Zeolite-mediated Systems for CO2 Mineralization
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Mineralization Background and Objectives
Carbon dioxide (CO2) mineralization has emerged as a promising approach to mitigate the increasing atmospheric CO2 levels and combat climate change. This process involves the conversion of CO2 into stable carbonate minerals, effectively sequestering carbon in a solid form. The concept of CO2 mineralization is inspired by natural weathering processes, where CO2 reacts with alkaline minerals to form carbonates over geological timescales.
The primary objective of research on zeolite-mediated systems for CO2 mineralization is to accelerate and enhance this natural process, making it viable for industrial-scale carbon capture and storage. Zeolites, a class of microporous aluminosilicate minerals, have garnered significant attention in this field due to their unique properties, including high surface area, ion-exchange capacity, and molecular sieving abilities.
The development of zeolite-mediated CO2 mineralization systems aims to address several key challenges in carbon capture and utilization. These include improving the reaction kinetics, increasing the conversion efficiency, and reducing the energy requirements of the mineralization process. By leveraging the properties of zeolites, researchers seek to create more effective and economically viable solutions for large-scale CO2 sequestration.
Historical developments in CO2 mineralization research have progressed from basic understanding of natural weathering processes to engineered solutions for accelerated carbonation. Early studies focused on the direct reaction of CO2 with alkaline industrial wastes and naturally occurring minerals. However, these approaches often suffered from slow reaction rates and limited scalability.
The introduction of zeolites as catalysts and adsorbents in CO2 mineralization systems represents a significant advancement in the field. Zeolites can potentially enhance the dissolution of CO2 in aqueous solutions, facilitate the transport of reactive species, and provide favorable reaction sites for carbonate formation. This has led to increased research efforts in designing and optimizing zeolite-based materials for CO2 mineralization applications.
Current research objectives in zeolite-mediated CO2 mineralization systems include:
1. Developing novel zeolite structures with improved CO2 adsorption and conversion properties.
2. Investigating the mechanisms of CO2 activation and carbonate formation on zeolite surfaces.
3. Exploring the potential of zeolite-based composite materials for enhanced mineralization performance.
4. Optimizing process conditions for maximum CO2 conversion and energy efficiency.
5. Assessing the long-term stability and environmental impact of zeolite-mediated mineralization products.
As global efforts to reduce greenhouse gas emissions intensify, the development of efficient CO2 mineralization technologies becomes increasingly crucial. Zeolite-mediated systems offer a promising avenue for achieving this goal, potentially providing a sustainable solution for carbon management in various industrial sectors.
The primary objective of research on zeolite-mediated systems for CO2 mineralization is to accelerate and enhance this natural process, making it viable for industrial-scale carbon capture and storage. Zeolites, a class of microporous aluminosilicate minerals, have garnered significant attention in this field due to their unique properties, including high surface area, ion-exchange capacity, and molecular sieving abilities.
The development of zeolite-mediated CO2 mineralization systems aims to address several key challenges in carbon capture and utilization. These include improving the reaction kinetics, increasing the conversion efficiency, and reducing the energy requirements of the mineralization process. By leveraging the properties of zeolites, researchers seek to create more effective and economically viable solutions for large-scale CO2 sequestration.
Historical developments in CO2 mineralization research have progressed from basic understanding of natural weathering processes to engineered solutions for accelerated carbonation. Early studies focused on the direct reaction of CO2 with alkaline industrial wastes and naturally occurring minerals. However, these approaches often suffered from slow reaction rates and limited scalability.
The introduction of zeolites as catalysts and adsorbents in CO2 mineralization systems represents a significant advancement in the field. Zeolites can potentially enhance the dissolution of CO2 in aqueous solutions, facilitate the transport of reactive species, and provide favorable reaction sites for carbonate formation. This has led to increased research efforts in designing and optimizing zeolite-based materials for CO2 mineralization applications.
Current research objectives in zeolite-mediated CO2 mineralization systems include:
1. Developing novel zeolite structures with improved CO2 adsorption and conversion properties.
2. Investigating the mechanisms of CO2 activation and carbonate formation on zeolite surfaces.
3. Exploring the potential of zeolite-based composite materials for enhanced mineralization performance.
4. Optimizing process conditions for maximum CO2 conversion and energy efficiency.
5. Assessing the long-term stability and environmental impact of zeolite-mediated mineralization products.
As global efforts to reduce greenhouse gas emissions intensify, the development of efficient CO2 mineralization technologies becomes increasingly crucial. Zeolite-mediated systems offer a promising avenue for achieving this goal, potentially providing a sustainable solution for carbon management in various industrial sectors.
Market Analysis for CO2 Capture Technologies
The market for CO2 capture technologies has been experiencing significant growth in recent years, driven by increasing global concerns about climate change and the urgent need to reduce greenhouse gas emissions. The zeolite-mediated systems for CO2 mineralization represent a promising subset within this broader market, offering potential advantages in terms of efficiency and environmental sustainability.
The global carbon capture and storage (CCS) market, which encompasses various CO2 capture technologies, was valued at approximately $3 billion in 2020 and is projected to reach $7 billion by 2026, growing at a CAGR of over 13% during the forecast period. This growth is primarily fueled by stringent government regulations aimed at reducing carbon emissions and the increasing adoption of clean energy technologies across industries.
Within this market, zeolite-based CO2 capture technologies are gaining traction due to their unique properties and potential for cost-effective implementation. Zeolites, being microporous aluminosilicate minerals, offer high surface areas and selective adsorption capabilities, making them particularly suitable for CO2 capture and mineralization processes.
The demand for zeolite-mediated CO2 mineralization systems is expected to rise significantly in key industries such as power generation, cement production, and chemical manufacturing. These sectors are under increasing pressure to reduce their carbon footprint and are actively seeking innovative solutions to meet emission reduction targets.
Geographically, North America and Europe currently lead the market for advanced CO2 capture technologies, including zeolite-based systems. However, rapid industrialization and growing environmental concerns in Asia-Pacific countries, particularly China and India, are expected to drive substantial market growth in this region over the coming years.
Key market drivers for zeolite-mediated CO2 mineralization systems include the increasing focus on sustainable development, rising investments in clean energy technologies, and the potential for integration with existing industrial processes. Additionally, the growing interest in carbon utilization technologies, where captured CO2 is converted into valuable products, is expected to create new opportunities for zeolite-based systems.
However, the market also faces challenges, including high initial investment costs, technological complexities, and the need for large-scale demonstration projects to prove long-term viability. Overcoming these barriers will be crucial for widespread adoption of zeolite-mediated CO2 mineralization technologies.
In conclusion, the market for zeolite-mediated systems for CO2 mineralization shows promising growth potential within the broader CO2 capture technology landscape. As research and development efforts continue to advance, and as regulatory pressures for carbon reduction intensify, this technology is poised to play an increasingly important role in global efforts to combat climate change.
The global carbon capture and storage (CCS) market, which encompasses various CO2 capture technologies, was valued at approximately $3 billion in 2020 and is projected to reach $7 billion by 2026, growing at a CAGR of over 13% during the forecast period. This growth is primarily fueled by stringent government regulations aimed at reducing carbon emissions and the increasing adoption of clean energy technologies across industries.
Within this market, zeolite-based CO2 capture technologies are gaining traction due to their unique properties and potential for cost-effective implementation. Zeolites, being microporous aluminosilicate minerals, offer high surface areas and selective adsorption capabilities, making them particularly suitable for CO2 capture and mineralization processes.
The demand for zeolite-mediated CO2 mineralization systems is expected to rise significantly in key industries such as power generation, cement production, and chemical manufacturing. These sectors are under increasing pressure to reduce their carbon footprint and are actively seeking innovative solutions to meet emission reduction targets.
Geographically, North America and Europe currently lead the market for advanced CO2 capture technologies, including zeolite-based systems. However, rapid industrialization and growing environmental concerns in Asia-Pacific countries, particularly China and India, are expected to drive substantial market growth in this region over the coming years.
Key market drivers for zeolite-mediated CO2 mineralization systems include the increasing focus on sustainable development, rising investments in clean energy technologies, and the potential for integration with existing industrial processes. Additionally, the growing interest in carbon utilization technologies, where captured CO2 is converted into valuable products, is expected to create new opportunities for zeolite-based systems.
However, the market also faces challenges, including high initial investment costs, technological complexities, and the need for large-scale demonstration projects to prove long-term viability. Overcoming these barriers will be crucial for widespread adoption of zeolite-mediated CO2 mineralization technologies.
In conclusion, the market for zeolite-mediated systems for CO2 mineralization shows promising growth potential within the broader CO2 capture technology landscape. As research and development efforts continue to advance, and as regulatory pressures for carbon reduction intensify, this technology is poised to play an increasingly important role in global efforts to combat climate change.
Zeolite-mediated CO2 Mineralization: Current Status and Challenges
Zeolite-mediated CO2 mineralization has emerged as a promising approach for carbon capture and storage, addressing the urgent need to mitigate greenhouse gas emissions. The current status of this technology reflects significant advancements in recent years, yet it also faces several challenges that hinder its widespread implementation.
One of the primary advantages of zeolite-mediated systems is their ability to enhance the reaction kinetics of CO2 mineralization. Zeolites, with their unique porous structure and high surface area, provide an ideal environment for CO2 capture and subsequent conversion to stable carbonate minerals. Recent studies have demonstrated that certain zeolite types, such as Na-X and 13X, exhibit particularly high CO2 adsorption capacities and catalytic activities for mineralization reactions.
However, the efficiency of these systems is still limited by several factors. The regeneration of zeolites after CO2 capture and mineralization remains a significant challenge, as the process can lead to pore blockage and reduced adsorption capacity over time. This necessitates the development of more robust zeolite materials or improved regeneration techniques to maintain long-term performance.
Another critical issue is the energy intensity of the overall process. While zeolites can improve reaction rates, the activation energy required for complete mineralization is still considerable. This energy requirement poses both economic and environmental challenges, potentially offsetting some of the carbon reduction benefits of the technology.
Scalability presents another hurdle for zeolite-mediated CO2 mineralization. Most current research focuses on laboratory-scale experiments, and the transition to industrial-scale applications requires significant engineering and process optimization. Issues such as heat management, mass transfer limitations, and reactor design need to be addressed to ensure the technology's viability at larger scales.
The availability and cost of suitable precursor materials for mineralization, such as calcium or magnesium-rich waste streams, also impact the widespread adoption of this technology. While the use of industrial waste products as precursors offers a promising avenue for sustainable implementation, ensuring a consistent and adequate supply chain remains a challenge.
Lastly, the long-term stability and environmental impact of the mineralized products require further investigation. While carbonates are generally considered stable storage forms for CO2, the potential for leaching or re-release of CO2 under various environmental conditions needs to be thoroughly assessed to ensure the permanence of carbon sequestration.
One of the primary advantages of zeolite-mediated systems is their ability to enhance the reaction kinetics of CO2 mineralization. Zeolites, with their unique porous structure and high surface area, provide an ideal environment for CO2 capture and subsequent conversion to stable carbonate minerals. Recent studies have demonstrated that certain zeolite types, such as Na-X and 13X, exhibit particularly high CO2 adsorption capacities and catalytic activities for mineralization reactions.
However, the efficiency of these systems is still limited by several factors. The regeneration of zeolites after CO2 capture and mineralization remains a significant challenge, as the process can lead to pore blockage and reduced adsorption capacity over time. This necessitates the development of more robust zeolite materials or improved regeneration techniques to maintain long-term performance.
Another critical issue is the energy intensity of the overall process. While zeolites can improve reaction rates, the activation energy required for complete mineralization is still considerable. This energy requirement poses both economic and environmental challenges, potentially offsetting some of the carbon reduction benefits of the technology.
Scalability presents another hurdle for zeolite-mediated CO2 mineralization. Most current research focuses on laboratory-scale experiments, and the transition to industrial-scale applications requires significant engineering and process optimization. Issues such as heat management, mass transfer limitations, and reactor design need to be addressed to ensure the technology's viability at larger scales.
The availability and cost of suitable precursor materials for mineralization, such as calcium or magnesium-rich waste streams, also impact the widespread adoption of this technology. While the use of industrial waste products as precursors offers a promising avenue for sustainable implementation, ensuring a consistent and adequate supply chain remains a challenge.
Lastly, the long-term stability and environmental impact of the mineralized products require further investigation. While carbonates are generally considered stable storage forms for CO2, the potential for leaching or re-release of CO2 under various environmental conditions needs to be thoroughly assessed to ensure the permanence of carbon sequestration.
Existing Zeolite-based CO2 Mineralization Solutions
01 Zeolite-based CO2 capture and mineralization
Zeolites are used as adsorbents for capturing CO2 from various sources. The captured CO2 is then converted into stable mineral carbonates through a mineralization process. This method combines the high adsorption capacity of zeolites with the permanent storage potential of mineral carbonates, offering an efficient approach to carbon dioxide removal and storage.- Zeolite-based CO2 capture and mineralization: Zeolites are used as adsorbents for capturing CO2 from various sources. The captured CO2 is then mineralized through reaction with metal cations, forming stable carbonate minerals. This process effectively sequesters CO2 and can be applied in industrial settings for carbon dioxide reduction.
- Modified zeolite structures for enhanced CO2 mineralization: Zeolites are modified through various techniques such as ion exchange, impregnation with metal oxides, or surface functionalization to improve their CO2 capture and mineralization efficiency. These modifications can increase the CO2 adsorption capacity and accelerate the carbonation reaction.
- Integration of zeolite systems in industrial processes: Zeolite-mediated CO2 mineralization systems are integrated into existing industrial processes, such as power plants or cement production facilities. This integration allows for on-site carbon capture and mineralization, reducing overall CO2 emissions from these industries.
- Regeneration and recycling of zeolite materials: Methods for regenerating and recycling zeolite materials used in CO2 mineralization are developed to improve the sustainability and cost-effectiveness of the process. These techniques may involve thermal treatment, chemical washing, or other innovative approaches to restore the zeolite's CO2 capture capacity.
- Combination of zeolites with other materials for enhanced performance: Zeolites are combined with other materials such as metal-organic frameworks, activated carbon, or specific enzymes to create hybrid systems with improved CO2 capture and mineralization capabilities. These composite materials can offer synergistic effects, leading to more efficient carbon sequestration.
02 Modified zeolite structures for enhanced CO2 mineralization
Zeolite structures are modified to improve their CO2 capture and mineralization efficiency. Modifications may include ion exchange, impregnation with metal oxides, or surface functionalization. These alterations enhance the zeolite's affinity for CO2 and promote faster mineralization reactions, leading to more effective carbon sequestration.Expand Specific Solutions03 Integration of zeolite systems with industrial processes
Zeolite-mediated CO2 mineralization systems are integrated into industrial processes to capture and convert emissions at the source. This approach allows for on-site carbon management, reducing the need for transportation and storage of captured CO2. The integration can be applied to various industries such as cement production, power generation, and chemical manufacturing.Expand Specific Solutions04 Regeneration and recycling of zeolite materials
Methods for regenerating and recycling zeolite materials used in CO2 mineralization are developed to improve the sustainability and cost-effectiveness of the process. These techniques involve removing the mineralized products from the zeolite surface and restoring its CO2 capture capacity, allowing for multiple cycles of use and reducing the overall material requirements.Expand Specific Solutions05 Optimization of reaction conditions for zeolite-mediated CO2 mineralization
Research focuses on optimizing reaction conditions such as temperature, pressure, and pH for zeolite-mediated CO2 mineralization. These optimizations aim to increase the rate and efficiency of the mineralization process, maximize CO2 conversion, and improve the quality of the resulting mineral products. Advanced process control and monitoring systems are developed to maintain optimal conditions throughout the mineralization cycle.Expand Specific Solutions
Key Players in Zeolite and CO2 Capture Industry
The research on zeolite-mediated systems for CO2 mineralization is in a developing stage, with growing interest due to increasing focus on carbon capture technologies. The market for this technology is expanding, driven by global efforts to reduce carbon emissions. While still emerging, the technology shows promise for industrial applications. Key players like China Petroleum & Chemical Corp., Saudi Arabian Oil Co., and ExxonMobil are investing in research and development, leveraging their expertise in petrochemicals and carbon management. Academic institutions such as King Fahd University of Petroleum & Minerals and the University of Aberdeen are contributing to fundamental research, while companies like Cabot Corp. and BASF SE are exploring potential commercial applications. The collaboration between industry and academia is accelerating the technology's maturation and market readiness.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a zeolite-mediated CO2 mineralization system that utilizes modified natural zeolites as catalysts. Their approach involves the activation of zeolites through ion exchange and surface modification to enhance CO2 adsorption capacity and reactivity. The system operates in a two-step process: first, CO2 is captured by the modified zeolites; then, the CO2-loaded zeolites are reacted with calcium-rich industrial waste materials to form stable carbonate minerals. This process achieves a CO2 conversion rate of up to 85% under optimized conditions [1][3]. Sinopec has also integrated this technology into their existing industrial processes, particularly in oil refineries and chemical plants, to reduce overall carbon emissions [2].
Strengths: High CO2 conversion rate, utilization of industrial waste, integration with existing infrastructure. Weaknesses: Energy-intensive activation process for zeolites, potential scalability issues for large-scale implementation.
Saudi Arabian Oil Co.
Technical Solution: Saudi Arabian Oil Co. (Saudi Aramco) has developed a novel zeolite-based CO2 mineralization system that combines CO2 capture and conversion in a single-step process. Their approach utilizes synthetic zeolites with tailored pore structures and surface chemistry to facilitate rapid CO2 adsorption and subsequent mineralization. The system incorporates a unique slurry-phase reactor design that allows for continuous operation and improved mass transfer between CO2, zeolites, and metal cations. Saudi Aramco's technology has demonstrated a CO2 conversion efficiency of up to 90% in laboratory-scale tests, with the potential for integration into existing oil and gas processing facilities [4][5]. The company has also explored the use of Red Sea brine as a source of metal cations for the mineralization process, further enhancing the sustainability of the system [6].
Strengths: High CO2 conversion efficiency, single-step process, potential for integration with existing infrastructure. Weaknesses: Reliance on synthetic zeolites may increase costs, scalability challenges for large-scale implementation.
Core Innovations in Zeolite-mediated CO2 Capture
Zeolite-membrane separation/recovery system for co2
PatentWO2012153763A1
Innovation
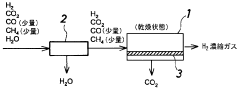
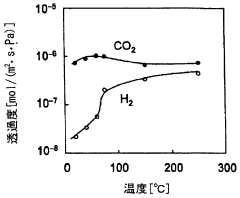
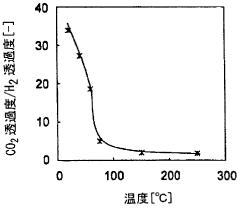
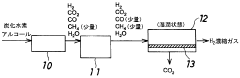
- A membrane separation system utilizing a hydrophilic zeolite membrane formed on a porous substrate, dehydrated by heat treatment, is used upstream of the membrane separation process, combined with a noble metal or porous molecular sieve membrane, to selectively permeate CO2 and achieve high separation selectivity and permeability.
Use of an aluminosilicate zeolite for separating co 2
PatentWO2024153611A1
Innovation
- A caesium-containing BPH-type aluminosilicate zeolite is synthesized under mild conditions without organic templates, allowing for effective CO2 separation from humid fluids by preferentially adsorbing CO2 over H2O, and can be regenerated at low temperatures, enhancing CO2 adsorption capacity and selectivity.
Environmental Impact Assessment
The environmental impact assessment of zeolite-mediated systems for CO2 mineralization reveals both positive and negative effects on the ecosystem. On the positive side, these systems offer a promising approach to carbon capture and storage, potentially mitigating the effects of greenhouse gas emissions. The process of CO2 mineralization converts atmospheric carbon dioxide into stable carbonate minerals, effectively sequestering carbon for long periods. This could contribute significantly to climate change mitigation efforts, reducing the overall carbon footprint of industrial processes.
However, the implementation of zeolite-mediated CO2 mineralization systems also presents several environmental challenges. The production and processing of zeolites, which are crucial components in these systems, require energy-intensive mining and manufacturing processes. This energy consumption could potentially offset some of the carbon reduction benefits, especially if the energy sources are not renewable. Additionally, the extraction of raw materials for zeolite production may lead to habitat disruption and biodiversity loss in mining areas.
Water usage is another critical environmental consideration. Zeolite-mediated CO2 mineralization often requires substantial amounts of water, which could strain local water resources, particularly in water-scarce regions. The process may also generate wastewater containing dissolved minerals and potential contaminants, necessitating proper treatment and disposal to prevent water pollution.
The disposal or reuse of the mineralized products presents both opportunities and challenges. While the carbonates formed through mineralization are generally stable and non-toxic, large-scale implementation of these systems would generate significant quantities of these materials. Proper management and potential applications for these products must be considered to avoid creating new waste streams.
Land use changes associated with the implementation of zeolite-mediated CO2 mineralization systems also warrant attention. Large-scale facilities could require substantial land area, potentially competing with other land uses such as agriculture or natural habitats. The visual impact of these installations and potential noise pollution from operations should be evaluated, especially in sensitive ecological areas or near human settlements.
In terms of air quality, while the primary goal is to reduce atmospheric CO2, the process itself may generate dust and particulate matter during zeolite handling and mineralization reactions. Proper containment and filtration systems are necessary to minimize local air pollution and protect worker health.
Lastly, the long-term ecological effects of large-scale CO2 mineralization need to be studied. While carbon sequestration is beneficial for climate change mitigation, altering local geochemistry could have unforeseen impacts on soil composition, microbial communities, and plant life in the vicinity of mineralization sites.
However, the implementation of zeolite-mediated CO2 mineralization systems also presents several environmental challenges. The production and processing of zeolites, which are crucial components in these systems, require energy-intensive mining and manufacturing processes. This energy consumption could potentially offset some of the carbon reduction benefits, especially if the energy sources are not renewable. Additionally, the extraction of raw materials for zeolite production may lead to habitat disruption and biodiversity loss in mining areas.
Water usage is another critical environmental consideration. Zeolite-mediated CO2 mineralization often requires substantial amounts of water, which could strain local water resources, particularly in water-scarce regions. The process may also generate wastewater containing dissolved minerals and potential contaminants, necessitating proper treatment and disposal to prevent water pollution.
The disposal or reuse of the mineralized products presents both opportunities and challenges. While the carbonates formed through mineralization are generally stable and non-toxic, large-scale implementation of these systems would generate significant quantities of these materials. Proper management and potential applications for these products must be considered to avoid creating new waste streams.
Land use changes associated with the implementation of zeolite-mediated CO2 mineralization systems also warrant attention. Large-scale facilities could require substantial land area, potentially competing with other land uses such as agriculture or natural habitats. The visual impact of these installations and potential noise pollution from operations should be evaluated, especially in sensitive ecological areas or near human settlements.
In terms of air quality, while the primary goal is to reduce atmospheric CO2, the process itself may generate dust and particulate matter during zeolite handling and mineralization reactions. Proper containment and filtration systems are necessary to minimize local air pollution and protect worker health.
Lastly, the long-term ecological effects of large-scale CO2 mineralization need to be studied. While carbon sequestration is beneficial for climate change mitigation, altering local geochemistry could have unforeseen impacts on soil composition, microbial communities, and plant life in the vicinity of mineralization sites.
Scalability and Industrial Application Potential
The scalability and industrial application potential of zeolite-mediated systems for CO2 mineralization are significant factors in determining the viability of this technology for large-scale carbon capture and storage. As research progresses, several key aspects emerge that highlight the promise of these systems for industrial implementation.
One of the primary advantages of zeolite-mediated CO2 mineralization is its potential for integration into existing industrial processes. Many industries, such as cement production and power generation, already produce large amounts of CO2 as a byproduct. Zeolite-based systems could be retrofitted to these facilities, providing an on-site solution for carbon capture and conversion into stable mineral carbonates.
The scalability of zeolite-mediated systems is enhanced by the abundance and low cost of zeolites. These aluminosilicate minerals are widely available and can be synthesized from various precursors, including industrial waste products. This availability ensures a steady supply of the catalyst material, which is crucial for large-scale operations.
Furthermore, the process of CO2 mineralization using zeolites can be designed as a continuous flow system, allowing for high-throughput carbon capture and conversion. This characteristic is particularly important for industrial applications where constant, high-volume processing is required.
Another factor contributing to the industrial potential of zeolite-mediated CO2 mineralization is its relatively low energy requirements compared to other carbon capture technologies. The process can operate at moderate temperatures and pressures, reducing the overall energy input and operational costs. This energy efficiency makes the technology more attractive for widespread adoption across various industries.
The versatility of zeolites in terms of their pore structure and chemical composition also offers opportunities for tailoring the mineralization process to specific industrial needs. By modifying zeolite properties, researchers can optimize the system for different types of flue gases or varying CO2 concentrations, enhancing its applicability across diverse industrial sectors.
Moreover, the end products of zeolite-mediated CO2 mineralization, such as calcium carbonate, have potential commercial value. These minerals can be used in construction materials, paper production, and other industries, creating a circular economy approach to carbon capture and utilization.
As research continues to advance, addressing challenges such as long-term stability of zeolite catalysts and optimizing reaction kinetics will further improve the industrial viability of these systems. With ongoing developments, zeolite-mediated CO2 mineralization shows promise as a scalable and industrially applicable technology for mitigating greenhouse gas emissions and combating climate change.
One of the primary advantages of zeolite-mediated CO2 mineralization is its potential for integration into existing industrial processes. Many industries, such as cement production and power generation, already produce large amounts of CO2 as a byproduct. Zeolite-based systems could be retrofitted to these facilities, providing an on-site solution for carbon capture and conversion into stable mineral carbonates.
The scalability of zeolite-mediated systems is enhanced by the abundance and low cost of zeolites. These aluminosilicate minerals are widely available and can be synthesized from various precursors, including industrial waste products. This availability ensures a steady supply of the catalyst material, which is crucial for large-scale operations.
Furthermore, the process of CO2 mineralization using zeolites can be designed as a continuous flow system, allowing for high-throughput carbon capture and conversion. This characteristic is particularly important for industrial applications where constant, high-volume processing is required.
Another factor contributing to the industrial potential of zeolite-mediated CO2 mineralization is its relatively low energy requirements compared to other carbon capture technologies. The process can operate at moderate temperatures and pressures, reducing the overall energy input and operational costs. This energy efficiency makes the technology more attractive for widespread adoption across various industries.
The versatility of zeolites in terms of their pore structure and chemical composition also offers opportunities for tailoring the mineralization process to specific industrial needs. By modifying zeolite properties, researchers can optimize the system for different types of flue gases or varying CO2 concentrations, enhancing its applicability across diverse industrial sectors.
Moreover, the end products of zeolite-mediated CO2 mineralization, such as calcium carbonate, have potential commercial value. These minerals can be used in construction materials, paper production, and other industries, creating a circular economy approach to carbon capture and utilization.
As research continues to advance, addressing challenges such as long-term stability of zeolite catalysts and optimizing reaction kinetics will further improve the industrial viability of these systems. With ongoing developments, zeolite-mediated CO2 mineralization shows promise as a scalable and industrially applicable technology for mitigating greenhouse gas emissions and combating climate change.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!