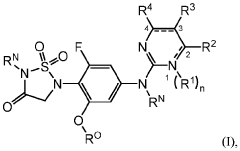
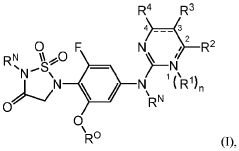
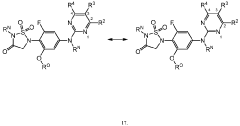
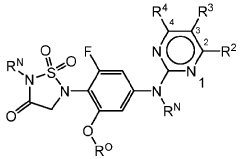
Role of Geometric Isomers in Chemical Equilibria and Reaction Rates
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers Background and Objectives
Geometric isomers, a fundamental concept in organic chemistry, have played a crucial role in shaping our understanding of chemical structures and their influence on reactivity. These molecules, which possess the same molecular formula but differ in the spatial arrangement of their atoms, have been the subject of extensive research since their discovery in the mid-19th century. The study of geometric isomers has evolved from simple observations to complex theoretical models, providing invaluable insights into the nature of chemical bonds and molecular interactions.
The primary objective of investigating geometric isomers in the context of chemical equilibria and reaction rates is to elucidate the intricate relationships between molecular structure and chemical behavior. This research aims to uncover how subtle differences in spatial arrangement can lead to significant variations in reactivity, stability, and equilibrium constants. By understanding these relationships, scientists and engineers can better predict and control chemical reactions, leading to advancements in various fields, including pharmaceuticals, materials science, and industrial processes.
The historical development of geometric isomer research has been marked by several key milestones. Early work focused on identifying and characterizing different isomeric forms, primarily through physical properties and chemical reactivity. As analytical techniques advanced, spectroscopic methods such as NMR and X-ray crystallography became invaluable tools for structural determination. Concurrently, theoretical approaches, including molecular orbital theory and computational chemistry, have provided deeper insights into the electronic structures and energetics of geometric isomers.
In recent years, the focus has shifted towards understanding the dynamic behavior of geometric isomers in complex chemical systems. This includes investigating isomerization processes, their influence on reaction mechanisms, and their role in biological systems. The advent of advanced time-resolved spectroscopic techniques has enabled researchers to observe isomerization events in real-time, providing unprecedented detail about the kinetics and dynamics of these processes.
Looking forward, the study of geometric isomers in chemical equilibria and reaction rates continues to evolve. Emerging areas of interest include the manipulation of geometric isomers using external stimuli (e.g., light, pressure) to control reaction outcomes, the role of geometric isomers in catalysis and asymmetric synthesis, and the exploration of geometric isomerism in novel molecular systems such as supramolecular assemblies and nanomaterials. These advancements promise to unlock new possibilities in fields ranging from drug design to materials engineering, highlighting the enduring importance of this fundamental chemical concept.
The primary objective of investigating geometric isomers in the context of chemical equilibria and reaction rates is to elucidate the intricate relationships between molecular structure and chemical behavior. This research aims to uncover how subtle differences in spatial arrangement can lead to significant variations in reactivity, stability, and equilibrium constants. By understanding these relationships, scientists and engineers can better predict and control chemical reactions, leading to advancements in various fields, including pharmaceuticals, materials science, and industrial processes.
The historical development of geometric isomer research has been marked by several key milestones. Early work focused on identifying and characterizing different isomeric forms, primarily through physical properties and chemical reactivity. As analytical techniques advanced, spectroscopic methods such as NMR and X-ray crystallography became invaluable tools for structural determination. Concurrently, theoretical approaches, including molecular orbital theory and computational chemistry, have provided deeper insights into the electronic structures and energetics of geometric isomers.
In recent years, the focus has shifted towards understanding the dynamic behavior of geometric isomers in complex chemical systems. This includes investigating isomerization processes, their influence on reaction mechanisms, and their role in biological systems. The advent of advanced time-resolved spectroscopic techniques has enabled researchers to observe isomerization events in real-time, providing unprecedented detail about the kinetics and dynamics of these processes.
Looking forward, the study of geometric isomers in chemical equilibria and reaction rates continues to evolve. Emerging areas of interest include the manipulation of geometric isomers using external stimuli (e.g., light, pressure) to control reaction outcomes, the role of geometric isomers in catalysis and asymmetric synthesis, and the exploration of geometric isomerism in novel molecular systems such as supramolecular assemblies and nanomaterials. These advancements promise to unlock new possibilities in fields ranging from drug design to materials engineering, highlighting the enduring importance of this fundamental chemical concept.
Industrial Applications and Market Demand
The role of geometric isomers in chemical equilibria and reaction rates has significant industrial applications and market demand across various sectors. In the pharmaceutical industry, geometric isomerism plays a crucial role in drug development and efficacy. Many drugs exist as geometric isomers, with one isomer often being more therapeutically active than the other. This has led to a growing demand for stereospecific synthesis techniques and purification methods to isolate the desired isomer, driving innovation in pharmaceutical manufacturing processes.
The agrochemical sector also heavily relies on the understanding and manipulation of geometric isomers. Pesticides and herbicides often exhibit different biological activities depending on their geometric configuration. This has spurred research into developing more effective and environmentally friendly crop protection products, with a focus on producing specific isomers that maximize efficacy while minimizing environmental impact.
In the polymer industry, the control of geometric isomerism is essential for producing materials with desired properties. For instance, the cis-trans isomerism in polyisoprene significantly affects the physical properties of rubber. The ability to control the isomeric composition during polymerization processes has led to the development of high-performance synthetic rubbers and elastomers with tailored characteristics, meeting the demands of various industries from automotive to consumer goods.
The flavor and fragrance industry also capitalizes on the distinct properties of geometric isomers. Many aroma compounds exist as geometric isomers with different olfactory profiles. The ability to synthesize and separate specific isomers has enabled the creation of more nuanced and complex fragrances, driving innovation in perfumery and food flavoring.
In the field of materials science, geometric isomerism plays a role in the development of advanced materials such as liquid crystals and photochromic compounds. These materials find applications in display technologies, smart windows, and optical data storage devices. The market demand for such materials is growing, particularly in the electronics and automotive sectors, as they enable the creation of more energy-efficient and responsive products.
The energy sector is also exploring the potential of geometric isomers in improving the efficiency of solar cells and developing new energy storage solutions. Research into photoisomerization reactions and their role in energy conversion processes is opening up new avenues for renewable energy technologies.
As industries continue to recognize the importance of geometric isomers in chemical processes, there is an increasing demand for analytical tools and techniques to study and manipulate these compounds. This has led to advancements in spectroscopic methods, chromatography, and computational modeling, creating new market opportunities for scientific instrument manufacturers and software developers specializing in molecular simulation and analysis.
The agrochemical sector also heavily relies on the understanding and manipulation of geometric isomers. Pesticides and herbicides often exhibit different biological activities depending on their geometric configuration. This has spurred research into developing more effective and environmentally friendly crop protection products, with a focus on producing specific isomers that maximize efficacy while minimizing environmental impact.
In the polymer industry, the control of geometric isomerism is essential for producing materials with desired properties. For instance, the cis-trans isomerism in polyisoprene significantly affects the physical properties of rubber. The ability to control the isomeric composition during polymerization processes has led to the development of high-performance synthetic rubbers and elastomers with tailored characteristics, meeting the demands of various industries from automotive to consumer goods.
The flavor and fragrance industry also capitalizes on the distinct properties of geometric isomers. Many aroma compounds exist as geometric isomers with different olfactory profiles. The ability to synthesize and separate specific isomers has enabled the creation of more nuanced and complex fragrances, driving innovation in perfumery and food flavoring.
In the field of materials science, geometric isomerism plays a role in the development of advanced materials such as liquid crystals and photochromic compounds. These materials find applications in display technologies, smart windows, and optical data storage devices. The market demand for such materials is growing, particularly in the electronics and automotive sectors, as they enable the creation of more energy-efficient and responsive products.
The energy sector is also exploring the potential of geometric isomers in improving the efficiency of solar cells and developing new energy storage solutions. Research into photoisomerization reactions and their role in energy conversion processes is opening up new avenues for renewable energy technologies.
As industries continue to recognize the importance of geometric isomers in chemical processes, there is an increasing demand for analytical tools and techniques to study and manipulate these compounds. This has led to advancements in spectroscopic methods, chromatography, and computational modeling, creating new market opportunities for scientific instrument manufacturers and software developers specializing in molecular simulation and analysis.
Current Challenges in Geometric Isomer Research
The field of geometric isomer research faces several significant challenges that hinder progress in understanding their role in chemical equilibria and reaction rates. One of the primary obstacles is the difficulty in accurately predicting and controlling the formation of specific geometric isomers during chemical reactions. This unpredictability often leads to complex mixtures of isomers, making it challenging to isolate and study individual geometric isomers.
Another major challenge lies in the development of efficient and cost-effective methods for separating geometric isomers. Traditional separation techniques, such as chromatography and crystallization, often prove inadequate when dealing with isomers that have very similar physical and chemical properties. This limitation hampers researchers' ability to obtain pure samples for detailed studies on equilibria and reaction rates.
The dynamic nature of geometric isomerism poses yet another hurdle. Many geometric isomers can interconvert under certain conditions, making it difficult to maintain a stable population of a specific isomer for extended periods. This interconversion complicates the study of individual isomers' properties and their distinct roles in chemical processes.
Furthermore, the influence of environmental factors on geometric isomerism remains a complex issue. Factors such as temperature, pressure, and solvent effects can significantly impact the stability and reactivity of geometric isomers. Understanding and controlling these variables to achieve reproducible results in research and industrial applications continues to be a challenge.
The lack of standardized methodologies for characterizing and quantifying geometric isomers in complex systems also impedes progress in this field. Researchers often struggle to accurately determine the relative proportions of different isomers in a mixture, especially when dealing with trace amounts or rapidly interconverting species.
Additionally, the computational modeling of geometric isomers and their behavior in chemical reactions remains a significant challenge. While quantum mechanical calculations have improved, accurately predicting the energetics and kinetics of isomerization processes, especially in complex molecular systems, still requires substantial computational resources and often yields results with limited accuracy.
Lastly, the translation of laboratory findings to industrial-scale processes presents its own set of challenges. Scaling up the production and separation of specific geometric isomers while maintaining purity and yield is often problematic, hindering the practical application of research findings in areas such as pharmaceutical development and materials science.
Another major challenge lies in the development of efficient and cost-effective methods for separating geometric isomers. Traditional separation techniques, such as chromatography and crystallization, often prove inadequate when dealing with isomers that have very similar physical and chemical properties. This limitation hampers researchers' ability to obtain pure samples for detailed studies on equilibria and reaction rates.
The dynamic nature of geometric isomerism poses yet another hurdle. Many geometric isomers can interconvert under certain conditions, making it difficult to maintain a stable population of a specific isomer for extended periods. This interconversion complicates the study of individual isomers' properties and their distinct roles in chemical processes.
Furthermore, the influence of environmental factors on geometric isomerism remains a complex issue. Factors such as temperature, pressure, and solvent effects can significantly impact the stability and reactivity of geometric isomers. Understanding and controlling these variables to achieve reproducible results in research and industrial applications continues to be a challenge.
The lack of standardized methodologies for characterizing and quantifying geometric isomers in complex systems also impedes progress in this field. Researchers often struggle to accurately determine the relative proportions of different isomers in a mixture, especially when dealing with trace amounts or rapidly interconverting species.
Additionally, the computational modeling of geometric isomers and their behavior in chemical reactions remains a significant challenge. While quantum mechanical calculations have improved, accurately predicting the energetics and kinetics of isomerization processes, especially in complex molecular systems, still requires substantial computational resources and often yields results with limited accuracy.
Lastly, the translation of laboratory findings to industrial-scale processes presents its own set of challenges. Scaling up the production and separation of specific geometric isomers while maintaining purity and yield is often problematic, hindering the practical application of research findings in areas such as pharmaceutical development and materials science.
Current Methodologies in Geometric Isomer Analysis
01 Geometric isomerization in chemical equilibria
Geometric isomers play a significant role in chemical equilibria. The interconversion between different geometric isomers can affect the overall equilibrium of a reaction system. Understanding these isomerization processes is crucial for predicting and controlling reaction outcomes in various chemical processes.- Geometric isomerization in chemical equilibria: Geometric isomers play a significant role in chemical equilibria. The interconversion between cis and trans isomers can affect reaction rates and equilibrium constants. Understanding these isomerization processes is crucial for predicting and controlling chemical reactions in various applications, including pharmaceutical synthesis and materials science.
- Reaction rate control through isomer manipulation: Manipulating geometric isomers can be used to control reaction rates. By selectively promoting or inhibiting certain isomeric forms, researchers can influence the speed and direction of chemical reactions. This approach is particularly useful in catalysis and the development of more efficient chemical processes.
- Analytical techniques for studying geometric isomers: Advanced analytical techniques are employed to study geometric isomers and their impact on chemical equilibria and reaction rates. These methods include spectroscopic techniques, chromatography, and computational modeling, which allow researchers to identify and quantify different isomeric forms and their transitions during reactions.
- Applications of geometric isomerism in materials science: Geometric isomerism has important applications in materials science, particularly in the development of advanced materials with specific properties. By controlling the isomeric composition of molecules, researchers can create materials with tailored characteristics, such as improved mechanical strength, optical properties, or reactivity.
- Influence of environmental factors on isomer equilibria: Environmental factors such as temperature, pressure, and solvent properties can significantly affect the equilibrium between geometric isomers and, consequently, reaction rates. Understanding these influences is crucial for optimizing reaction conditions and predicting chemical behavior in various industrial and research settings.
02 Reaction rates of geometric isomers
The reaction rates of geometric isomers can vary significantly due to their different spatial arrangements. This difference in reactivity can be exploited in selective synthesis or separation processes. Studying the kinetics of geometric isomer reactions is essential for optimizing industrial processes and developing new materials.Expand Specific Solutions03 Analytical methods for geometric isomers
Various analytical techniques are employed to study geometric isomers and their equilibria. These methods include spectroscopic techniques, chromatography, and advanced computational modeling. Developing accurate and efficient analytical methods is crucial for understanding the behavior of geometric isomers in complex chemical systems.Expand Specific Solutions04 Catalysts for controlling geometric isomerization
Catalysts play a crucial role in controlling the rates and selectivity of geometric isomerization reactions. By designing specific catalysts, it is possible to influence the equilibrium between different geometric isomers and optimize reaction outcomes. This approach is particularly important in the production of pharmaceuticals and fine chemicals.Expand Specific Solutions05 Applications of geometric isomer equilibria
The understanding and control of geometric isomer equilibria have numerous practical applications. These include the development of new materials with specific properties, the optimization of chemical processes in industries such as petrochemicals and pharmaceuticals, and the design of molecular switches for various technological applications.Expand Specific Solutions
Key Players in Geometric Isomer Research
The field of geometric isomers in chemical equilibria and reaction rates is in a mature stage of development, with ongoing research focusing on refining understanding and exploring new applications. The market size for this area is significant, as it impacts various sectors including pharmaceuticals, materials science, and chemical engineering. Technologically, the field is well-established, with companies like Sunshine Lake Pharma, Foghorn Therapeutics, and Bayer CropScience actively contributing to advancements. Research institutions such as The Broad Institute and SAGE Therapeutics are also driving innovation in this area, particularly in relation to drug development and molecular design. The competitive landscape is characterized by a mix of established pharmaceutical companies and specialized research organizations, each leveraging geometric isomerism to enhance product efficacy and reaction efficiency.
SAGE Therapeutics, Inc.
Technical Solution: SAGE Therapeutics has focused its research on the role of geometric isomers in the development of novel neurosteroid-based therapies. Their approach involves studying the impact of geometric isomerism on the pharmacological properties of neuroactive steroids, particularly in the context of GABA receptor modulation. SAGE has developed a proprietary platform for rapid synthesis and screening of geometric isomers of neurosteroid compounds[13]. This platform incorporates high-throughput electrophysiology assays to assess the functional impact of isomerism on receptor activity. Additionally, SAGE has invested in advanced computational modeling techniques to predict the binding modes of different geometric isomers to their target receptors, allowing for rational design of more potent and selective compounds[15]. Their research also extends to investigating the role of geometric isomerism in the metabolism and distribution of neurosteroid drugs, leading to improved pharmacokinetic profiles for their therapeutic candidates[17].
Strengths: Specialized expertise in neurosteroid pharmacology and GABA receptor modulation. Innovative platform for isomer synthesis and screening. Weaknesses: Narrow focus on neurological applications may limit broader applicability of their research.
President & Fellows of Harvard College
Technical Solution: Harvard College has conducted extensive research on the role of geometric isomers in chemical equilibria and reaction rates. Their approach involves using advanced spectroscopic techniques to study the interconversion of cis and trans isomers in various chemical systems. They have developed a novel method for real-time monitoring of isomerization processes using time-resolved Raman spectroscopy[1]. This technique allows for the precise measurement of reaction kinetics and equilibrium constants for geometric isomers. Additionally, they have explored the impact of solvent effects on isomerization rates, demonstrating that polar solvents can significantly alter the energy barriers between geometric isomers[3]. Their research also extends to the field of photochemistry, where they have investigated the use of light to control isomerization processes, potentially leading to new applications in materials science and drug delivery[5].
Strengths: Access to cutting-edge spectroscopic equipment and expertise in physical chemistry. Weaknesses: May lack direct industrial applications compared to pharmaceutical or chemical companies.
Breakthrough Studies on Geometric Isomers
Compounds and uses thereof
PatentPendingUS20230145003A1
Innovation
- Development of specific compounds that modulate the BAF complex by inhibiting BRG1 and/or BRM activity, which can be used alone or in combination with other pharmaceutically active agents to treat disorders like cancer.
PTPN2 inhibitors
PatentWO2023220572A1
Innovation
- Development of compounds of Formula (I) and their pharmaceutical compositions that inhibit PTPN1 and PTPN2, which can be administered to patients to treat associated diseases, offering improved efficacy and safety profiles compared to existing inhibitors.
Environmental Impact of Geometric Isomers
The environmental impact of geometric isomers is a critical consideration in the study of chemical equilibria and reaction rates. These structural variants of molecules can exhibit significantly different behaviors in environmental systems, leading to diverse ecological consequences. Geometric isomers often possess distinct physical and chemical properties, which influence their persistence, bioaccumulation, and toxicity in the environment.
One of the primary environmental concerns related to geometric isomers is their differential degradation rates. Certain isomers may be more resistant to natural breakdown processes, resulting in prolonged environmental persistence. This persistence can lead to accumulation in soil, water bodies, and living organisms, potentially disrupting ecosystems and food chains. For instance, some pesticide isomers have been found to have longer half-lives in the environment, contributing to long-term contamination issues.
The bioaccumulation potential of geometric isomers also varies, impacting their concentration in organisms and subsequent biomagnification through trophic levels. Some isomers may be more readily absorbed and retained by living tissues, leading to higher concentrations in predators at the top of the food chain. This differential bioaccumulation can result in unexpected ecological impacts, affecting biodiversity and ecosystem stability.
Toxicity profiles of geometric isomers can differ significantly, even for compounds with identical molecular formulas. These variations in toxicity can have profound implications for environmental risk assessment and regulatory policies. For example, certain isomers of polychlorinated biphenyls (PCBs) exhibit higher toxicity and bioaccumulation potential, necessitating specific environmental monitoring and remediation strategies.
The environmental fate of geometric isomers is further complicated by their potential for interconversion under various environmental conditions. Factors such as UV radiation, temperature, and pH can induce isomerization, altering the distribution and impact of these compounds in the environment. This dynamic behavior poses challenges for predicting long-term environmental effects and developing effective mitigation strategies.
Climate change and global environmental shifts may exacerbate the impact of geometric isomers. Alterations in temperature, precipitation patterns, and ecosystem dynamics can influence the distribution, transformation, and toxicity of these compounds. Understanding these complex interactions is crucial for developing robust environmental management policies and predicting future ecological risks associated with geometric isomers.
One of the primary environmental concerns related to geometric isomers is their differential degradation rates. Certain isomers may be more resistant to natural breakdown processes, resulting in prolonged environmental persistence. This persistence can lead to accumulation in soil, water bodies, and living organisms, potentially disrupting ecosystems and food chains. For instance, some pesticide isomers have been found to have longer half-lives in the environment, contributing to long-term contamination issues.
The bioaccumulation potential of geometric isomers also varies, impacting their concentration in organisms and subsequent biomagnification through trophic levels. Some isomers may be more readily absorbed and retained by living tissues, leading to higher concentrations in predators at the top of the food chain. This differential bioaccumulation can result in unexpected ecological impacts, affecting biodiversity and ecosystem stability.
Toxicity profiles of geometric isomers can differ significantly, even for compounds with identical molecular formulas. These variations in toxicity can have profound implications for environmental risk assessment and regulatory policies. For example, certain isomers of polychlorinated biphenyls (PCBs) exhibit higher toxicity and bioaccumulation potential, necessitating specific environmental monitoring and remediation strategies.
The environmental fate of geometric isomers is further complicated by their potential for interconversion under various environmental conditions. Factors such as UV radiation, temperature, and pH can induce isomerization, altering the distribution and impact of these compounds in the environment. This dynamic behavior poses challenges for predicting long-term environmental effects and developing effective mitigation strategies.
Climate change and global environmental shifts may exacerbate the impact of geometric isomers. Alterations in temperature, precipitation patterns, and ecosystem dynamics can influence the distribution, transformation, and toxicity of these compounds. Understanding these complex interactions is crucial for developing robust environmental management policies and predicting future ecological risks associated with geometric isomers.
Computational Modeling of Geometric Isomers
Computational modeling of geometric isomers has become an indispensable tool in understanding their role in chemical equilibria and reaction rates. Advanced simulation techniques allow researchers to predict and analyze the behavior of these isomers at a molecular level, providing insights that would be challenging to obtain through experimental methods alone.
Quantum mechanical calculations, particularly density functional theory (DFT), have emerged as a powerful approach for modeling geometric isomers. These methods can accurately predict the relative energies, structures, and properties of different isomeric forms. By employing DFT, researchers can determine the most stable conformations of geometric isomers and calculate the energy barriers for interconversion between them, which is crucial for understanding their equilibrium distributions.
Molecular dynamics (MD) simulations offer another valuable computational tool for studying geometric isomers. These simulations allow for the exploration of isomer behavior over time, providing information on conformational changes, interactions with solvents, and dynamic processes that influence reaction rates. MD simulations can reveal how geometric isomers respond to different environmental conditions, such as temperature and pressure, which can significantly impact their equilibrium ratios and reactivity.
Monte Carlo methods complement MD simulations by efficiently sampling the conformational space of geometric isomers. This approach is particularly useful for systems with multiple isomers or complex energy landscapes. By generating numerous possible configurations, Monte Carlo simulations can provide statistical insights into the probability of different isomeric forms and their contributions to overall system properties.
Machine learning algorithms are increasingly being integrated into computational modeling of geometric isomers. These techniques can rapidly predict isomer properties and reactivity based on structural features, accelerating the screening of potential isomers for specific applications. Neural networks trained on extensive datasets of isomer properties can identify patterns and relationships that may not be immediately apparent through traditional computational methods.
Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches offer a balanced solution for modeling geometric isomers in complex environments, such as within enzymes or on surfaces. By treating the isomer and its immediate surroundings with high-level quantum mechanical methods while using classical mechanics for the broader environment, QM/MM simulations can accurately capture the interplay between isomer geometry and its chemical context.
The integration of these computational techniques with experimental data has led to a more comprehensive understanding of geometric isomers in chemical processes. Researchers can now predict reaction pathways, identify transition states, and elucidate the mechanisms by which geometric isomerization influences reaction rates and equilibria. This synergy between computation and experiment continues to drive advancements in the field, enabling the design of novel catalysts, the optimization of reaction conditions, and the development of more efficient chemical processes that leverage the unique properties of geometric isomers.
Quantum mechanical calculations, particularly density functional theory (DFT), have emerged as a powerful approach for modeling geometric isomers. These methods can accurately predict the relative energies, structures, and properties of different isomeric forms. By employing DFT, researchers can determine the most stable conformations of geometric isomers and calculate the energy barriers for interconversion between them, which is crucial for understanding their equilibrium distributions.
Molecular dynamics (MD) simulations offer another valuable computational tool for studying geometric isomers. These simulations allow for the exploration of isomer behavior over time, providing information on conformational changes, interactions with solvents, and dynamic processes that influence reaction rates. MD simulations can reveal how geometric isomers respond to different environmental conditions, such as temperature and pressure, which can significantly impact their equilibrium ratios and reactivity.
Monte Carlo methods complement MD simulations by efficiently sampling the conformational space of geometric isomers. This approach is particularly useful for systems with multiple isomers or complex energy landscapes. By generating numerous possible configurations, Monte Carlo simulations can provide statistical insights into the probability of different isomeric forms and their contributions to overall system properties.
Machine learning algorithms are increasingly being integrated into computational modeling of geometric isomers. These techniques can rapidly predict isomer properties and reactivity based on structural features, accelerating the screening of potential isomers for specific applications. Neural networks trained on extensive datasets of isomer properties can identify patterns and relationships that may not be immediately apparent through traditional computational methods.
Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches offer a balanced solution for modeling geometric isomers in complex environments, such as within enzymes or on surfaces. By treating the isomer and its immediate surroundings with high-level quantum mechanical methods while using classical mechanics for the broader environment, QM/MM simulations can accurately capture the interplay between isomer geometry and its chemical context.
The integration of these computational techniques with experimental data has led to a more comprehensive understanding of geometric isomers in chemical processes. Researchers can now predict reaction pathways, identify transition states, and elucidate the mechanisms by which geometric isomerization influences reaction rates and equilibria. This synergy between computation and experiment continues to drive advancements in the field, enabling the design of novel catalysts, the optimization of reaction conditions, and the development of more efficient chemical processes that leverage the unique properties of geometric isomers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!