1 μg/g tissue.
Emerging standards are beginning to incorporate requirements for antimicrobial properties of anodized surfaces, recognizing the potential for modified anodization processes to inhibit bacterial colonization. These standards typically specify minimum zones of inhibition against common pathogens such as S. aureus and E. coli, providing an additional safety dimension beyond traditional biocompatibility parameters.
Recent research indicates that properly anodized aluminum surfaces exhibit excellent biocompatibility characteristics. The anodization process creates a controlled oxide layer that significantly reduces ion leaching—a primary concern with metallic implants. Studies have shown that Type II and Type III anodized aluminum surfaces demonstrate minimal cytotoxicity in vitro, with cell viability rates exceeding 90% in standardized tests. The thickness and integrity of the anodic oxide layer directly correlate with biocompatibility outcomes, with optimal results observed in layers between 10-25 μm.
Safety standards for anodized implants have evolved to address specific concerns related to aluminum's potential neurotoxicity. The ASTM F1295 standard specifically addresses the performance requirements for anodized titanium and aluminum alloys, establishing thresholds for acceptable ion release rates. European regulations under MDR 2017/745 impose stricter requirements for implantable devices, mandating extensive clinical evidence before market approval for anodized aluminum components.
Surface characterization requirements constitute another critical aspect of safety standards. Manufacturers must demonstrate consistent surface properties, including roughness parameters (Ra values typically between 0.5-2.0 μm), oxide layer uniformity, and chemical composition. Electron microscopy and X-ray photoelectron spectroscopy are commonly required analytical methods to verify compliance with these standards.
Long-term implantation studies represent the gold standard for safety assessment. Current regulations require in vivo testing periods of 26-52 weeks for permanent implants containing anodized aluminum components. These studies must demonstrate the absence of adverse local tissue reactions, systemic effects, and material degradation. Particular attention is given to potential aluminum accumulation in distant organs, especially the brain and kidneys, with established safety thresholds typically set below 1 μg/g tissue.
Emerging standards are beginning to incorporate requirements for antimicrobial properties of anodized surfaces, recognizing the potential for modified anodization processes to inhibit bacterial colonization. These standards typically specify minimum zones of inhibition against common pathogens such as S. aureus and E. coli, providing an additional safety dimension beyond traditional biocompatibility parameters.
Surface Modification Strategies for Enhanced Osseointegration
Surface modification of anodized aluminum implants represents a critical frontier in biomedical engineering, with osseointegration enhancement being a primary focus. The anodization process creates a controlled oxide layer with unique topographical and chemical properties that can be further modified to promote bone-implant integration. Current strategies for enhancing osseointegration can be categorized into physical, chemical, and biological approaches, each targeting different aspects of the bone-implant interface.
Physical modification techniques include micro-arc oxidation (MAO), which creates porous structures with roughness parameters that closely mimic natural bone architecture. Laser surface texturing has emerged as another promising approach, allowing precise control over surface patterns at micro and nano scales. These physical modifications provide mechanical interlocking between the implant and surrounding bone tissue, significantly improving primary stability.
Chemical modifications focus on altering the surface chemistry to enhance bioactivity. Hydroxyapatite coating remains the gold standard due to its chemical similarity to natural bone mineral. Recent advances include the incorporation of bioactive elements such as calcium, phosphorus, and magnesium into the anodic oxide layer through electrolyte manipulation during anodization. Sol-gel derived coatings offer another avenue for introducing bioactive compounds with controlled release profiles.
Biological functionalization represents the cutting-edge of osseointegration enhancement. This approach involves immobilizing bioactive molecules such as bone morphogenetic proteins (BMPs), growth factors, and cell-adhesion peptides onto the anodized surface. RGD peptide sequences have shown particular promise by mimicking the cell-binding domain of extracellular matrix proteins, thereby promoting osteoblast adhesion and proliferation.
Hybrid approaches combining multiple modification strategies have demonstrated synergistic effects. For instance, hierarchical surface structures created through combined anodization and hydrothermal treatment, followed by biomolecule immobilization, show superior osseointegration compared to single-strategy modifications. These multi-functional surfaces can simultaneously address multiple requirements: mechanical stability, bioactivity, and controlled biological responses.
Antibacterial surface modifications have gained increasing attention as infection remains a significant cause of implant failure. Incorporation of silver nanoparticles, copper ions, or antibiotic-loaded nanotubes into the anodized layer provides localized antimicrobial activity without compromising osseointegration. Dual-function surfaces that both promote bone growth and inhibit bacterial colonization represent an important advancement in implant technology.
The long-term stability of these surface modifications remains a challenge, with degradation kinetics and mechanical integrity under physiological conditions requiring further investigation. Emerging research focuses on "smart" surfaces that can respond dynamically to the implantation environment, potentially adapting their properties throughout the healing process.
Physical modification techniques include micro-arc oxidation (MAO), which creates porous structures with roughness parameters that closely mimic natural bone architecture. Laser surface texturing has emerged as another promising approach, allowing precise control over surface patterns at micro and nano scales. These physical modifications provide mechanical interlocking between the implant and surrounding bone tissue, significantly improving primary stability.
Chemical modifications focus on altering the surface chemistry to enhance bioactivity. Hydroxyapatite coating remains the gold standard due to its chemical similarity to natural bone mineral. Recent advances include the incorporation of bioactive elements such as calcium, phosphorus, and magnesium into the anodic oxide layer through electrolyte manipulation during anodization. Sol-gel derived coatings offer another avenue for introducing bioactive compounds with controlled release profiles.
Biological functionalization represents the cutting-edge of osseointegration enhancement. This approach involves immobilizing bioactive molecules such as bone morphogenetic proteins (BMPs), growth factors, and cell-adhesion peptides onto the anodized surface. RGD peptide sequences have shown particular promise by mimicking the cell-binding domain of extracellular matrix proteins, thereby promoting osteoblast adhesion and proliferation.
Hybrid approaches combining multiple modification strategies have demonstrated synergistic effects. For instance, hierarchical surface structures created through combined anodization and hydrothermal treatment, followed by biomolecule immobilization, show superior osseointegration compared to single-strategy modifications. These multi-functional surfaces can simultaneously address multiple requirements: mechanical stability, bioactivity, and controlled biological responses.
Antibacterial surface modifications have gained increasing attention as infection remains a significant cause of implant failure. Incorporation of silver nanoparticles, copper ions, or antibiotic-loaded nanotubes into the anodized layer provides localized antimicrobial activity without compromising osseointegration. Dual-function surfaces that both promote bone growth and inhibit bacterial colonization represent an important advancement in implant technology.
The long-term stability of these surface modifications remains a challenge, with degradation kinetics and mechanical integrity under physiological conditions requiring further investigation. Emerging research focuses on "smart" surfaces that can respond dynamically to the implantation environment, potentially adapting their properties throughout the healing process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!