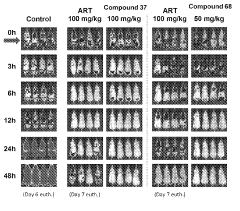
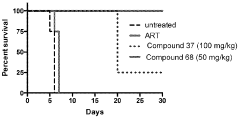
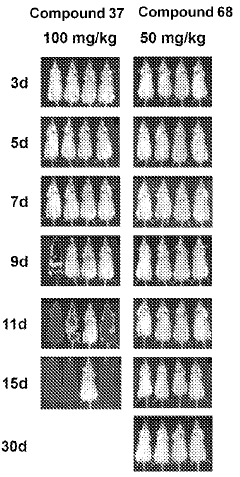
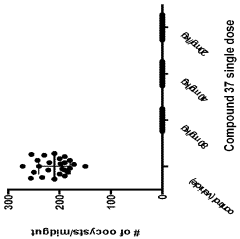
The Role of Geometric Isomers in Drug Metabolite Profiling
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Drug Development
Geometric isomers play a crucial role in drug development, significantly impacting the pharmacological properties and metabolic profiles of pharmaceutical compounds. These isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit vastly different biological activities and metabolic fates within the human body.
In drug discovery and development, understanding the geometric isomerism of potential drug candidates is essential for optimizing their efficacy and safety profiles. The spatial configuration of a molecule can affect its binding affinity to target receptors, influencing its therapeutic potency and selectivity. Moreover, geometric isomers can undergo distinct metabolic pathways, leading to variations in drug bioavailability, half-life, and potential side effects.
One of the primary challenges in drug development is the identification and characterization of geometric isomers. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are employed to separate and identify these isomers. These methods allow researchers to determine the relative abundance of different isomers in a drug formulation and assess their individual pharmacokinetic properties.
The importance of geometric isomerism is particularly evident in the field of stereochemistry-based drug design. By carefully controlling the stereochemistry of drug molecules, pharmaceutical companies can develop more effective and safer medications. This approach has led to the development of single-isomer drugs, which contain only one specific geometric isomer, rather than a mixture of isomers.
In metabolite profiling, geometric isomers present both challenges and opportunities. The metabolic transformation of drugs can result in the formation of new geometric isomers, each with potentially different biological activities. Understanding these metabolic processes is crucial for predicting drug-drug interactions, assessing toxicity risks, and optimizing dosing regimens.
Recent advancements in computational chemistry and molecular modeling have significantly enhanced our ability to predict and analyze the behavior of geometric isomers in drug development. These tools allow researchers to simulate the interactions between drug isomers and biological targets, providing valuable insights into structure-activity relationships and guiding the design of more effective drug candidates.
As the pharmaceutical industry continues to evolve, the consideration of geometric isomerism in drug development is becoming increasingly sophisticated. Regulatory agencies now require thorough characterization of all isomers present in drug formulations, including their potential metabolites. This heightened scrutiny has led to more rigorous testing and quality control measures throughout the drug development process, ultimately resulting in safer and more effective medications for patients.
In drug discovery and development, understanding the geometric isomerism of potential drug candidates is essential for optimizing their efficacy and safety profiles. The spatial configuration of a molecule can affect its binding affinity to target receptors, influencing its therapeutic potency and selectivity. Moreover, geometric isomers can undergo distinct metabolic pathways, leading to variations in drug bioavailability, half-life, and potential side effects.
One of the primary challenges in drug development is the identification and characterization of geometric isomers. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are employed to separate and identify these isomers. These methods allow researchers to determine the relative abundance of different isomers in a drug formulation and assess their individual pharmacokinetic properties.
The importance of geometric isomerism is particularly evident in the field of stereochemistry-based drug design. By carefully controlling the stereochemistry of drug molecules, pharmaceutical companies can develop more effective and safer medications. This approach has led to the development of single-isomer drugs, which contain only one specific geometric isomer, rather than a mixture of isomers.
In metabolite profiling, geometric isomers present both challenges and opportunities. The metabolic transformation of drugs can result in the formation of new geometric isomers, each with potentially different biological activities. Understanding these metabolic processes is crucial for predicting drug-drug interactions, assessing toxicity risks, and optimizing dosing regimens.
Recent advancements in computational chemistry and molecular modeling have significantly enhanced our ability to predict and analyze the behavior of geometric isomers in drug development. These tools allow researchers to simulate the interactions between drug isomers and biological targets, providing valuable insights into structure-activity relationships and guiding the design of more effective drug candidates.
As the pharmaceutical industry continues to evolve, the consideration of geometric isomerism in drug development is becoming increasingly sophisticated. Regulatory agencies now require thorough characterization of all isomers present in drug formulations, including their potential metabolites. This heightened scrutiny has led to more rigorous testing and quality control measures throughout the drug development process, ultimately resulting in safer and more effective medications for patients.
Market Demand for Metabolite Profiling
The market demand for metabolite profiling, particularly in the context of geometric isomers in drug development, has been steadily increasing over the past decade. This growth is primarily driven by the pharmaceutical industry's need for more comprehensive and accurate drug safety assessments, as well as the rising emphasis on personalized medicine.
Pharmaceutical companies are increasingly recognizing the importance of understanding the metabolic fate of drugs, including the formation and behavior of geometric isomers. This knowledge is crucial for predicting drug efficacy, toxicity, and potential drug-drug interactions. As a result, there is a growing demand for advanced metabolite profiling techniques that can distinguish between geometric isomers and provide detailed information about their pharmacokinetic and pharmacodynamic properties.
The global metabolomics market, which encompasses metabolite profiling, was valued at approximately $2.2 billion in 2020 and is projected to reach $4.5 billion by 2025, with a compound annual growth rate (CAGR) of 15.4%. A significant portion of this growth is attributed to the increasing adoption of metabolite profiling in drug discovery and development processes.
In the pharmaceutical industry, there is a particular focus on metabolite profiling during the early stages of drug development. This trend is driven by regulatory requirements, such as the FDA's Metabolites in Safety Testing (MIST) guidance, which emphasizes the importance of identifying and characterizing drug metabolites, including geometric isomers, to ensure drug safety and efficacy.
The demand for metabolite profiling is also being fueled by advancements in analytical technologies, such as high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy. These technologies enable more precise identification and quantification of geometric isomers in complex biological matrices, meeting the industry's need for detailed metabolic information.
Furthermore, the growing field of pharmacometabolomics is creating new opportunities for metabolite profiling. This approach combines metabolomics with pharmacology to predict drug response and toxicity based on an individual's metabolic profile. The ability to distinguish between geometric isomers is crucial in this context, as these isomers can have significantly different biological activities and metabolic fates.
The increasing focus on rare diseases and orphan drugs is another factor driving the demand for metabolite profiling. In these cases, understanding the metabolic profile of drugs, including the role of geometric isomers, is essential for developing effective treatments for small patient populations.
Pharmaceutical companies are increasingly recognizing the importance of understanding the metabolic fate of drugs, including the formation and behavior of geometric isomers. This knowledge is crucial for predicting drug efficacy, toxicity, and potential drug-drug interactions. As a result, there is a growing demand for advanced metabolite profiling techniques that can distinguish between geometric isomers and provide detailed information about their pharmacokinetic and pharmacodynamic properties.
The global metabolomics market, which encompasses metabolite profiling, was valued at approximately $2.2 billion in 2020 and is projected to reach $4.5 billion by 2025, with a compound annual growth rate (CAGR) of 15.4%. A significant portion of this growth is attributed to the increasing adoption of metabolite profiling in drug discovery and development processes.
In the pharmaceutical industry, there is a particular focus on metabolite profiling during the early stages of drug development. This trend is driven by regulatory requirements, such as the FDA's Metabolites in Safety Testing (MIST) guidance, which emphasizes the importance of identifying and characterizing drug metabolites, including geometric isomers, to ensure drug safety and efficacy.
The demand for metabolite profiling is also being fueled by advancements in analytical technologies, such as high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy. These technologies enable more precise identification and quantification of geometric isomers in complex biological matrices, meeting the industry's need for detailed metabolic information.
Furthermore, the growing field of pharmacometabolomics is creating new opportunities for metabolite profiling. This approach combines metabolomics with pharmacology to predict drug response and toxicity based on an individual's metabolic profile. The ability to distinguish between geometric isomers is crucial in this context, as these isomers can have significantly different biological activities and metabolic fates.
The increasing focus on rare diseases and orphan drugs is another factor driving the demand for metabolite profiling. In these cases, understanding the metabolic profile of drugs, including the role of geometric isomers, is essential for developing effective treatments for small patient populations.
Current Challenges in Isomer Analysis
The analysis of geometric isomers in drug metabolite profiling faces several significant challenges that hinder accurate identification and quantification. One of the primary obstacles is the structural similarity between isomers, which makes them difficult to distinguish using conventional analytical techniques. This similarity often results in overlapping peaks in chromatographic separations, leading to ambiguous results and potential misidentification of metabolites.
Another major challenge is the limited resolution of many analytical instruments when dealing with complex biological matrices. The presence of numerous endogenous compounds in biological samples can interfere with the detection and characterization of isomeric drug metabolites, especially when they are present in low concentrations. This issue is particularly pronounced in high-throughput screening methods, where the need for rapid analysis may compromise the ability to resolve closely related isomers.
The dynamic nature of isomerization processes in biological systems adds another layer of complexity to metabolite profiling. Some geometric isomers can interconvert under physiological conditions or during sample preparation, making it challenging to determine the original form of the metabolite in vivo. This interconversion can lead to inaccurate assessments of metabolite profiles and potentially misleading conclusions about drug metabolism pathways.
Furthermore, the lack of comprehensive spectral libraries and databases for geometric isomers of drug metabolites poses a significant challenge in their identification. Many existing libraries are incomplete or do not adequately represent the diversity of isomeric structures, making it difficult to match experimental data with reference spectra. This gap in reference data often necessitates time-consuming and resource-intensive de novo structure elucidation processes.
The development of robust and reliable quantification methods for geometric isomers is also challenging. Many current analytical techniques struggle to provide accurate quantitative data for isomeric mixtures, especially when dealing with complex biological matrices. This limitation can lead to errors in pharmacokinetic studies and potentially impact the assessment of drug efficacy and safety.
Lastly, the interpretation of mass spectrometry data for geometric isomers remains a significant challenge. Fragmentation patterns of isomers can be very similar, making it difficult to differentiate between them based on MS/MS spectra alone. This similarity often requires the integration of multiple analytical techniques and sophisticated data analysis algorithms to achieve reliable isomer identification and characterization.
Another major challenge is the limited resolution of many analytical instruments when dealing with complex biological matrices. The presence of numerous endogenous compounds in biological samples can interfere with the detection and characterization of isomeric drug metabolites, especially when they are present in low concentrations. This issue is particularly pronounced in high-throughput screening methods, where the need for rapid analysis may compromise the ability to resolve closely related isomers.
The dynamic nature of isomerization processes in biological systems adds another layer of complexity to metabolite profiling. Some geometric isomers can interconvert under physiological conditions or during sample preparation, making it challenging to determine the original form of the metabolite in vivo. This interconversion can lead to inaccurate assessments of metabolite profiles and potentially misleading conclusions about drug metabolism pathways.
Furthermore, the lack of comprehensive spectral libraries and databases for geometric isomers of drug metabolites poses a significant challenge in their identification. Many existing libraries are incomplete or do not adequately represent the diversity of isomeric structures, making it difficult to match experimental data with reference spectra. This gap in reference data often necessitates time-consuming and resource-intensive de novo structure elucidation processes.
The development of robust and reliable quantification methods for geometric isomers is also challenging. Many current analytical techniques struggle to provide accurate quantitative data for isomeric mixtures, especially when dealing with complex biological matrices. This limitation can lead to errors in pharmacokinetic studies and potentially impact the assessment of drug efficacy and safety.
Lastly, the interpretation of mass spectrometry data for geometric isomers remains a significant challenge. Fragmentation patterns of isomers can be very similar, making it difficult to differentiate between them based on MS/MS spectra alone. This similarity often requires the integration of multiple analytical techniques and sophisticated data analysis algorithms to achieve reliable isomer identification and characterization.
Existing Methods for Isomer Separation
01 Analytical methods for geometric isomer identification
Various analytical techniques are employed to identify and characterize geometric isomers in drug metabolite profiling. These methods may include chromatography, spectroscopy, and mass spectrometry. The combination of these techniques allows for the separation and identification of different geometric isomers, which is crucial for understanding drug metabolism and potential biological effects.- Analytical techniques for geometric isomer identification: Various analytical techniques are employed to identify and characterize geometric isomers in drug metabolite profiling. These methods may include chromatography, spectroscopy, and mass spectrometry. The combination of these techniques allows for the separation and identification of different geometric isomers, which is crucial for understanding drug metabolism and potential biological effects.
- Metabolite profiling of geometric isomers in drug development: Geometric isomer metabolite profiling plays a crucial role in drug development. This process involves identifying and quantifying different geometric isomers of drug metabolites, which can have varying biological activities and pharmacokinetic properties. Understanding the metabolic fate of geometric isomers helps in predicting drug efficacy, toxicity, and potential drug-drug interactions.
- Synthesis and separation of geometric isomers: Methods for synthesizing and separating geometric isomers are essential in drug metabolite profiling. These techniques may involve stereoselective synthesis, isomerization reactions, and advanced separation methods. The ability to produce and isolate specific geometric isomers allows for more accurate profiling and assessment of their individual metabolic properties.
- In vivo and in vitro studies of geometric isomer metabolism: Both in vivo and in vitro studies are conducted to investigate the metabolism of geometric isomers. These studies help elucidate metabolic pathways, identify key enzymes involved in isomer interconversion, and determine the pharmacological and toxicological implications of different geometric isomers. Such information is vital for drug safety and efficacy assessments.
- Computational methods in geometric isomer metabolite prediction: Computational approaches, including molecular modeling and machine learning, are increasingly used to predict the metabolic fate of geometric isomers. These methods can help in identifying potential metabolites, predicting their structures, and estimating their biological activities. Computational tools complement experimental techniques and can accelerate the drug development process by providing early insights into metabolite profiles.
02 Metabolite profiling of geometric isomers in drug development
Geometric isomer metabolite profiling plays a crucial role in drug development. This process involves identifying and quantifying different geometric isomers of drug metabolites, which can have varying biological activities and pharmacokinetic properties. Understanding the metabolic fate of geometric isomers helps in assessing drug efficacy, safety, and potential side effects.Expand Specific Solutions03 Synthesis and isolation of geometric isomers for metabolite studies
Researchers develop methods to synthesize and isolate specific geometric isomers of drug compounds and their metabolites. These pure isomers are essential for conducting accurate metabolite profiling studies and understanding the pharmacological properties of each isomer. Techniques may include stereoselective synthesis, chiral separation, and purification methods.Expand Specific Solutions04 In vitro and in vivo models for geometric isomer metabolism
Various in vitro and in vivo models are utilized to study the metabolism of geometric isomers. These models help researchers understand how different isomers are metabolized in the body, their distribution, and their potential biological effects. Such studies are crucial for predicting drug behavior and optimizing drug design.Expand Specific Solutions05 Computational methods for predicting geometric isomer metabolism
Advanced computational techniques are employed to predict the metabolism of geometric isomers. These methods may include molecular modeling, machine learning algorithms, and quantitative structure-activity relationship (QSAR) studies. Such predictive tools aid in the early stages of drug development by providing insights into potential metabolic pathways and metabolite profiles of geometric isomers.Expand Specific Solutions
Key Players in Metabolomics Industry
The field of geometric isomers in drug metabolite profiling is in a mature stage of development, with significant market potential due to its crucial role in pharmaceutical research and development. The global market for metabolite profiling is estimated to be worth billions of dollars, driven by the increasing demand for personalized medicine and drug safety assessments. Companies like Novo Nordisk, Vertex Pharmaceuticals, and Bristol Myers Squibb are at the forefront of this technology, leveraging advanced analytical techniques and AI-driven platforms to enhance drug discovery and development processes. Smaller specialized firms such as Metabolon and BIOCRATES Life Sciences are also making significant contributions, offering cutting-edge metabolomics solutions to pharmaceutical companies and research institutions.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has developed a comprehensive approach to drug metabolite profiling that incorporates geometric isomer analysis. Their method utilizes advanced liquid chromatography-mass spectrometry (LC-MS) techniques to separate and identify geometric isomers of drug metabolites. The company employs a combination of high-resolution mass spectrometry and ion mobility spectrometry to distinguish between geometric isomers with similar molecular masses but different spatial arrangements[1]. This approach allows for the detection and characterization of previously unidentified metabolites, including those resulting from geometric isomerization. Vertex has also implemented computational modeling to predict potential geometric isomers and their metabolic pathways, enhancing the efficiency of their metabolite profiling process[3].
Strengths: Advanced analytical techniques for isomer separation, comprehensive metabolite identification, and predictive modeling capabilities. Weaknesses: Potentially high cost and complexity of implementation, may require specialized expertise to interpret results.
Metabolon, Inc.
Technical Solution: Metabolon has developed a proprietary metabolomics platform that incorporates geometric isomer analysis in drug metabolite profiling. Their approach combines ultra-high-performance liquid chromatography (UHPLC) with high-resolution mass spectrometry to achieve superior separation and identification of geometric isomers. The company utilizes a vast spectral library and advanced machine learning algorithms to accurately identify and quantify metabolites, including geometric isomers, in complex biological samples[2]. Metabolon's platform also integrates multi-omic data analysis, allowing for a more comprehensive understanding of drug metabolism and the role of geometric isomers in pharmacokinetics and pharmacodynamics[4].
Strengths: Comprehensive metabolomics platform, extensive spectral library, and integration of multi-omic data. Weaknesses: Potential reliance on proprietary databases and algorithms, which may limit transparency and external validation.
Innovations in Geometric Isomer Detection
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Compounds and uses thereof
PatentPendingUS20230145003A1
Innovation
- Development of specific compounds that modulate the BAF complex by inhibiting BRG1 and/or BRM activity, which can be used alone or in combination with other pharmaceutically active agents to treat disorders like cancer.
Regulatory Guidelines for Metabolite Profiling
Regulatory guidelines for metabolite profiling play a crucial role in ensuring the safety and efficacy of pharmaceutical products. These guidelines are established by regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to provide a framework for the identification, characterization, and assessment of drug metabolites.
The FDA's Guidance for Industry on Safety Testing of Drug Metabolites emphasizes the importance of identifying and characterizing metabolites that are present at exposures greater than 10% of the parent drug systemic exposure at steady state. This guideline also recommends conducting safety assessments on these metabolites to evaluate their potential toxicity.
Similarly, the EMA's Guideline on the Investigation of Drug Interactions outlines the requirements for metabolite profiling studies. It emphasizes the need to identify metabolites that contribute significantly to the pharmacological activity or toxicity of the drug. The EMA guideline also recommends investigating the potential for drug-drug interactions involving metabolites.
In the context of geometric isomers, regulatory guidelines require thorough characterization of all isomeric forms of drug metabolites. This is particularly important as geometric isomers can exhibit different pharmacological and toxicological properties. The guidelines emphasize the need to develop analytical methods capable of distinguishing and quantifying individual isomers.
Regulatory agencies also require the assessment of metabolite exposure in humans compared to animal species used in toxicology studies. This comparison helps identify any human metabolites that may not have been adequately evaluated in preclinical safety studies, potentially necessitating additional toxicological evaluations.
The International Conference on Harmonisation (ICH) guideline M3(R2) provides recommendations on the timing of metabolite profiling studies during drug development. It suggests that metabolite profiling should be conducted early in the development process to inform subsequent safety assessments and clinical trial designs.
Regulatory guidelines also address the need for metabolite profiling in special populations, such as patients with hepatic or renal impairment. These studies are crucial for understanding potential differences in metabolite formation and elimination in these populations, which may impact drug safety and efficacy.
In conclusion, regulatory guidelines for metabolite profiling provide a comprehensive framework for investigating drug metabolism, including the role of geometric isomers. Adherence to these guidelines ensures a thorough understanding of drug metabolites, their potential impact on safety and efficacy, and informs appropriate risk assessment and management strategies throughout the drug development process.
The FDA's Guidance for Industry on Safety Testing of Drug Metabolites emphasizes the importance of identifying and characterizing metabolites that are present at exposures greater than 10% of the parent drug systemic exposure at steady state. This guideline also recommends conducting safety assessments on these metabolites to evaluate their potential toxicity.
Similarly, the EMA's Guideline on the Investigation of Drug Interactions outlines the requirements for metabolite profiling studies. It emphasizes the need to identify metabolites that contribute significantly to the pharmacological activity or toxicity of the drug. The EMA guideline also recommends investigating the potential for drug-drug interactions involving metabolites.
In the context of geometric isomers, regulatory guidelines require thorough characterization of all isomeric forms of drug metabolites. This is particularly important as geometric isomers can exhibit different pharmacological and toxicological properties. The guidelines emphasize the need to develop analytical methods capable of distinguishing and quantifying individual isomers.
Regulatory agencies also require the assessment of metabolite exposure in humans compared to animal species used in toxicology studies. This comparison helps identify any human metabolites that may not have been adequately evaluated in preclinical safety studies, potentially necessitating additional toxicological evaluations.
The International Conference on Harmonisation (ICH) guideline M3(R2) provides recommendations on the timing of metabolite profiling studies during drug development. It suggests that metabolite profiling should be conducted early in the development process to inform subsequent safety assessments and clinical trial designs.
Regulatory guidelines also address the need for metabolite profiling in special populations, such as patients with hepatic or renal impairment. These studies are crucial for understanding potential differences in metabolite formation and elimination in these populations, which may impact drug safety and efficacy.
In conclusion, regulatory guidelines for metabolite profiling provide a comprehensive framework for investigating drug metabolism, including the role of geometric isomers. Adherence to these guidelines ensures a thorough understanding of drug metabolites, their potential impact on safety and efficacy, and informs appropriate risk assessment and management strategies throughout the drug development process.
Impact on Drug Safety and Efficacy
The impact of geometric isomers on drug safety and efficacy is a critical consideration in drug metabolite profiling. Geometric isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit significantly different pharmacological properties. This variation can lead to profound implications for drug safety and efficacy, necessitating careful analysis during drug development and metabolite profiling.
One of the primary concerns regarding geometric isomers in drug metabolism is their potential to produce unexpected metabolites. These metabolites may have different biological activities compared to the parent compound, potentially leading to altered efficacy or unforeseen side effects. For instance, the cis and trans isomers of a drug may be metabolized at different rates or through different pathways, resulting in varying concentrations of active metabolites in the body.
The presence of geometric isomers can also affect drug-drug interactions, a crucial aspect of drug safety. Isomers may interact differently with metabolizing enzymes, such as cytochrome P450, leading to altered metabolism of other drugs. This can result in increased or decreased efficacy of co-administered medications, potentially compromising patient safety or treatment outcomes.
Furthermore, geometric isomers can influence drug absorption, distribution, and excretion profiles. The spatial arrangement of atoms in different isomers can affect their lipophilicity, solubility, and binding affinity to transport proteins. These factors directly impact the drug's pharmacokinetics, potentially altering its bioavailability and therapeutic window.
In terms of efficacy, geometric isomers may exhibit varying degrees of potency or selectivity for their intended targets. This can lead to differences in therapeutic effects, with one isomer potentially being more effective than the other. In some cases, one isomer may be responsible for the desired therapeutic effect, while the other may contribute to side effects or have no significant activity.
The impact of geometric isomers on drug safety and efficacy underscores the importance of comprehensive metabolite profiling during drug development. Advanced analytical techniques, such as chiral chromatography and mass spectrometry, are essential for identifying and quantifying geometric isomers and their metabolites. This information is crucial for optimizing drug formulations, predicting potential safety issues, and developing more effective therapeutic strategies.
Regulatory agencies have recognized the significance of geometric isomers in drug development. Guidelines now require thorough characterization of isomeric compounds and their metabolites as part of the drug approval process. This emphasis on isomer-specific analysis has led to improved drug safety profiles and more targeted therapeutic approaches, ultimately benefiting patient outcomes.
One of the primary concerns regarding geometric isomers in drug metabolism is their potential to produce unexpected metabolites. These metabolites may have different biological activities compared to the parent compound, potentially leading to altered efficacy or unforeseen side effects. For instance, the cis and trans isomers of a drug may be metabolized at different rates or through different pathways, resulting in varying concentrations of active metabolites in the body.
The presence of geometric isomers can also affect drug-drug interactions, a crucial aspect of drug safety. Isomers may interact differently with metabolizing enzymes, such as cytochrome P450, leading to altered metabolism of other drugs. This can result in increased or decreased efficacy of co-administered medications, potentially compromising patient safety or treatment outcomes.
Furthermore, geometric isomers can influence drug absorption, distribution, and excretion profiles. The spatial arrangement of atoms in different isomers can affect their lipophilicity, solubility, and binding affinity to transport proteins. These factors directly impact the drug's pharmacokinetics, potentially altering its bioavailability and therapeutic window.
In terms of efficacy, geometric isomers may exhibit varying degrees of potency or selectivity for their intended targets. This can lead to differences in therapeutic effects, with one isomer potentially being more effective than the other. In some cases, one isomer may be responsible for the desired therapeutic effect, while the other may contribute to side effects or have no significant activity.
The impact of geometric isomers on drug safety and efficacy underscores the importance of comprehensive metabolite profiling during drug development. Advanced analytical techniques, such as chiral chromatography and mass spectrometry, are essential for identifying and quantifying geometric isomers and their metabolites. This information is crucial for optimizing drug formulations, predicting potential safety issues, and developing more effective therapeutic strategies.
Regulatory agencies have recognized the significance of geometric isomers in drug development. Guidelines now require thorough characterization of isomeric compounds and their metabolites as part of the drug approval process. This emphasis on isomer-specific analysis has led to improved drug safety profiles and more targeted therapeutic approaches, ultimately benefiting patient outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!