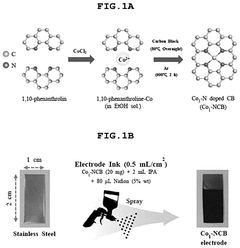
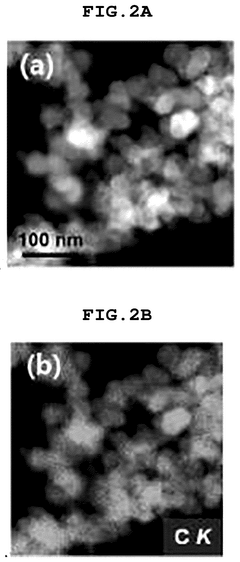
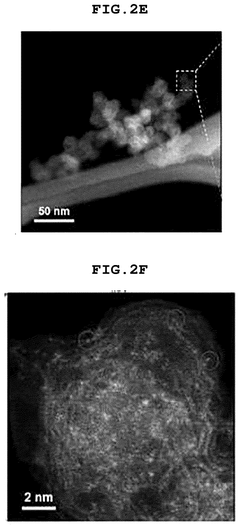
SAC Integration With Gas Diffusion Electrodes
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SAC-GDE Technology Background and Objectives
Single-atom catalysts (SAC) integrated with gas diffusion electrodes (GDE) represent a cutting-edge technological convergence that has emerged from decades of research in heterogeneous catalysis and electrochemical engineering. This integration combines the atomic efficiency of single-atom catalysts with the high mass transport capabilities of gas diffusion electrodes, creating systems with unprecedented potential for clean energy applications and industrial electrochemical processes.
The evolution of this technology can be traced back to the early 2000s when single-atom catalysis was first conceptualized, followed by significant breakthroughs in 2011 when researchers demonstrated the feasibility of anchoring individual metal atoms on supports. Concurrently, gas diffusion electrodes have evolved since the 1960s, primarily in fuel cell applications, before finding broader applications in CO2 reduction and other electrochemical conversions.
The convergence of these technologies has accelerated in the past five years, driven by urgent global needs for carbon-neutral technologies and efficient energy conversion systems. The integration addresses fundamental limitations in conventional catalytic systems, particularly regarding atom utilization efficiency, mass transport limitations, and reaction selectivity.
The primary technical objectives of SAC-GDE integration include maximizing the atomic efficiency of precious metal catalysts, enhancing the three-phase (solid-liquid-gas) interface critical for electrochemical reactions, and developing scalable manufacturing processes for commercial viability. Researchers aim to achieve near 100% atom utilization while maintaining catalyst stability under operating conditions—a significant challenge given the tendency of single atoms to aggregate under reaction conditions.
Another crucial objective is to optimize the microstructure of GDEs to accommodate single-atom catalysts without compromising their unique electronic properties or mass transport capabilities. This requires precise control over pore size distribution, hydrophobicity, and electrical conductivity throughout the electrode structure.
From a performance perspective, the technology targets substantial improvements in energy efficiency, reaction selectivity, and operational stability compared to conventional catalytic systems. Specific metrics include achieving Faradaic efficiencies exceeding 90% for target reactions, reducing overpotentials by at least 200mV, and demonstrating operational stability beyond 1000 hours without significant performance degradation.
The long-term vision for SAC-GDE technology encompasses transformative applications in renewable energy storage, carbon dioxide utilization, green hydrogen production, and sustainable chemical manufacturing. These applications align with global sustainability goals and represent potential solutions to some of the most pressing environmental challenges of our time.
The evolution of this technology can be traced back to the early 2000s when single-atom catalysis was first conceptualized, followed by significant breakthroughs in 2011 when researchers demonstrated the feasibility of anchoring individual metal atoms on supports. Concurrently, gas diffusion electrodes have evolved since the 1960s, primarily in fuel cell applications, before finding broader applications in CO2 reduction and other electrochemical conversions.
The convergence of these technologies has accelerated in the past five years, driven by urgent global needs for carbon-neutral technologies and efficient energy conversion systems. The integration addresses fundamental limitations in conventional catalytic systems, particularly regarding atom utilization efficiency, mass transport limitations, and reaction selectivity.
The primary technical objectives of SAC-GDE integration include maximizing the atomic efficiency of precious metal catalysts, enhancing the three-phase (solid-liquid-gas) interface critical for electrochemical reactions, and developing scalable manufacturing processes for commercial viability. Researchers aim to achieve near 100% atom utilization while maintaining catalyst stability under operating conditions—a significant challenge given the tendency of single atoms to aggregate under reaction conditions.
Another crucial objective is to optimize the microstructure of GDEs to accommodate single-atom catalysts without compromising their unique electronic properties or mass transport capabilities. This requires precise control over pore size distribution, hydrophobicity, and electrical conductivity throughout the electrode structure.
From a performance perspective, the technology targets substantial improvements in energy efficiency, reaction selectivity, and operational stability compared to conventional catalytic systems. Specific metrics include achieving Faradaic efficiencies exceeding 90% for target reactions, reducing overpotentials by at least 200mV, and demonstrating operational stability beyond 1000 hours without significant performance degradation.
The long-term vision for SAC-GDE technology encompasses transformative applications in renewable energy storage, carbon dioxide utilization, green hydrogen production, and sustainable chemical manufacturing. These applications align with global sustainability goals and represent potential solutions to some of the most pressing environmental challenges of our time.
Market Analysis for SAC-GDE Applications
The global market for Single Atom Catalysts (SAC) integrated with Gas Diffusion Electrodes (GDEs) is experiencing significant growth, driven by increasing demand for sustainable energy solutions and green chemistry applications. This integration represents a convergence of advanced catalytic technology with practical electrochemical engineering, creating substantial market opportunities across multiple sectors.
The renewable energy sector currently represents the largest market segment for SAC-GDE applications, particularly in fuel cells and electrolyzers. The global fuel cell market, valued at approximately $5.9 billion in 2022, is projected to reach $32 billion by 2030, with SAC-GDE technology positioned to capture a growing share of this expansion. Hydrogen production through water electrolysis using SAC-GDEs offers enhanced efficiency and reduced precious metal loading, addressing critical cost barriers in green hydrogen adoption.
Carbon dioxide reduction represents another rapidly expanding application area, with the CO2 utilization market expected to grow at a CAGR of 24% through 2028. SAC-GDEs enable selective conversion of CO2 to valuable chemicals and fuels, creating economic incentives for carbon capture while addressing climate concerns. Major chemical companies are increasingly investing in this technology to develop carbon-neutral production pathways.
Industrial electrochemistry presents substantial growth potential, particularly in chlor-alkali processes, organic electrosynthesis, and wastewater treatment. The precision of single-atom catalysts enables unprecedented selectivity in these processes, potentially reducing energy consumption by 15-30% compared to conventional methods while minimizing waste generation.
Regional analysis reveals Asia-Pacific as the fastest-growing market for SAC-GDE technology, with China, Japan, and South Korea making substantial investments in research and commercialization. North America and Europe maintain strong positions through established industrial partnerships and academic research networks focused on scaling these technologies.
Customer segmentation shows three primary market tiers: large industrial corporations seeking efficiency improvements and sustainability credentials; specialized clean technology companies developing next-generation energy systems; and research institutions advancing fundamental capabilities. Each segment presents distinct requirements and adoption timelines.
Market barriers include high initial production costs, scaling challenges for consistent single-atom distribution, and competition from established technologies. However, declining precious metal requirements through SAC technology is progressively improving economic feasibility, with production costs decreasing approximately 18% annually as manufacturing processes mature.
The renewable energy sector currently represents the largest market segment for SAC-GDE applications, particularly in fuel cells and electrolyzers. The global fuel cell market, valued at approximately $5.9 billion in 2022, is projected to reach $32 billion by 2030, with SAC-GDE technology positioned to capture a growing share of this expansion. Hydrogen production through water electrolysis using SAC-GDEs offers enhanced efficiency and reduced precious metal loading, addressing critical cost barriers in green hydrogen adoption.
Carbon dioxide reduction represents another rapidly expanding application area, with the CO2 utilization market expected to grow at a CAGR of 24% through 2028. SAC-GDEs enable selective conversion of CO2 to valuable chemicals and fuels, creating economic incentives for carbon capture while addressing climate concerns. Major chemical companies are increasingly investing in this technology to develop carbon-neutral production pathways.
Industrial electrochemistry presents substantial growth potential, particularly in chlor-alkali processes, organic electrosynthesis, and wastewater treatment. The precision of single-atom catalysts enables unprecedented selectivity in these processes, potentially reducing energy consumption by 15-30% compared to conventional methods while minimizing waste generation.
Regional analysis reveals Asia-Pacific as the fastest-growing market for SAC-GDE technology, with China, Japan, and South Korea making substantial investments in research and commercialization. North America and Europe maintain strong positions through established industrial partnerships and academic research networks focused on scaling these technologies.
Customer segmentation shows three primary market tiers: large industrial corporations seeking efficiency improvements and sustainability credentials; specialized clean technology companies developing next-generation energy systems; and research institutions advancing fundamental capabilities. Each segment presents distinct requirements and adoption timelines.
Market barriers include high initial production costs, scaling challenges for consistent single-atom distribution, and competition from established technologies. However, declining precious metal requirements through SAC technology is progressively improving economic feasibility, with production costs decreasing approximately 18% annually as manufacturing processes mature.
Technical Challenges in SAC-GDE Integration
The integration of Single-Atom Catalysts (SAC) with Gas Diffusion Electrodes (GDE) presents several significant technical challenges that must be addressed for successful implementation. One of the primary difficulties lies in achieving uniform dispersion of single-atom active sites across the GDE substrate. Unlike conventional nanoparticle catalysts, SACs require atomic-level precision in their distribution to maintain their unique catalytic properties, making the manufacturing process considerably more complex.
Interface stability represents another major challenge, as the dynamic operating conditions of GDEs can lead to migration and aggregation of single atoms, potentially causing the formation of clusters or nanoparticles. This transformation fundamentally alters the catalytic behavior and negates the advantages of single-atom architecture. The three-phase boundary where gas, liquid, and solid phases meet in GDEs creates a particularly challenging environment for maintaining SAC stability.
Mass transport limitations significantly impact SAC-GDE performance. The intricate porous structure of GDEs must be carefully engineered to facilitate efficient reactant delivery to catalytic sites while allowing product removal. When integrating SACs, this balance becomes even more critical as the atomic dispersion creates unique mass transport dynamics that differ from conventional catalyst systems.
Durability under operating conditions presents substantial obstacles. SACs are inherently susceptible to deactivation mechanisms including poisoning, leaching, and structural reorganization. The high-current density environments typical in industrial applications place extreme demands on catalyst stability. Additionally, the presence of contaminants in feed streams can preferentially adsorb on single-atom active sites, rapidly diminishing catalytic activity.
Scalable manufacturing techniques represent perhaps the most significant barrier to widespread adoption. Current laboratory methods for SAC synthesis often involve complex procedures that are difficult to scale industrially. The precise control required for atomic dispersion becomes increasingly challenging at larger production volumes, leading to inconsistent performance and higher manufacturing costs.
Characterization and quality control of SAC-GDE systems present unique challenges. Traditional analytical techniques may lack the resolution to accurately assess single-atom dispersion within the complex three-dimensional structure of GDEs. This limitation hampers both research advancement and quality assurance in production settings.
Optimizing the electronic interaction between single atoms and the support material requires sophisticated engineering approaches. The electron transfer dynamics at the atomic level significantly influence catalytic performance, necessitating precise tuning of the local electronic environment around each catalytic site.
Interface stability represents another major challenge, as the dynamic operating conditions of GDEs can lead to migration and aggregation of single atoms, potentially causing the formation of clusters or nanoparticles. This transformation fundamentally alters the catalytic behavior and negates the advantages of single-atom architecture. The three-phase boundary where gas, liquid, and solid phases meet in GDEs creates a particularly challenging environment for maintaining SAC stability.
Mass transport limitations significantly impact SAC-GDE performance. The intricate porous structure of GDEs must be carefully engineered to facilitate efficient reactant delivery to catalytic sites while allowing product removal. When integrating SACs, this balance becomes even more critical as the atomic dispersion creates unique mass transport dynamics that differ from conventional catalyst systems.
Durability under operating conditions presents substantial obstacles. SACs are inherently susceptible to deactivation mechanisms including poisoning, leaching, and structural reorganization. The high-current density environments typical in industrial applications place extreme demands on catalyst stability. Additionally, the presence of contaminants in feed streams can preferentially adsorb on single-atom active sites, rapidly diminishing catalytic activity.
Scalable manufacturing techniques represent perhaps the most significant barrier to widespread adoption. Current laboratory methods for SAC synthesis often involve complex procedures that are difficult to scale industrially. The precise control required for atomic dispersion becomes increasingly challenging at larger production volumes, leading to inconsistent performance and higher manufacturing costs.
Characterization and quality control of SAC-GDE systems present unique challenges. Traditional analytical techniques may lack the resolution to accurately assess single-atom dispersion within the complex three-dimensional structure of GDEs. This limitation hampers both research advancement and quality assurance in production settings.
Optimizing the electronic interaction between single atoms and the support material requires sophisticated engineering approaches. The electron transfer dynamics at the atomic level significantly influence catalytic performance, necessitating precise tuning of the local electronic environment around each catalytic site.
Current SAC-GDE Integration Solutions
01 SAC integration methods for gas diffusion electrodes
Various methods can be used to integrate single-atom catalysts (SACs) with gas diffusion electrodes (GDEs). These methods include atomic layer deposition, wet impregnation, and electrodeposition techniques that allow for precise placement of isolated metal atoms on support materials. The integration process typically involves anchoring metal atoms to nitrogen or oxygen functional groups on carbon-based substrates to create stable M-N-C or M-O-C structures that prevent aggregation during electrochemical reactions.- SAC integration methods for gas diffusion electrodes: Various methods can be used to integrate single-atom catalysts (SACs) with gas diffusion electrodes (GDEs). These methods include atomic layer deposition, wet impregnation, and electrodeposition techniques that allow for precise placement of isolated metal atoms on support materials. The integration process typically involves anchoring metal atoms to nitrogen or oxygen sites on carbon-based substrates to create stable M-N-C or M-O-C structures that prevent aggregation during electrochemical reactions.
- Carbon-based supports for SAC in GDEs: Carbon-based materials serve as excellent supports for single-atom catalysts in gas diffusion electrodes due to their high surface area, electrical conductivity, and ability to host coordinating sites. Materials such as graphene, carbon nanotubes, porous carbon, and nitrogen-doped carbon are commonly used. These supports provide the necessary structural framework for anchoring isolated metal atoms while facilitating electron transfer and gas diffusion properties essential for electrochemical reactions.
- Metal selection and loading strategies for SACs: The selection of metal species and their loading strategies significantly impact the performance of single-atom catalysts in gas diffusion electrodes. Transition metals such as Fe, Co, Ni, Cu, and noble metals like Pt, Pd, and Ir are commonly used as active sites. Precise control of metal loading is crucial to maintain the single-atom state and prevent clustering. Various techniques including controlled pyrolysis, coordination chemistry approaches, and spatial confinement methods are employed to achieve optimal metal dispersion and loading.
- Performance enhancement of SAC-GDE systems: Various strategies can enhance the performance of single-atom catalyst integrated gas diffusion electrodes. These include tuning the electronic structure of the metal centers through ligand effects, optimizing the porosity and hydrophobicity of the electrode to improve mass transport, and incorporating secondary components that can work synergistically with the single-atom sites. Additionally, structural modifications to the gas diffusion layer can improve the three-phase boundary, enhancing reactant accessibility and product removal efficiency.
- Applications of SAC-GDE systems in electrochemical processes: Single-atom catalyst integrated gas diffusion electrodes find applications in various electrochemical processes. These include CO2 electroreduction to valuable chemicals, oxygen reduction reaction for fuel cells, hydrogen evolution reaction for water splitting, and nitrogen reduction for ammonia synthesis. The high atom efficiency, selectivity, and activity of SACs combined with the mass transport benefits of GDEs make these systems particularly effective for industrial-scale electrochemical conversions and energy storage applications.
02 Carbon-based supports for SAC in GDEs
Carbon-based materials serve as excellent supports for single-atom catalysts in gas diffusion electrodes due to their high surface area, electrical conductivity, and ability to be functionalized. Materials such as graphene, carbon nanotubes, porous carbon, and nitrogen-doped carbon are commonly used. These supports provide abundant anchoring sites for metal atoms and facilitate electron transfer during electrochemical reactions, while their porous structure allows for efficient gas diffusion, which is crucial for electrode performance.Expand Specific Solutions03 Metal selection and coordination environment for SACs
The choice of metal and its coordination environment significantly impacts the catalytic performance of SACs in gas diffusion electrodes. Transition metals such as Fe, Co, Ni, Cu, and noble metals like Pt, Pd, and Ru are commonly used. The coordination environment, typically involving nitrogen, oxygen, or sulfur atoms, determines the electronic structure of the metal center and thus its catalytic activity. By tailoring the metal type and coordination sphere, researchers can optimize selectivity and activity for specific electrochemical reactions.Expand Specific Solutions04 Performance enhancement strategies for SAC-GDE systems
Various strategies can enhance the performance of SAC-integrated gas diffusion electrodes, including the creation of dual-atom or cluster sites, introduction of secondary components, and optimization of the electrode structure. Techniques such as hierarchical pore design, hydrophobic treatment, and incorporation of ionomer binders improve mass transport and reaction kinetics. Additionally, the use of promoters or co-catalysts can create synergistic effects that boost catalytic activity, selectivity, and stability during long-term operation.Expand Specific Solutions05 Applications of SAC-GDE systems in electrochemical devices
SAC-integrated gas diffusion electrodes find applications in various electrochemical devices including fuel cells, electrolyzers, and CO2 reduction systems. In fuel cells, they serve as efficient cathodes for oxygen reduction reactions with minimal platinum loading. In water electrolysis, they catalyze hydrogen and oxygen evolution reactions. For CO2 reduction, they enable selective conversion to valuable products like carbon monoxide, formate, or hydrocarbons. These applications benefit from the high atom utilization efficiency of SACs combined with the mass transport advantages of gas diffusion electrodes.Expand Specific Solutions
Key Industry Players in SAC-GDE Technology
The SAC (Solid Acid Catalyst) integration with Gas Diffusion Electrodes market is currently in an early growth phase, with increasing adoption across electrochemical applications. The global market size is estimated to be expanding at a CAGR of 8-10%, driven by rising demand for sustainable energy solutions and carbon capture technologies. From a technical maturity perspective, companies like Mitsui Chemicals, Tosoh Corp, and Industrie De Nora are leading commercial development, while research institutions such as Dalian Institute of Chemical Physics are advancing fundamental innovations. Japanese firms including Kaneka Corp and NGK Insulators have established strong patent positions, while newer entrants like Verdox are disrupting with novel electro-swing processes that promise significant efficiency improvements. The competitive landscape remains fragmented with specialized expertise distributed across chemical, materials, and electronics sectors.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has developed advanced SAC (Solid Alkaline Conductor) integration with gas diffusion electrodes (GDEs) for chlor-alkali production. Their technology utilizes ion-exchange membranes with specialized fluoropolymer structures that enhance ionic conductivity while maintaining mechanical stability. The company's approach incorporates nanoscale catalyst particles (typically platinum group metals or mixed metal oxides) directly embedded within a three-dimensional electrode matrix, creating an optimized triple-phase boundary. This design maximizes electrochemical reaction efficiency by facilitating simultaneous gas diffusion, electron transfer, and ion transport. Mitsui's proprietary binder system ensures long-term adhesion between the SAC layer and GDE components, addressing a common failure point in such systems. Their manufacturing process includes precision coating techniques that create uniform catalyst distribution and controlled porosity gradients across the electrode structure.
Strengths: Superior ionic conductivity and mechanical durability in harsh alkaline environments; excellent integration between SAC and GDE components resulting in reduced interfacial resistance; proven scalability for industrial applications. Weaknesses: Higher manufacturing costs compared to conventional systems; requires specialized materials that may face supply chain constraints; performance degradation under certain impurity conditions.
Chlorine Engineers Co. Ltd.
Technical Solution: Chlorine Engineers has pioneered a comprehensive SAC integration system with gas diffusion electrodes specifically optimized for chlor-alkali electrolysis. Their technology features a multi-layer electrode architecture where the SAC component is chemically bonded to a high-surface-area carbon substrate through proprietary surface functionalization techniques. The GDE structure incorporates hydrophobic PTFE regions alongside hydrophilic catalyst zones, creating an optimized pathway for both gas permeation and electrolyte access. Their innovation includes a gradient porosity design that transitions from larger pores at the gas interface to nanoscale pores at the electrolyte interface, maximizing mass transport efficiency. Chlorine Engineers' system also incorporates specialized current collectors with corrosion-resistant coatings that maintain electrical conductivity even after thousands of operational hours in aggressive chlorine environments. The company has developed automated manufacturing processes that ensure consistent electrode quality across large production volumes.
Strengths: Exceptional chlorine evolution efficiency with minimal side reactions; outstanding durability in industrial chlor-alkali conditions; proven track record in large-scale implementations with documented performance data. Weaknesses: System complexity requires specialized maintenance protocols; higher initial capital investment compared to traditional technologies; performance sensitivity to feed gas composition fluctuations.
Critical Patents and Research in SAC-GDE Field
Single-atom catalyst and method of preparing same
PatentPendingUS20250146149A1
Innovation
- A single-atom catalyst (SAC) is developed, comprising a nitrogen-doped carbon structure and a single-atom metal, such as cobalt, that forms a coordination bond with nitrogen atoms, preventing hydroxyl radical adsorption and extending the optimal pH range.
Acid-base reaction catalyst, gas diffusion electrode, and co 2 permeable device
PatentWO2013089112A1
Innovation
- An acid-base reaction catalyst comprising a support and a metal complex with a ligand having sp3 structure nitrogen, which enhances the catalytic performance by maintaining higher cationicity and susceptibility to nucleophilic attack, is developed. This catalyst is integrated into a gas diffusion electrode for efficient CO2 permeation devices.
Environmental Impact Assessment of SAC-GDE Technology
The integration of Single-Atom Catalysts (SAC) with Gas Diffusion Electrodes (GDE) represents a significant advancement in electrochemical technologies, but its environmental implications require thorough assessment. The SAC-GDE technology demonstrates considerable potential for reducing environmental footprints compared to conventional catalytic systems. Primary environmental benefits include substantial reductions in precious metal usage—often by 90-95%—due to the atomic-level dispersion of catalytic metals, addressing resource scarcity concerns and reducing mining-related environmental damage.
Energy efficiency improvements constitute another significant environmental advantage. SAC-GDE systems typically demonstrate 15-30% higher energy efficiency than traditional catalytic electrodes, translating to reduced carbon emissions when powered by conventional energy sources. When coupled with renewable energy, these systems can approach carbon-neutral operation for electrochemical processes like CO2 reduction or hydrogen evolution.
Waste reduction represents a third key environmental benefit. The enhanced selectivity of SAC-GDE systems (often exceeding 90% for target reactions) minimizes byproduct formation, reducing downstream separation requirements and associated energy consumption. Additionally, the extended catalyst lifetime—typically 2-3 times longer than conventional catalysts—reduces replacement frequency and associated manufacturing impacts.
However, several environmental challenges warrant attention. The synthesis of SAC-GDE components often involves specialized chemicals and energy-intensive processes. Life cycle assessments indicate that manufacturing impacts may offset operational benefits unless production methods are optimized. Preliminary studies suggest that manufacturing energy requirements are 30-50% higher than conventional electrode production, though this difference diminishes when considering full operational lifespans.
End-of-life management presents additional concerns. While precious metal recovery from spent SAC-GDE systems is technically feasible, the atomically dispersed nature of catalysts complicates recycling processes. Current recovery rates average 60-75%, compared to 85-95% for conventional catalysts, indicating an area requiring technological improvement.
Water usage impacts vary significantly by application. In water electrolysis applications, SAC-GDE systems demonstrate 20-25% lower water consumption than conventional approaches. However, in CO2 electroreduction applications, water consumption may increase by 10-15% due to specific reaction requirements, necessitating application-specific environmental assessments.
Overall, while SAC-GDE technology offers promising environmental benefits through resource efficiency and reduced operational impacts, optimizing manufacturing processes and developing effective recycling methods remain critical challenges for maximizing its net environmental benefit across full product lifecycles.
Energy efficiency improvements constitute another significant environmental advantage. SAC-GDE systems typically demonstrate 15-30% higher energy efficiency than traditional catalytic electrodes, translating to reduced carbon emissions when powered by conventional energy sources. When coupled with renewable energy, these systems can approach carbon-neutral operation for electrochemical processes like CO2 reduction or hydrogen evolution.
Waste reduction represents a third key environmental benefit. The enhanced selectivity of SAC-GDE systems (often exceeding 90% for target reactions) minimizes byproduct formation, reducing downstream separation requirements and associated energy consumption. Additionally, the extended catalyst lifetime—typically 2-3 times longer than conventional catalysts—reduces replacement frequency and associated manufacturing impacts.
However, several environmental challenges warrant attention. The synthesis of SAC-GDE components often involves specialized chemicals and energy-intensive processes. Life cycle assessments indicate that manufacturing impacts may offset operational benefits unless production methods are optimized. Preliminary studies suggest that manufacturing energy requirements are 30-50% higher than conventional electrode production, though this difference diminishes when considering full operational lifespans.
End-of-life management presents additional concerns. While precious metal recovery from spent SAC-GDE systems is technically feasible, the atomically dispersed nature of catalysts complicates recycling processes. Current recovery rates average 60-75%, compared to 85-95% for conventional catalysts, indicating an area requiring technological improvement.
Water usage impacts vary significantly by application. In water electrolysis applications, SAC-GDE systems demonstrate 20-25% lower water consumption than conventional approaches. However, in CO2 electroreduction applications, water consumption may increase by 10-15% due to specific reaction requirements, necessitating application-specific environmental assessments.
Overall, while SAC-GDE technology offers promising environmental benefits through resource efficiency and reduced operational impacts, optimizing manufacturing processes and developing effective recycling methods remain critical challenges for maximizing its net environmental benefit across full product lifecycles.
Scalability and Manufacturing Considerations
The scalability of SAC (Single-Atom Catalyst) integration with Gas Diffusion Electrodes (GDEs) represents a critical challenge for industrial implementation. Current laboratory-scale synthesis methods often involve complex procedures that are difficult to translate to mass production environments. Batch-to-batch consistency remains problematic, with variations in catalyst loading, distribution, and performance metrics frequently observed when scaling up from milligram to gram quantities.
Manufacturing considerations must address several key aspects to enable commercial viability. The precise control of single-atom dispersion during large-scale production requires sophisticated process monitoring techniques that can verify atomic-level distribution in real-time. Traditional manufacturing equipment may need significant modifications to accommodate the specialized conditions necessary for maintaining single-atom configurations without clustering or agglomeration.
Cost factors present substantial barriers to widespread adoption. The precious metals commonly used as SACs (platinum, palladium, ruthenium) contribute significantly to overall production expenses. Developing cost-effective alternatives using earth-abundant metals while maintaining comparable catalytic activity represents an important research direction. Additionally, the specialized equipment and controlled environments required for SAC synthesis add capital and operational expenditures that must be optimized.
Process integration challenges emerge when incorporating SAC-GDE manufacturing into existing production lines. The sensitivity of single-atom catalysts to environmental contaminants necessitates stringent quality control measures throughout the manufacturing workflow. Cross-contamination risks between different catalyst formulations require dedicated production lines or thorough cleaning protocols that may reduce overall manufacturing efficiency.
Standardization efforts are currently insufficient across the industry. The lack of universally accepted testing protocols and performance benchmarks makes it difficult to compare different manufacturing approaches objectively. Establishing standardized characterization methods specifically designed for SAC-GDEs would accelerate industrial adoption by providing clear quality metrics and performance expectations.
Environmental and safety considerations must also be addressed in manufacturing scale-up. The nanoscale nature of these materials raises questions about worker exposure during production and potential environmental impacts throughout the product lifecycle. Developing green synthesis routes that minimize hazardous reagents and waste streams will be essential for sustainable large-scale implementation of SAC-GDE technology.
Manufacturing considerations must address several key aspects to enable commercial viability. The precise control of single-atom dispersion during large-scale production requires sophisticated process monitoring techniques that can verify atomic-level distribution in real-time. Traditional manufacturing equipment may need significant modifications to accommodate the specialized conditions necessary for maintaining single-atom configurations without clustering or agglomeration.
Cost factors present substantial barriers to widespread adoption. The precious metals commonly used as SACs (platinum, palladium, ruthenium) contribute significantly to overall production expenses. Developing cost-effective alternatives using earth-abundant metals while maintaining comparable catalytic activity represents an important research direction. Additionally, the specialized equipment and controlled environments required for SAC synthesis add capital and operational expenditures that must be optimized.
Process integration challenges emerge when incorporating SAC-GDE manufacturing into existing production lines. The sensitivity of single-atom catalysts to environmental contaminants necessitates stringent quality control measures throughout the manufacturing workflow. Cross-contamination risks between different catalyst formulations require dedicated production lines or thorough cleaning protocols that may reduce overall manufacturing efficiency.
Standardization efforts are currently insufficient across the industry. The lack of universally accepted testing protocols and performance benchmarks makes it difficult to compare different manufacturing approaches objectively. Establishing standardized characterization methods specifically designed for SAC-GDEs would accelerate industrial adoption by providing clear quality metrics and performance expectations.
Environmental and safety considerations must also be addressed in manufacturing scale-up. The nanoscale nature of these materials raises questions about worker exposure during production and potential environmental impacts throughout the product lifecycle. Developing green synthesis routes that minimize hazardous reagents and waste streams will be essential for sustainable large-scale implementation of SAC-GDE technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!