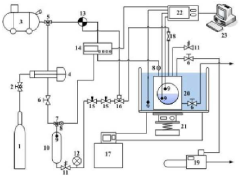
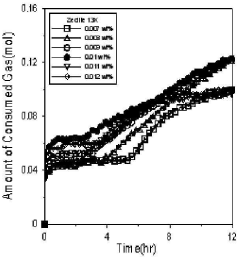
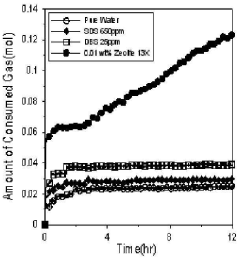
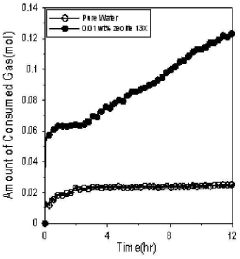
Use of Zeolites in Gas Hydrate Formation and Dissociation
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeolite-Hydrate Interaction Background and Objectives
Gas hydrates, crystalline compounds formed by water and gas molecules, have garnered significant attention in recent years due to their potential as an energy resource and their role in climate change. Zeolites, microporous aluminosilicate minerals, have emerged as a promising material for enhancing gas hydrate formation and controlling their dissociation. This technological intersection presents a unique opportunity for addressing energy and environmental challenges.
The use of zeolites in gas hydrate formation and dissociation has evolved from initial observations of their promoting effects to more sophisticated applications in gas storage, separation, and carbon dioxide sequestration. Early studies focused on the ability of zeolites to act as nucleation sites for hydrate formation, significantly reducing induction times and increasing formation rates. As research progressed, the specific properties of zeolites, such as their high surface area, uniform pore structure, and ion-exchange capabilities, were recognized as key factors in their effectiveness.
The primary objective of this technological approach is to harness the synergistic relationship between zeolites and gas hydrates to develop more efficient and controllable methods for hydrate formation and dissociation. This includes improving the kinetics of hydrate formation, enhancing gas storage capacity, and developing novel techniques for selective gas capture and release. Additionally, researchers aim to utilize this interaction to mitigate the risks associated with naturally occurring gas hydrates, such as their potential to destabilize seafloor sediments or contribute to greenhouse gas emissions.
Recent advancements in zeolite synthesis and modification have opened new avenues for tailoring their properties to specific hydrate-related applications. This includes the development of hierarchical zeolites with enhanced mass transfer properties, functionalized zeolites for improved gas selectivity, and composite materials that combine the advantages of zeolites with other promoters or inhibitors of hydrate formation.
The exploration of zeolite-hydrate interactions also extends to understanding the fundamental mechanisms governing their relationship. This involves investigating the role of zeolite surface chemistry, pore geometry, and framework composition in hydrate nucleation and growth. Such knowledge is crucial for designing optimized zeolite materials and predicting their performance in various hydrate-related processes.
As research in this field progresses, the ultimate goal is to translate laboratory findings into practical, large-scale applications. This includes developing zeolite-based technologies for natural gas storage and transportation, CO2 capture and sequestration, and potentially even extraterrestrial resource utilization. The success of these endeavors could have far-reaching implications for energy security, environmental protection, and space exploration.
The use of zeolites in gas hydrate formation and dissociation has evolved from initial observations of their promoting effects to more sophisticated applications in gas storage, separation, and carbon dioxide sequestration. Early studies focused on the ability of zeolites to act as nucleation sites for hydrate formation, significantly reducing induction times and increasing formation rates. As research progressed, the specific properties of zeolites, such as their high surface area, uniform pore structure, and ion-exchange capabilities, were recognized as key factors in their effectiveness.
The primary objective of this technological approach is to harness the synergistic relationship between zeolites and gas hydrates to develop more efficient and controllable methods for hydrate formation and dissociation. This includes improving the kinetics of hydrate formation, enhancing gas storage capacity, and developing novel techniques for selective gas capture and release. Additionally, researchers aim to utilize this interaction to mitigate the risks associated with naturally occurring gas hydrates, such as their potential to destabilize seafloor sediments or contribute to greenhouse gas emissions.
Recent advancements in zeolite synthesis and modification have opened new avenues for tailoring their properties to specific hydrate-related applications. This includes the development of hierarchical zeolites with enhanced mass transfer properties, functionalized zeolites for improved gas selectivity, and composite materials that combine the advantages of zeolites with other promoters or inhibitors of hydrate formation.
The exploration of zeolite-hydrate interactions also extends to understanding the fundamental mechanisms governing their relationship. This involves investigating the role of zeolite surface chemistry, pore geometry, and framework composition in hydrate nucleation and growth. Such knowledge is crucial for designing optimized zeolite materials and predicting their performance in various hydrate-related processes.
As research in this field progresses, the ultimate goal is to translate laboratory findings into practical, large-scale applications. This includes developing zeolite-based technologies for natural gas storage and transportation, CO2 capture and sequestration, and potentially even extraterrestrial resource utilization. The success of these endeavors could have far-reaching implications for energy security, environmental protection, and space exploration.
Market Analysis for Zeolite-Based Gas Hydrate Technologies
The market for zeolite-based gas hydrate technologies is experiencing significant growth, driven by the increasing demand for natural gas and the need for more efficient and environmentally friendly extraction methods. Gas hydrates, crystalline compounds composed of water and natural gas molecules, represent a vast untapped energy resource. Zeolites, with their unique porous structure and catalytic properties, play a crucial role in both the formation and dissociation of gas hydrates.
The global zeolite market, valued at $33.8 billion in 2020, is projected to reach $47.5 billion by 2028, growing at a CAGR of 4.3% from 2021 to 2028. This growth is partly attributed to the increasing application of zeolites in gas hydrate technologies. The Asia-Pacific region, particularly China and Japan, leads the market due to their extensive research and development activities in gas hydrate extraction.
In the context of gas hydrate technologies, zeolites are primarily used for two purposes: promoting gas hydrate formation for storage and transportation, and enhancing gas hydrate dissociation for extraction. The market for zeolite-based storage and transportation solutions is expected to grow rapidly, driven by the need for more efficient natural gas logistics in regions lacking pipeline infrastructure.
The gas hydrate extraction market, while still in its early stages, shows immense potential. Countries with significant gas hydrate reserves, such as Japan, China, and the United States, are investing heavily in research and development of extraction technologies. The U.S. Department of Energy has allocated substantial funding for gas hydrate research, with a focus on zeolite-based extraction methods.
Environmental concerns and regulations are also shaping the market landscape. Zeolite-based technologies offer a more environmentally friendly alternative to traditional extraction methods, aligning with global efforts to reduce carbon emissions. This factor is expected to drive adoption in environmentally conscious markets, particularly in Europe and North America.
The competitive landscape of the zeolite-based gas hydrate technology market is characterized by a mix of established chemical companies and innovative startups. Key players include Honeywell UOP, BASF, and Clariant, who are investing in research and development to improve zeolite performance in gas hydrate applications. Emerging companies like Zeochem and Zeolyst International are also making significant contributions to the field.
Despite the promising outlook, challenges remain. The high initial investment required for research and development, coupled with the technical complexities of gas hydrate extraction, may slow market growth. However, as technology advances and becomes more cost-effective, the market is expected to expand rapidly, potentially revolutionizing the natural gas industry.
The global zeolite market, valued at $33.8 billion in 2020, is projected to reach $47.5 billion by 2028, growing at a CAGR of 4.3% from 2021 to 2028. This growth is partly attributed to the increasing application of zeolites in gas hydrate technologies. The Asia-Pacific region, particularly China and Japan, leads the market due to their extensive research and development activities in gas hydrate extraction.
In the context of gas hydrate technologies, zeolites are primarily used for two purposes: promoting gas hydrate formation for storage and transportation, and enhancing gas hydrate dissociation for extraction. The market for zeolite-based storage and transportation solutions is expected to grow rapidly, driven by the need for more efficient natural gas logistics in regions lacking pipeline infrastructure.
The gas hydrate extraction market, while still in its early stages, shows immense potential. Countries with significant gas hydrate reserves, such as Japan, China, and the United States, are investing heavily in research and development of extraction technologies. The U.S. Department of Energy has allocated substantial funding for gas hydrate research, with a focus on zeolite-based extraction methods.
Environmental concerns and regulations are also shaping the market landscape. Zeolite-based technologies offer a more environmentally friendly alternative to traditional extraction methods, aligning with global efforts to reduce carbon emissions. This factor is expected to drive adoption in environmentally conscious markets, particularly in Europe and North America.
The competitive landscape of the zeolite-based gas hydrate technology market is characterized by a mix of established chemical companies and innovative startups. Key players include Honeywell UOP, BASF, and Clariant, who are investing in research and development to improve zeolite performance in gas hydrate applications. Emerging companies like Zeochem and Zeolyst International are also making significant contributions to the field.
Despite the promising outlook, challenges remain. The high initial investment required for research and development, coupled with the technical complexities of gas hydrate extraction, may slow market growth. However, as technology advances and becomes more cost-effective, the market is expected to expand rapidly, potentially revolutionizing the natural gas industry.
Current Challenges in Zeolite-Assisted Hydrate Formation
Despite the promising potential of zeolites in gas hydrate formation and dissociation, several challenges persist in their practical application. One of the primary obstacles is the optimization of zeolite pore size and structure for specific gas hydrate systems. While zeolites offer a range of pore sizes, finding the ideal match for different gas molecules and hydrate structures remains complex, often requiring extensive experimentation and computational modeling.
Another significant challenge lies in the scalability of zeolite-assisted hydrate formation processes. Laboratory-scale successes have been achieved, but translating these results to industrial-scale operations presents numerous engineering hurdles. Issues such as uniform distribution of zeolites within large-scale reactors, maintaining consistent temperature and pressure conditions, and ensuring efficient heat transfer throughout the system need to be addressed.
The long-term stability and reusability of zeolites in hydrate formation cycles also pose challenges. Repeated hydrate formation and dissociation can lead to structural changes in zeolites, potentially altering their performance over time. Understanding and mitigating these effects are crucial for developing economically viable processes.
Water management in zeolite-assisted hydrate formation is another area of concern. Zeolites' hydrophilic nature can lead to competitive adsorption between water and gas molecules, potentially reducing hydrate formation efficiency. Balancing water content to promote hydrate growth while preventing zeolite saturation requires careful control and innovative solutions.
Furthermore, the selectivity of zeolites for specific gas components in mixed gas streams presents both opportunities and challenges. While this selectivity can be advantageous for gas separation applications, it may complicate hydrate formation processes involving multi-component gas mixtures, necessitating tailored zeolite designs or multi-stage processes.
The environmental impact and cost-effectiveness of large-scale zeolite production and application in hydrate formation also need careful consideration. Developing sustainable synthesis methods and exploring the use of natural zeolites are areas requiring further research to address these concerns.
Lastly, integrating zeolite-assisted hydrate formation technology with existing industrial processes and infrastructure presents logistical and technical challenges. Adapting current systems to incorporate zeolite-based methods while maintaining operational efficiency and safety standards requires significant engineering efforts and potential redesigns of established processes.
Another significant challenge lies in the scalability of zeolite-assisted hydrate formation processes. Laboratory-scale successes have been achieved, but translating these results to industrial-scale operations presents numerous engineering hurdles. Issues such as uniform distribution of zeolites within large-scale reactors, maintaining consistent temperature and pressure conditions, and ensuring efficient heat transfer throughout the system need to be addressed.
The long-term stability and reusability of zeolites in hydrate formation cycles also pose challenges. Repeated hydrate formation and dissociation can lead to structural changes in zeolites, potentially altering their performance over time. Understanding and mitigating these effects are crucial for developing economically viable processes.
Water management in zeolite-assisted hydrate formation is another area of concern. Zeolites' hydrophilic nature can lead to competitive adsorption between water and gas molecules, potentially reducing hydrate formation efficiency. Balancing water content to promote hydrate growth while preventing zeolite saturation requires careful control and innovative solutions.
Furthermore, the selectivity of zeolites for specific gas components in mixed gas streams presents both opportunities and challenges. While this selectivity can be advantageous for gas separation applications, it may complicate hydrate formation processes involving multi-component gas mixtures, necessitating tailored zeolite designs or multi-stage processes.
The environmental impact and cost-effectiveness of large-scale zeolite production and application in hydrate formation also need careful consideration. Developing sustainable synthesis methods and exploring the use of natural zeolites are areas requiring further research to address these concerns.
Lastly, integrating zeolite-assisted hydrate formation technology with existing industrial processes and infrastructure presents logistical and technical challenges. Adapting current systems to incorporate zeolite-based methods while maintaining operational efficiency and safety standards requires significant engineering efforts and potential redesigns of established processes.
Existing Zeolite Solutions for Hydrate Formation Control
01 Zeolite synthesis methods
Various methods for synthesizing zeolites, including hydrothermal synthesis, sol-gel processes, and template-assisted growth. These techniques involve controlling factors such as temperature, pressure, pH, and reactant concentrations to achieve desired zeolite structures and properties.- Zeolite synthesis methods: Various methods for synthesizing zeolites, including hydrothermal synthesis, sol-gel processes, and template-assisted growth. These techniques involve controlling factors such as temperature, pressure, pH, and reaction time to produce zeolites with specific structures and properties.
- Zeolite structure characterization: Techniques for analyzing and characterizing zeolite structures, including X-ray diffraction, electron microscopy, and spectroscopic methods. These approaches help determine the crystalline structure, pore size distribution, and chemical composition of zeolites.
- Zeolite modification and functionalization: Methods for modifying zeolites to enhance their properties or introduce new functionalities. This includes ion exchange, dealumination, and incorporation of metal nanoparticles. These modifications can improve catalytic activity, selectivity, and stability of zeolites for various applications.
- Zeolite dissociation and regeneration: Processes for breaking down zeolite structures and regenerating them for reuse. This includes thermal treatments, chemical treatments, and combinations thereof to remove trapped molecules, restore porosity, and maintain catalytic activity.
- Applications of zeolites: Various industrial and commercial applications of zeolites, including catalysis, gas separation, water purification, and ion exchange. The unique properties of zeolites, such as their high surface area and selective adsorption capabilities, make them valuable in diverse fields.
02 Zeolite structure characterization
Techniques for analyzing and characterizing zeolite structures, including X-ray diffraction, electron microscopy, and spectroscopic methods. These approaches help determine crystal structure, pore size distribution, and chemical composition of zeolites, which are crucial for understanding their formation and properties.Expand Specific Solutions03 Zeolite modification and functionalization
Methods for modifying zeolites to enhance their properties or introduce new functionalities. This includes ion exchange, dealumination, and incorporation of metal ions or organic molecules into the zeolite framework, which can alter their catalytic activity, adsorption capacity, or selectivity.Expand Specific Solutions04 Zeolite dissociation and regeneration
Processes for breaking down zeolite structures and regenerating them for reuse. This includes thermal treatments, chemical treatments, and mechanical methods to remove trapped molecules, repair structural defects, or completely dissolve and recrystallize zeolites.Expand Specific Solutions05 Applications of zeolites in various industries
Diverse applications of zeolites in industries such as catalysis, gas separation, water treatment, and agriculture. The unique properties of zeolites, including their porous structure and ion-exchange capabilities, make them valuable in numerous processes and technologies.Expand Specific Solutions
Key Players in Zeolite and Gas Hydrate Industries
The use of zeolites in gas hydrate formation and dissociation is an emerging field with significant potential in energy and environmental applications. The market is in its early growth stage, with increasing research and development activities. Key players like UOP LLC, Asahi Kasei Corp., and Johnson Matthey Plc are leveraging their expertise in catalysts and adsorbents to explore zeolite applications in gas hydrate processes. The technology is still evolving, with academic institutions such as Xi'an University of Architecture & Technology and King Fahd University of Petroleum & Minerals contributing to fundamental research. As the technology matures, it is expected to attract more industrial players, potentially revolutionizing gas storage and transportation methods.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has developed advanced zeolite-based technologies for gas hydrate management in natural gas processing. Their approach involves using specially designed zeolite catalysts to promote controlled hydrate formation and dissociation. The company's patented ZeoGas™ process utilizes zeolite adsorbents to selectively remove water and heavy hydrocarbons from natural gas streams, effectively preventing hydrate formation in pipelines and processing equipment[1]. Additionally, UOP has engineered zeolite-based membranes that can be used for gas separation and purification, which indirectly aids in hydrate management by removing potential hydrate-forming components[2].
Strengths: Extensive experience in industrial-scale zeolite applications, proprietary ZeoGas™ technology, and integrated solutions for natural gas processing. Weaknesses: Potential high initial investment costs for implementing zeolite-based systems in existing infrastructure.
Saudi Arabian Oil Co.
Technical Solution: Saudi Aramco, the world's largest oil company, has invested significantly in zeolite-based technologies for gas hydrate management. Their research focuses on developing novel zeolite formulations tailored for the specific conditions of their gas fields. The company has patented a method using hydrophobic zeolites to inhibit gas hydrate formation in offshore pipelines[3]. This approach involves coating pipeline interiors with zeolite-based materials that repel water molecules, thereby preventing the nucleation of hydrate crystals. Furthermore, Saudi Aramco has explored the use of zeolite-supported metal nanoparticles as catalysts for rapid hydrate dissociation in emergency scenarios[4]. Their ongoing research also investigates the potential of zeolite-based sensors for early detection of hydrate formation conditions in gas processing facilities.
Strengths: Substantial R&D resources, extensive field testing capabilities, and integration of zeolite technologies with existing oil and gas infrastructure. Weaknesses: Technologies primarily optimized for specific regional conditions, which may limit global applicability.
Core Innovations in Zeolite-Hydrate Interface Engineering
Methane hydrate using zeolite and manufacturing method thereof
PatentInactiveKR1020120026210A
Innovation
- A mixed fluid is prepared by dispersing zeolite in water using an ultrasonic dispersion method, and methane gas is injected into this fluid to accelerate the formation of methane hydrate.
Process for treating unsaturated hydrocarbon-containing gases or liquids by adsorption on zeolites
PatentInactiveEP0511885A1
Innovation
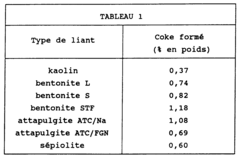
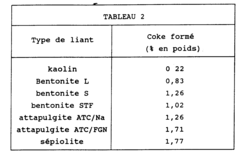
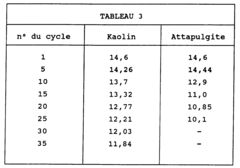
- Using zeolites bound with kaolin as the agglomeration binder, which significantly enhances the resistance to coke loading, allowing for prolonged adsorbent activity through a process involving dry-mixing zeolite and kaolin powders with lignosulphonate and polysaccharide agents, followed by extrusion and calcination, to create zeolite agglomerates suitable for thermal swing adsorption processes.
Environmental Impact of Zeolite Use in Hydrate Systems
The use of zeolites in gas hydrate formation and dissociation processes has significant environmental implications that warrant careful consideration. Zeolites, as microporous aluminosilicate minerals, possess unique properties that make them valuable in hydrate systems, but their application also raises important environmental concerns.
One of the primary environmental benefits of using zeolites in hydrate systems is their potential to enhance the efficiency of gas storage and transportation. By promoting the formation of gas hydrates, zeolites can facilitate the storage of large volumes of gas in a compact, stable form. This can lead to reduced energy consumption and greenhouse gas emissions associated with traditional gas transportation methods.
However, the extraction and processing of natural zeolites can have negative environmental impacts. Mining operations for zeolite deposits may result in habitat destruction, soil erosion, and water pollution if not properly managed. Additionally, the energy-intensive processes required to synthesize artificial zeolites contribute to carbon emissions and resource depletion.
In hydrate dissociation applications, zeolites can potentially mitigate some of the environmental risks associated with uncontrolled methane release from natural gas hydrates. By providing a controlled environment for hydrate decomposition, zeolites may help prevent sudden, large-scale methane emissions that could exacerbate climate change.
The use of zeolites in hydrate systems also raises questions about their long-term environmental fate. While zeolites are generally considered stable and non-toxic, their persistence in the environment after use in hydrate applications is not fully understood. There is a need for further research to assess the potential for zeolite accumulation in marine or terrestrial ecosystems and any associated ecological impacts.
Water quality is another important consideration in the environmental impact of zeolite use in hydrate systems. Zeolites have the capacity to adsorb various ions and molecules, which can be beneficial for water treatment purposes. However, this property also raises concerns about the potential release of trapped contaminants during hydrate formation or dissociation processes, particularly in marine environments.
The recyclability and reusability of zeolites in hydrate systems present both opportunities and challenges from an environmental perspective. Effective recycling of zeolites can reduce the demand for new material production and minimize waste. However, the processes involved in regenerating used zeolites may require additional energy and resources, potentially offsetting some of the environmental benefits.
One of the primary environmental benefits of using zeolites in hydrate systems is their potential to enhance the efficiency of gas storage and transportation. By promoting the formation of gas hydrates, zeolites can facilitate the storage of large volumes of gas in a compact, stable form. This can lead to reduced energy consumption and greenhouse gas emissions associated with traditional gas transportation methods.
However, the extraction and processing of natural zeolites can have negative environmental impacts. Mining operations for zeolite deposits may result in habitat destruction, soil erosion, and water pollution if not properly managed. Additionally, the energy-intensive processes required to synthesize artificial zeolites contribute to carbon emissions and resource depletion.
In hydrate dissociation applications, zeolites can potentially mitigate some of the environmental risks associated with uncontrolled methane release from natural gas hydrates. By providing a controlled environment for hydrate decomposition, zeolites may help prevent sudden, large-scale methane emissions that could exacerbate climate change.
The use of zeolites in hydrate systems also raises questions about their long-term environmental fate. While zeolites are generally considered stable and non-toxic, their persistence in the environment after use in hydrate applications is not fully understood. There is a need for further research to assess the potential for zeolite accumulation in marine or terrestrial ecosystems and any associated ecological impacts.
Water quality is another important consideration in the environmental impact of zeolite use in hydrate systems. Zeolites have the capacity to adsorb various ions and molecules, which can be beneficial for water treatment purposes. However, this property also raises concerns about the potential release of trapped contaminants during hydrate formation or dissociation processes, particularly in marine environments.
The recyclability and reusability of zeolites in hydrate systems present both opportunities and challenges from an environmental perspective. Effective recycling of zeolites can reduce the demand for new material production and minimize waste. However, the processes involved in regenerating used zeolites may require additional energy and resources, potentially offsetting some of the environmental benefits.
Economic Feasibility of Zeolite-Based Hydrate Technologies
The economic feasibility of zeolite-based hydrate technologies hinges on several key factors that must be carefully evaluated. Firstly, the cost of zeolite production and modification plays a crucial role in determining the overall economic viability. While zeolites are naturally occurring minerals, synthetic zeolites with tailored properties are often required for specific hydrate applications, potentially increasing production costs.
The efficiency of zeolite-based hydrate formation and dissociation processes is another critical factor. Enhanced formation rates and improved gas recovery during dissociation can significantly impact the economic attractiveness of these technologies. Research has shown that zeolites can accelerate hydrate formation and increase gas storage capacity, potentially reducing operational costs and improving overall system efficiency.
Energy requirements for hydrate formation and dissociation processes must also be considered. Zeolites may help lower the energy input needed for hydrate formation, but the energy costs associated with regeneration and recycling of zeolite materials should be factored into the economic analysis.
The scalability of zeolite-based hydrate technologies is crucial for their commercial viability. While laboratory-scale experiments have demonstrated promising results, the transition to industrial-scale applications may present challenges that could affect economic feasibility. Factors such as equipment design, process optimization, and material handling at larger scales need to be carefully evaluated.
Market demand for gas hydrate-based applications, such as natural gas storage and transportation, carbon dioxide sequestration, and water desalination, will significantly influence the economic potential of zeolite-based technologies. As global energy demands continue to rise and environmental concerns grow, the market for efficient and sustainable hydrate technologies is likely to expand.
Regulatory frameworks and environmental considerations also play a role in determining economic feasibility. Compliance with environmental regulations and potential incentives for clean energy technologies could impact the cost-benefit analysis of zeolite-based hydrate systems.
Lastly, the long-term durability and reusability of zeolite materials in hydrate applications must be assessed. The ability to maintain performance over multiple cycles and minimize material degradation will directly affect operational costs and overall economic viability.
The efficiency of zeolite-based hydrate formation and dissociation processes is another critical factor. Enhanced formation rates and improved gas recovery during dissociation can significantly impact the economic attractiveness of these technologies. Research has shown that zeolites can accelerate hydrate formation and increase gas storage capacity, potentially reducing operational costs and improving overall system efficiency.
Energy requirements for hydrate formation and dissociation processes must also be considered. Zeolites may help lower the energy input needed for hydrate formation, but the energy costs associated with regeneration and recycling of zeolite materials should be factored into the economic analysis.
The scalability of zeolite-based hydrate technologies is crucial for their commercial viability. While laboratory-scale experiments have demonstrated promising results, the transition to industrial-scale applications may present challenges that could affect economic feasibility. Factors such as equipment design, process optimization, and material handling at larger scales need to be carefully evaluated.
Market demand for gas hydrate-based applications, such as natural gas storage and transportation, carbon dioxide sequestration, and water desalination, will significantly influence the economic potential of zeolite-based technologies. As global energy demands continue to rise and environmental concerns grow, the market for efficient and sustainable hydrate technologies is likely to expand.
Regulatory frameworks and environmental considerations also play a role in determining economic feasibility. Compliance with environmental regulations and potential incentives for clean energy technologies could impact the cost-benefit analysis of zeolite-based hydrate systems.
Lastly, the long-term durability and reusability of zeolite materials in hydrate applications must be assessed. The ability to maintain performance over multiple cycles and minimize material degradation will directly affect operational costs and overall economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!