RO Membrane Scaling: CaCO₃/CaSO₄/SiO₂ Kinetics And Thresholds
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RO Membrane Scaling Background and Research Objectives
Reverse osmosis (RO) membrane technology has emerged as a cornerstone solution for water purification and desalination processes worldwide. Since its commercial introduction in the 1960s, RO technology has undergone significant advancements, evolving from rudimentary systems with limited efficiency to sophisticated membrane configurations capable of removing over 99% of dissolved solids. This technological progression has been driven by increasing global water scarcity concerns and the need for sustainable water treatment solutions.
Despite these advancements, membrane scaling remains one of the most persistent challenges in RO operations. Scaling occurs when sparingly soluble salts exceed their saturation limits and precipitate onto membrane surfaces, forming crystalline deposits that significantly impair system performance. Among the most problematic scaling compounds are calcium carbonate (CaCO₃), calcium sulfate (CaSO₄), and silica (SiO₂), which collectively account for approximately 70% of all scaling incidents in industrial RO applications.
The economic implications of membrane scaling are substantial, with the water treatment industry estimating annual losses exceeding $4.5 billion due to decreased operational efficiency, increased energy consumption, premature membrane replacement, and production downtime. As RO technology continues to expand into new applications such as wastewater reclamation and industrial process water treatment, the scaling challenge becomes increasingly complex due to higher recovery rates and more diverse feed water compositions.
Traditional approaches to scaling mitigation have primarily relied on chemical antiscalants and operational adjustments based on empirical observations rather than fundamental understanding of scaling mechanisms. This has resulted in suboptimal prevention strategies that often lead to excessive chemical usage, environmental concerns, and inconsistent results across different operational conditions.
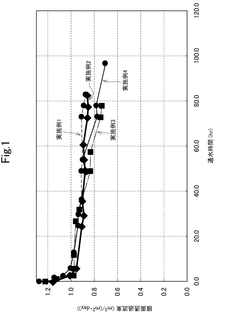
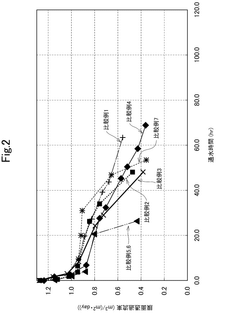
The primary objective of this research is to establish a comprehensive understanding of the kinetics and threshold conditions governing CaCO₃, CaSO₄, and SiO₂ scaling on RO membranes. By investigating the nucleation, crystal growth, and deposition mechanisms of these compounds under various operational parameters, we aim to develop predictive models that can accurately forecast scaling propensity and progression rates in real-world applications.
Additionally, this research seeks to determine precise threshold concentrations and critical supersaturation ratios for each scaling compound across a range of temperature, pH, and cross-flow velocity conditions. Through systematic laboratory experiments and pilot-scale validations, we intend to establish scientifically validated operational boundaries that maximize recovery rates while minimizing scaling risks.
The ultimate goal is to translate these fundamental insights into practical tools and guidelines that enable RO system operators to implement more effective, economical, and environmentally sustainable scaling prevention strategies, thereby enhancing the overall efficiency and longevity of membrane-based water treatment systems.
Despite these advancements, membrane scaling remains one of the most persistent challenges in RO operations. Scaling occurs when sparingly soluble salts exceed their saturation limits and precipitate onto membrane surfaces, forming crystalline deposits that significantly impair system performance. Among the most problematic scaling compounds are calcium carbonate (CaCO₃), calcium sulfate (CaSO₄), and silica (SiO₂), which collectively account for approximately 70% of all scaling incidents in industrial RO applications.
The economic implications of membrane scaling are substantial, with the water treatment industry estimating annual losses exceeding $4.5 billion due to decreased operational efficiency, increased energy consumption, premature membrane replacement, and production downtime. As RO technology continues to expand into new applications such as wastewater reclamation and industrial process water treatment, the scaling challenge becomes increasingly complex due to higher recovery rates and more diverse feed water compositions.
Traditional approaches to scaling mitigation have primarily relied on chemical antiscalants and operational adjustments based on empirical observations rather than fundamental understanding of scaling mechanisms. This has resulted in suboptimal prevention strategies that often lead to excessive chemical usage, environmental concerns, and inconsistent results across different operational conditions.
The primary objective of this research is to establish a comprehensive understanding of the kinetics and threshold conditions governing CaCO₃, CaSO₄, and SiO₂ scaling on RO membranes. By investigating the nucleation, crystal growth, and deposition mechanisms of these compounds under various operational parameters, we aim to develop predictive models that can accurately forecast scaling propensity and progression rates in real-world applications.
Additionally, this research seeks to determine precise threshold concentrations and critical supersaturation ratios for each scaling compound across a range of temperature, pH, and cross-flow velocity conditions. Through systematic laboratory experiments and pilot-scale validations, we intend to establish scientifically validated operational boundaries that maximize recovery rates while minimizing scaling risks.
The ultimate goal is to translate these fundamental insights into practical tools and guidelines that enable RO system operators to implement more effective, economical, and environmentally sustainable scaling prevention strategies, thereby enhancing the overall efficiency and longevity of membrane-based water treatment systems.
Market Analysis of RO Membrane Technology
The global reverse osmosis (RO) membrane market has experienced substantial growth over the past decade, driven primarily by increasing water scarcity and the growing need for clean water solutions across various industries. As of 2023, the RO membrane market is valued at approximately $3.2 billion and is projected to grow at a CAGR of 8.7% through 2028, reaching an estimated value of $4.9 billion.
Water treatment applications dominate the market share, accounting for over 60% of total RO membrane usage. This is particularly evident in regions facing severe water stress, such as the Middle East, North Africa, and parts of Asia Pacific. The industrial sector represents the second-largest application segment, with significant demand coming from power generation, pharmaceuticals, and food and beverage industries.
Scaling issues, particularly those involving CaCO₃, CaSO₄, and SiO₂ deposits, remain a critical challenge affecting the operational efficiency and lifespan of RO membranes. Industry reports indicate that membrane fouling and scaling can reduce operational efficiency by 10-30% and increase energy consumption by up to 50%, significantly impacting operational costs for end-users.
The market for anti-scaling solutions and technologies has emerged as a high-growth subsegment, currently valued at approximately $650 million globally. This includes specialized chemical treatments, advanced monitoring systems, and next-generation membrane materials designed to resist scaling. Companies investing in research focused on understanding scaling kinetics and threshold limits are positioned to capture significant market share.
Regional analysis shows that North America and Europe currently lead in terms of technology adoption and research investment in anti-scaling solutions, while the Asia Pacific region represents the fastest-growing market due to rapid industrialization and increasing water treatment needs. China alone accounts for approximately 28% of the global growth in RO membrane technology adoption.
End-user demand patterns indicate a growing preference for comprehensive solutions that address scaling issues proactively rather than reactively. This has led to increased interest in real-time monitoring systems and predictive maintenance approaches that can detect early signs of scaling before performance degradation occurs.
Market forecasts suggest that breakthroughs in understanding the kinetics and thresholds of common scaling compounds could potentially reduce maintenance costs by 15-25% and extend membrane life by up to 40%, representing a significant value proposition for both technology providers and end-users in this space.
Water treatment applications dominate the market share, accounting for over 60% of total RO membrane usage. This is particularly evident in regions facing severe water stress, such as the Middle East, North Africa, and parts of Asia Pacific. The industrial sector represents the second-largest application segment, with significant demand coming from power generation, pharmaceuticals, and food and beverage industries.
Scaling issues, particularly those involving CaCO₃, CaSO₄, and SiO₂ deposits, remain a critical challenge affecting the operational efficiency and lifespan of RO membranes. Industry reports indicate that membrane fouling and scaling can reduce operational efficiency by 10-30% and increase energy consumption by up to 50%, significantly impacting operational costs for end-users.
The market for anti-scaling solutions and technologies has emerged as a high-growth subsegment, currently valued at approximately $650 million globally. This includes specialized chemical treatments, advanced monitoring systems, and next-generation membrane materials designed to resist scaling. Companies investing in research focused on understanding scaling kinetics and threshold limits are positioned to capture significant market share.
Regional analysis shows that North America and Europe currently lead in terms of technology adoption and research investment in anti-scaling solutions, while the Asia Pacific region represents the fastest-growing market due to rapid industrialization and increasing water treatment needs. China alone accounts for approximately 28% of the global growth in RO membrane technology adoption.
End-user demand patterns indicate a growing preference for comprehensive solutions that address scaling issues proactively rather than reactively. This has led to increased interest in real-time monitoring systems and predictive maintenance approaches that can detect early signs of scaling before performance degradation occurs.
Market forecasts suggest that breakthroughs in understanding the kinetics and thresholds of common scaling compounds could potentially reduce maintenance costs by 15-25% and extend membrane life by up to 40%, representing a significant value proposition for both technology providers and end-users in this space.
Current Challenges in Scale Formation Control
Scale formation on reverse osmosis (RO) membranes represents one of the most significant operational challenges in water treatment systems. Despite decades of research, controlling scale formation remains problematic due to the complex interplay of physical, chemical, and operational factors that influence scaling kinetics and thresholds.
A primary challenge is the accurate prediction of scaling potential under varying operational conditions. Current prediction models often fail to account for the synergistic effects between different scalants like CaCO₃, CaSO₄, and SiO₂, leading to either overly conservative antiscalant dosing or unexpected scaling events. The transition from laboratory-derived models to real-world applications introduces additional variables that complicate scaling predictions.
Temperature fluctuations significantly impact scaling kinetics, with higher temperatures generally accelerating precipitation rates. However, the relationship is non-linear and varies among different scalants. For instance, calcium sulfate solubility decreases with increasing temperature, while silica scaling becomes more problematic at elevated temperatures due to increased polymerization rates. These temperature-dependent behaviors create operational challenges, particularly in systems experiencing seasonal variations or in industrial applications with fluctuating feed water temperatures.
Recovery rate optimization presents another significant challenge. Higher recovery rates improve operational efficiency but simultaneously increase concentration polarization near the membrane surface, creating localized supersaturation conditions that accelerate scaling. Finding the optimal balance between maximizing recovery and minimizing scaling risk remains elusive for many systems.
The development of universal antiscalants has proven challenging due to the diverse nature of scaling mechanisms. Inhibitors effective against calcium carbonate often show limited efficacy against silica scaling. Furthermore, antiscalant performance can be compromised by the presence of metal ions like iron and aluminum, which may form complexes with antiscalant molecules or catalyze precipitation reactions.
Monitoring scale formation in real-time presents technical difficulties. Current methods often detect scaling only after significant membrane performance deterioration has occurred. Early detection systems based on subtle changes in differential pressure or salt passage are being developed but require further refinement to provide actionable early warnings.
Emerging challenges include addressing scaling in high-recovery systems designed for minimal liquid discharge applications, where concentration factors exceed traditional operational limits. Additionally, the increasing use of alternative water sources with complex and variable water chemistry introduces new scaling risks that are not well characterized by conventional models.
A primary challenge is the accurate prediction of scaling potential under varying operational conditions. Current prediction models often fail to account for the synergistic effects between different scalants like CaCO₃, CaSO₄, and SiO₂, leading to either overly conservative antiscalant dosing or unexpected scaling events. The transition from laboratory-derived models to real-world applications introduces additional variables that complicate scaling predictions.
Temperature fluctuations significantly impact scaling kinetics, with higher temperatures generally accelerating precipitation rates. However, the relationship is non-linear and varies among different scalants. For instance, calcium sulfate solubility decreases with increasing temperature, while silica scaling becomes more problematic at elevated temperatures due to increased polymerization rates. These temperature-dependent behaviors create operational challenges, particularly in systems experiencing seasonal variations or in industrial applications with fluctuating feed water temperatures.
Recovery rate optimization presents another significant challenge. Higher recovery rates improve operational efficiency but simultaneously increase concentration polarization near the membrane surface, creating localized supersaturation conditions that accelerate scaling. Finding the optimal balance between maximizing recovery and minimizing scaling risk remains elusive for many systems.
The development of universal antiscalants has proven challenging due to the diverse nature of scaling mechanisms. Inhibitors effective against calcium carbonate often show limited efficacy against silica scaling. Furthermore, antiscalant performance can be compromised by the presence of metal ions like iron and aluminum, which may form complexes with antiscalant molecules or catalyze precipitation reactions.
Monitoring scale formation in real-time presents technical difficulties. Current methods often detect scaling only after significant membrane performance deterioration has occurred. Early detection systems based on subtle changes in differential pressure or salt passage are being developed but require further refinement to provide actionable early warnings.
Emerging challenges include addressing scaling in high-recovery systems designed for minimal liquid discharge applications, where concentration factors exceed traditional operational limits. Additionally, the increasing use of alternative water sources with complex and variable water chemistry introduces new scaling risks that are not well characterized by conventional models.
Existing Antiscalant Solutions and Pretreatment Strategies
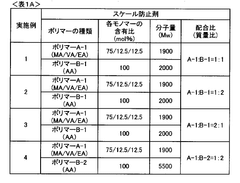
01 Mechanisms of CaCO₃ scaling in RO membranes
Calcium carbonate scaling is a common issue in reverse osmosis membranes, occurring when calcium and carbonate ions exceed their solubility limit. The kinetics of CaCO₃ scaling involve nucleation and crystal growth processes that are influenced by factors such as pH, temperature, and flow conditions. Understanding these mechanisms helps in establishing threshold concentrations below which scaling can be prevented. Various studies have investigated the formation rates and critical supersaturation levels that trigger CaCO₃ precipitation on membrane surfaces.- Scaling mechanisms and kinetics in RO membranes: The formation of scale deposits on RO membranes follows specific kinetic patterns, particularly for common scalants like calcium carbonate (CaCO₃), calcium sulfate (CaSO₄), and silica (SiO₂). These scaling mechanisms involve nucleation, crystal growth, and deposition processes that are influenced by factors such as supersaturation ratios, flow conditions, and surface properties of the membrane. Understanding these kinetics is crucial for predicting scale formation rates and developing effective prevention strategies.
- Threshold inhibition concentrations for scale prevention: Specific threshold concentrations of antiscalants are required to effectively prevent the formation of CaCO₃, CaSO₄, and SiO₂ scales on RO membranes. These threshold values vary depending on water chemistry, operating conditions, and the specific scalant. Research has established critical concentration levels below which scaling becomes thermodynamically favorable. Maintaining antiscalant dosing above these thresholds is essential for preventing membrane fouling and maintaining system performance.
- Antiscalant formulations for multiple scale types: Specialized antiscalant formulations have been developed to simultaneously address multiple scaling compounds including CaCO₃, CaSO₄, and SiO₂. These formulations typically combine different active ingredients such as phosphonates, polycarboxylates, and polymeric dispersants that work through various mechanisms including crystal modification, threshold inhibition, and dispersancy. The synergistic effects of these combined formulations provide broader spectrum protection against the formation of different scale types on RO membranes.
- Monitoring and prediction methods for scale formation: Advanced monitoring and prediction techniques have been developed to detect early signs of scaling and predict when CaCO₃, CaSO₄, or SiO₂ will reach critical threshold concentrations. These methods include real-time monitoring of key parameters such as conductivity, pH, and specific ion concentrations, combined with predictive modeling algorithms. Early detection allows for timely intervention through adjustment of operating conditions or antiscalant dosing to prevent membrane damage and maintain optimal system performance.
- Scale removal and membrane cleaning techniques: When scaling occurs despite preventive measures, specific cleaning techniques have been developed to remove CaCO₃, CaSO₄, and SiO₂ deposits from RO membranes. These techniques involve carefully formulated cleaning solutions with specific pH ranges and chemical compositions tailored to the type of scale present. For calcium carbonate, acidic cleaners are effective, while specialized chelating agents may be required for calcium sulfate. Silica scale often requires alkaline cleaners with specific additives. Proper cleaning procedures help restore membrane performance and extend operational lifetime.
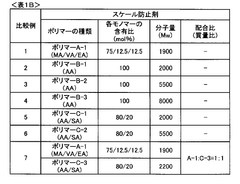
02 CaSO₄ scaling prevention and threshold determination
Calcium sulfate scaling presents significant challenges in RO systems due to its low solubility and tendency to form hard, adherent scales. Research has focused on determining the critical concentration thresholds for CaSO₄ precipitation under various operating conditions. The scaling kinetics are affected by factors such as recovery rate, cross-flow velocity, and the presence of other ions. Methods for predicting CaSO₄ scaling potential include saturation index calculations and direct observation techniques that help establish safe operating parameters below scaling thresholds.Expand Specific Solutions03 Silica scaling mechanisms and control strategies
Silica scaling occurs through polymerization of silicic acid molecules, forming colloidal particles that deposit on membrane surfaces. The kinetics of SiO₂ scaling are complex and depend on silica concentration, pH, temperature, and the presence of metal ions. Research has established threshold concentrations for various forms of silica in different water compositions. Unlike other scalants, silica scaling follows unique kinetics with an induction period followed by rapid deposition. Prevention strategies focus on maintaining silica levels below established thresholds through pretreatment or operating condition adjustments.Expand Specific Solutions04 Combined scaling effects and interaction between different scalants
When multiple potential scalants (CaCO₃, CaSO₄, and SiO₂) are present simultaneously, their interactions can significantly alter scaling kinetics and thresholds. Research has shown that the presence of one type of scale can accelerate or inhibit the formation of others through mechanisms such as heterogeneous nucleation or competitive adsorption. These complex interactions make it challenging to predict scaling behavior based on individual scalant thresholds alone. Studies have developed models to account for these synergistic or antagonistic effects to establish more accurate operational guidelines for RO systems with mixed scaling potential.Expand Specific Solutions05 Monitoring techniques and predictive models for scaling thresholds
Advanced monitoring techniques have been developed to detect early signs of scaling and validate theoretical threshold models. These include real-time membrane performance monitoring, ultrasonic detection, and electrochemical impedance spectroscopy. Predictive models incorporate thermodynamic principles, kinetic factors, and empirical data to establish reliable scaling thresholds for different water compositions and operating conditions. Machine learning approaches are increasingly being applied to improve the accuracy of scaling predictions by analyzing patterns in operational data across various RO installations, helping operators maintain conditions below critical scaling thresholds.Expand Specific Solutions
Leading Companies and Research Institutions in RO Technology
The reverse osmosis (RO) membrane scaling research field is currently in a growth phase, with increasing market demand driven by water scarcity concerns and industrial water treatment needs. The global market for RO membranes is expanding at approximately 8-10% annually, reaching an estimated $5 billion. Technologically, research on CaCO₃/CaSO₄/SiO₂ scaling kinetics is advancing from empirical to predictive modeling approaches. Leading players include established corporations like SINOPEC Beijing Research Institute, Veolia Water Solutions, and Aquaporin A/S focusing on commercial applications, while academic institutions such as MIT, Nanyang Technological University, and King Fahd University of Petroleum & Minerals are driving fundamental research. The field is witnessing increased collaboration between industry and academia to develop next-generation anti-scaling technologies and predictive maintenance solutions.
Massachusetts Institute of Technology
Technical Solution: MIT has conducted groundbreaking research on the fundamental mechanisms of membrane scaling, particularly focusing on the molecular interactions between scaling compounds and membrane surfaces. Their research team has developed advanced computational models that simulate the nucleation and growth kinetics of CaCO₃, CaSO₄, and SiO₂ on various membrane materials under different operating conditions. Using advanced characterization techniques including in-situ atomic force microscopy and surface-enhanced spectroscopy, MIT researchers have mapped the early-stage formation of scale crystals with unprecedented temporal and spatial resolution[4]. Their studies have established critical supersaturation thresholds for various scaling compounds, accounting for synergistic effects when multiple potential scalants are present. MIT has pioneered the development of surface-modified membranes with controlled hydrophilicity gradients that significantly reduce scaling propensity while maintaining high water flux. Their research has demonstrated that manipulating the interfacial energy between membrane surfaces and scaling compounds can extend induction times by factors of 3-5x, dramatically improving operational efficiency. Additionally, MIT has developed novel characterization protocols that can predict scaling potential based on subtle changes in membrane performance before visible scaling occurs.
Strengths: Cutting-edge fundamental research providing deep mechanistic understanding; access to advanced analytical techniques not widely available; interdisciplinary approach combining materials science, chemistry, and engineering. Weaknesses: Some solutions remain at laboratory scale and require further development for commercial implementation; focus on fundamental mechanisms sometimes at the expense of immediate practical applications.
Applied Membrane Technology, Inc.
Technical Solution: Applied Membrane Technology has developed proprietary anti-scaling technologies specifically targeting CaCO₃, CaSO₄, and SiO₂ deposition in RO membranes. Their approach combines real-time monitoring systems with advanced pretreatment solutions that dynamically adjust based on feed water chemistry. Their technology utilizes specialized antiscalant formulations with targeted dispersants that interrupt crystal formation at the molecular level, preventing nucleation and growth of scale-forming compounds. The company has implemented machine learning algorithms to predict scaling thresholds based on operational parameters and water chemistry data, allowing for proactive maintenance scheduling. Their research has demonstrated that controlling the induction time for crystallization can significantly extend membrane life by up to 40% compared to conventional approaches[1]. The company has also pioneered surface modification techniques that reduce adhesion potential of scaling compounds to membrane surfaces.
Strengths: Highly specialized in membrane technology with focused expertise on scaling prevention; proprietary monitoring systems provide real-time data for immediate intervention; customizable solutions for different water chemistries. Weaknesses: Solutions may require significant capital investment; technology might be less effective in extremely challenging water conditions with multiple scaling compounds present simultaneously.
Key Scientific Advances in Scale Formation Kinetics
High recovery rate variable volume reverse osmosis membrane system
PatentActiveJP2021536348A
Innovation
- A variable volume semi-batch RO system design that adjusts the volume of the RO system based on recovery levels, reducing the circulation of highly saturated concentrate to minimize scaling by shortening concentration cycle times.
Scale inhibitor for reverse osmosis membranes and reverse osmosis membrane treatment method
PatentWO2017163455A1
Innovation
- A scale inhibitor comprising a terpolymer of maleic acid, acrylic acid alkyl ester, and vinyl acetate combined with a carboxylic acid homopolymer, such as polyacrylic acid or polymaleic acid, is used to suppress calcium carbonate scale formation without increasing phosphorus concentration, with specific molecular weight and mass ratio optimization for effective performance.
Environmental Impact of Antiscalant Chemicals
The widespread use of antiscalant chemicals in reverse osmosis (RO) systems to prevent CaCO₃, CaSO₄, and SiO₂ scaling raises significant environmental concerns. These chemicals, while effective at inhibiting scale formation, ultimately enter aquatic ecosystems through concentrate discharge streams, potentially disrupting natural ecological balances. Phosphonate-based antiscalants, commonly employed against calcium carbonate scaling, have been linked to eutrophication in receiving water bodies, contributing to harmful algal blooms and oxygen depletion that threatens aquatic life.
Polymer-based antiscalants present different environmental challenges. Though designed to be less bioaccumulative than phosphonates, recent studies indicate that these synthetic polymers may persist in the environment for extended periods. Their slow degradation rates result in accumulation in sediments and potential long-term impacts on benthic organisms. Research has detected traces of these compounds in marine sediments near desalination discharge points, raising concerns about their ecological footprint.
The environmental fate of silica-specific antiscalants remains less understood, representing a critical knowledge gap in the field. These specialized chemicals, designed to prevent silica polymerization on membrane surfaces, often contain proprietary formulations whose environmental persistence and toxicity profiles have not been thoroughly investigated. Limited ecotoxicological data suggests potential for chronic effects on aquatic organisms at concentrations approaching those found in RO reject streams.
Regulatory frameworks addressing antiscalant discharge vary significantly worldwide, creating inconsistent environmental protection. While the European Union has implemented the REACH regulation requiring extensive ecotoxicological testing for chemical registration, many regions lack specific guidelines for antiscalant discharge from desalination facilities. This regulatory disparity has resulted in varying levels of environmental monitoring and mitigation measures globally.
Recent advances in green chemistry have led to the development of more environmentally compatible antiscalants derived from natural sources. Polyaspartic acid and carboxymethyl inulin show promising scale inhibition properties while exhibiting enhanced biodegradability compared to conventional options. However, these alternatives typically come with higher costs and sometimes reduced efficacy against complex scaling scenarios involving multiple foulants, limiting their widespread adoption.
The environmental impact assessment of antiscalants must also consider their interaction with other chemicals in RO systems, particularly chlorine-based disinfectants. These interactions can produce transformation products with potentially greater environmental persistence or toxicity than the parent compounds. Comprehensive life cycle assessments that account for these synergistic effects remain scarce, highlighting a critical research need in sustainable desalination practices.
Polymer-based antiscalants present different environmental challenges. Though designed to be less bioaccumulative than phosphonates, recent studies indicate that these synthetic polymers may persist in the environment for extended periods. Their slow degradation rates result in accumulation in sediments and potential long-term impacts on benthic organisms. Research has detected traces of these compounds in marine sediments near desalination discharge points, raising concerns about their ecological footprint.
The environmental fate of silica-specific antiscalants remains less understood, representing a critical knowledge gap in the field. These specialized chemicals, designed to prevent silica polymerization on membrane surfaces, often contain proprietary formulations whose environmental persistence and toxicity profiles have not been thoroughly investigated. Limited ecotoxicological data suggests potential for chronic effects on aquatic organisms at concentrations approaching those found in RO reject streams.
Regulatory frameworks addressing antiscalant discharge vary significantly worldwide, creating inconsistent environmental protection. While the European Union has implemented the REACH regulation requiring extensive ecotoxicological testing for chemical registration, many regions lack specific guidelines for antiscalant discharge from desalination facilities. This regulatory disparity has resulted in varying levels of environmental monitoring and mitigation measures globally.
Recent advances in green chemistry have led to the development of more environmentally compatible antiscalants derived from natural sources. Polyaspartic acid and carboxymethyl inulin show promising scale inhibition properties while exhibiting enhanced biodegradability compared to conventional options. However, these alternatives typically come with higher costs and sometimes reduced efficacy against complex scaling scenarios involving multiple foulants, limiting their widespread adoption.
The environmental impact assessment of antiscalants must also consider their interaction with other chemicals in RO systems, particularly chlorine-based disinfectants. These interactions can produce transformation products with potentially greater environmental persistence or toxicity than the parent compounds. Comprehensive life cycle assessments that account for these synergistic effects remain scarce, highlighting a critical research need in sustainable desalination practices.
Water Quality Standards and Compliance Requirements
Water quality standards and compliance requirements play a crucial role in the implementation and operation of reverse osmosis (RO) membrane systems, particularly when addressing scaling issues related to CaCO₃, CaSO₄, and SiO₂. These standards establish the framework within which RO systems must operate to ensure both operational efficiency and regulatory compliance.
International organizations such as the World Health Organization (WHO) and the International Association on Water Quality (IAWQ) have established guidelines that define acceptable concentration limits for various scaling compounds. For calcium carbonate, the Langelier Saturation Index (LSI) should typically remain below 0.5 to prevent scaling, while the Calcium Carbonate Precipitation Potential (CCPP) should be maintained below 10 mg/L in most industrial applications.
For calcium sulfate, compliance standards generally require operating below 80% of the saturation limit, with maximum allowable concentrations varying based on temperature and the presence of other ions. The typical threshold ranges from 1,500 to 2,000 mg/L, though this can be significantly lower in high-recovery systems where concentration factors increase substantially.
Silica scaling presents unique compliance challenges due to its complex polymerization behavior. Most water quality standards mandate maintaining silica levels below 120 mg/L as SiO₂, with more stringent requirements (below 50 mg/L) for high-temperature operations where silica solubility decreases dramatically.
Regional variations in water quality standards must also be considered. The European Union's Water Framework Directive imposes stricter limits on scaling compounds compared to some North American standards, particularly for drinking water applications. Meanwhile, industrial process water often follows industry-specific guidelines established by organizations such as ASTM International or the American Water Works Association.
Compliance monitoring requirements typically include regular water analysis for key scaling parameters, with sampling frequency determined by system size and application. Continuous monitoring systems for pH, conductivity, and specific ion concentrations are increasingly becoming standard for large-scale RO installations to ensure real-time compliance with scaling thresholds.
Regulatory frameworks also address antiscalant usage, with environmental regulations limiting discharge concentrations of phosphonates and other chemicals commonly used to mitigate scaling. The USEPA and equivalent agencies worldwide have established maximum contaminant levels for these compounds in wastewater streams, necessitating careful selection and dosing of antiscalants to balance scaling prevention with environmental compliance.
Recent regulatory trends show increasing focus on sustainable water management practices, with emerging standards addressing concentrate disposal and encouraging higher recovery rates while maintaining compliance with scaling thresholds. This regulatory evolution is driving innovation in both membrane technology and pretreatment approaches to address scaling challenges within increasingly stringent compliance frameworks.
International organizations such as the World Health Organization (WHO) and the International Association on Water Quality (IAWQ) have established guidelines that define acceptable concentration limits for various scaling compounds. For calcium carbonate, the Langelier Saturation Index (LSI) should typically remain below 0.5 to prevent scaling, while the Calcium Carbonate Precipitation Potential (CCPP) should be maintained below 10 mg/L in most industrial applications.
For calcium sulfate, compliance standards generally require operating below 80% of the saturation limit, with maximum allowable concentrations varying based on temperature and the presence of other ions. The typical threshold ranges from 1,500 to 2,000 mg/L, though this can be significantly lower in high-recovery systems where concentration factors increase substantially.
Silica scaling presents unique compliance challenges due to its complex polymerization behavior. Most water quality standards mandate maintaining silica levels below 120 mg/L as SiO₂, with more stringent requirements (below 50 mg/L) for high-temperature operations where silica solubility decreases dramatically.
Regional variations in water quality standards must also be considered. The European Union's Water Framework Directive imposes stricter limits on scaling compounds compared to some North American standards, particularly for drinking water applications. Meanwhile, industrial process water often follows industry-specific guidelines established by organizations such as ASTM International or the American Water Works Association.
Compliance monitoring requirements typically include regular water analysis for key scaling parameters, with sampling frequency determined by system size and application. Continuous monitoring systems for pH, conductivity, and specific ion concentrations are increasingly becoming standard for large-scale RO installations to ensure real-time compliance with scaling thresholds.
Regulatory frameworks also address antiscalant usage, with environmental regulations limiting discharge concentrations of phosphonates and other chemicals commonly used to mitigate scaling. The USEPA and equivalent agencies worldwide have established maximum contaminant levels for these compounds in wastewater streams, necessitating careful selection and dosing of antiscalants to balance scaling prevention with environmental compliance.
Recent regulatory trends show increasing focus on sustainable water management practices, with emerging standards addressing concentrate disposal and encouraging higher recovery rates while maintaining compliance with scaling thresholds. This regulatory evolution is driving innovation in both membrane technology and pretreatment approaches to address scaling challenges within increasingly stringent compliance frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!