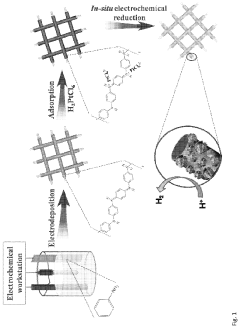
Synthesis Optimization For High Single-Atom Loading
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Single-Atom Catalysis Background and Objectives
Single-atom catalysis (SAC) represents a revolutionary frontier in heterogeneous catalysis that has emerged over the past decade. This innovative approach involves dispersing individual metal atoms on suitable supports, maximizing atomic efficiency while delivering exceptional catalytic performance. The concept was first formally introduced in 2011, though earlier studies had observed similar phenomena without explicitly defining the field.
The evolution of SAC technology has been driven by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated metal atoms. These developments have transformed our understanding of catalytic processes at the atomic level, revealing unique properties that differ significantly from traditional nanoparticle catalysts.
Single-atom catalysts exhibit distinctive advantages including 100% atom utilization, uniform active sites, and often superior selectivity compared to conventional catalysts. These characteristics make them particularly valuable for applications in energy conversion, environmental remediation, and fine chemical synthesis. The field has witnessed exponential growth in research publications, with annual papers increasing from fewer than 10 in 2011 to several hundred in recent years.
Despite these advances, a persistent challenge limiting industrial application is the difficulty in achieving high metal loadings while maintaining atomic dispersion. Conventional synthesis methods typically achieve loadings below 1 wt%, insufficient for many practical applications. This limitation stems from the thermodynamic tendency of metal atoms to aggregate during synthesis or catalytic operation, driven by their high surface energy.
The technical objective of this research is to develop innovative synthesis strategies that enable high single-atom loading (>5 wt%) while maintaining atomic dispersion and stability under reaction conditions. This goal requires fundamental understanding of metal-support interactions, novel precursor design, and advanced synthesis protocols that can overcome thermodynamic limitations.
Recent breakthroughs suggest several promising directions, including the use of defect engineering, confinement strategies, and coordination chemistry approaches. These methods aim to create strong anchoring sites that can stabilize individual metal atoms even at high loadings, preventing aggregation through kinetic or thermodynamic control mechanisms.
Achieving high single-atom loading would dramatically enhance the practical viability of SACs, potentially enabling their implementation in industrial processes where high volumetric activity is required. This advancement would represent a significant step toward more sustainable catalytic technologies with reduced precious metal consumption and improved process efficiency.
The evolution of SAC technology has been driven by advances in characterization techniques, particularly aberration-corrected electron microscopy and X-ray absorption spectroscopy, which have enabled direct visualization and electronic structure analysis of isolated metal atoms. These developments have transformed our understanding of catalytic processes at the atomic level, revealing unique properties that differ significantly from traditional nanoparticle catalysts.
Single-atom catalysts exhibit distinctive advantages including 100% atom utilization, uniform active sites, and often superior selectivity compared to conventional catalysts. These characteristics make them particularly valuable for applications in energy conversion, environmental remediation, and fine chemical synthesis. The field has witnessed exponential growth in research publications, with annual papers increasing from fewer than 10 in 2011 to several hundred in recent years.
Despite these advances, a persistent challenge limiting industrial application is the difficulty in achieving high metal loadings while maintaining atomic dispersion. Conventional synthesis methods typically achieve loadings below 1 wt%, insufficient for many practical applications. This limitation stems from the thermodynamic tendency of metal atoms to aggregate during synthesis or catalytic operation, driven by their high surface energy.
The technical objective of this research is to develop innovative synthesis strategies that enable high single-atom loading (>5 wt%) while maintaining atomic dispersion and stability under reaction conditions. This goal requires fundamental understanding of metal-support interactions, novel precursor design, and advanced synthesis protocols that can overcome thermodynamic limitations.
Recent breakthroughs suggest several promising directions, including the use of defect engineering, confinement strategies, and coordination chemistry approaches. These methods aim to create strong anchoring sites that can stabilize individual metal atoms even at high loadings, preventing aggregation through kinetic or thermodynamic control mechanisms.
Achieving high single-atom loading would dramatically enhance the practical viability of SACs, potentially enabling their implementation in industrial processes where high volumetric activity is required. This advancement would represent a significant step toward more sustainable catalytic technologies with reduced precious metal consumption and improved process efficiency.
Market Analysis for High-Loading SACs
The single-atom catalyst (SAC) market is experiencing significant growth, driven by increasing demand for sustainable and efficient catalytic solutions across various industries. The global market for advanced catalysts, including SACs, was valued at approximately $25.6 billion in 2022 and is projected to reach $35.4 billion by 2028, representing a compound annual growth rate of 5.5%. Within this broader market, high-loading SACs are emerging as a particularly promising segment due to their enhanced catalytic performance and reduced precious metal usage.
The primary market drivers for high-loading SACs include stringent environmental regulations, growing focus on green chemistry, and increasing industrial demand for more efficient catalytic processes. Industries such as petrochemicals, fine chemicals, pharmaceuticals, and automotive are actively seeking advanced catalytic solutions to reduce energy consumption, minimize waste generation, and comply with tightening emissions standards. The automotive sector, in particular, represents a substantial market opportunity as manufacturers work to meet increasingly strict emissions regulations worldwide.
Regional analysis indicates that Asia-Pacific currently dominates the market for advanced catalytic materials, accounting for approximately 40% of global demand. This is primarily due to rapid industrialization in countries like China and India, coupled with significant investments in clean energy technologies. North America and Europe follow closely, with strong demand driven by established chemical and pharmaceutical industries and supportive regulatory frameworks for sustainable technologies.
Market segmentation reveals that environmental applications, particularly in pollution control and clean energy production, represent the fastest-growing segment for high-loading SACs, with an estimated annual growth rate of 7.8%. Industrial catalysis applications in chemical synthesis follow closely at 6.5% growth, while automotive applications are expanding at approximately 5.9% annually.
Customer needs analysis indicates that end-users prioritize catalyst stability, selectivity, and cost-effectiveness. High-loading SACs address these needs by offering enhanced atom utilization efficiency and potentially lower overall costs despite higher initial investment. Market surveys suggest that customers are willing to pay a premium of 15-20% for catalysts that demonstrate superior performance and longevity.
The market for high-loading SACs faces certain challenges, including high production costs, scalability issues, and competition from alternative catalytic technologies. However, these challenges are offset by the significant performance advantages and potential for cost reduction through economies of scale and continued technological advancement. Industry experts project that as synthesis optimization techniques improve, production costs could decrease by 30-40% over the next five years, further accelerating market adoption.
The primary market drivers for high-loading SACs include stringent environmental regulations, growing focus on green chemistry, and increasing industrial demand for more efficient catalytic processes. Industries such as petrochemicals, fine chemicals, pharmaceuticals, and automotive are actively seeking advanced catalytic solutions to reduce energy consumption, minimize waste generation, and comply with tightening emissions standards. The automotive sector, in particular, represents a substantial market opportunity as manufacturers work to meet increasingly strict emissions regulations worldwide.
Regional analysis indicates that Asia-Pacific currently dominates the market for advanced catalytic materials, accounting for approximately 40% of global demand. This is primarily due to rapid industrialization in countries like China and India, coupled with significant investments in clean energy technologies. North America and Europe follow closely, with strong demand driven by established chemical and pharmaceutical industries and supportive regulatory frameworks for sustainable technologies.
Market segmentation reveals that environmental applications, particularly in pollution control and clean energy production, represent the fastest-growing segment for high-loading SACs, with an estimated annual growth rate of 7.8%. Industrial catalysis applications in chemical synthesis follow closely at 6.5% growth, while automotive applications are expanding at approximately 5.9% annually.
Customer needs analysis indicates that end-users prioritize catalyst stability, selectivity, and cost-effectiveness. High-loading SACs address these needs by offering enhanced atom utilization efficiency and potentially lower overall costs despite higher initial investment. Market surveys suggest that customers are willing to pay a premium of 15-20% for catalysts that demonstrate superior performance and longevity.
The market for high-loading SACs faces certain challenges, including high production costs, scalability issues, and competition from alternative catalytic technologies. However, these challenges are offset by the significant performance advantages and potential for cost reduction through economies of scale and continued technological advancement. Industry experts project that as synthesis optimization techniques improve, production costs could decrease by 30-40% over the next five years, further accelerating market adoption.
Current Synthesis Challenges and Limitations
Despite significant advancements in single-atom catalyst (SAC) synthesis, achieving high metal loading remains one of the most formidable challenges in the field. Conventional synthesis methods typically yield metal loadings below 1 wt%, which severely limits industrial applicability and economic viability. This low loading is primarily attributed to the thermodynamic instability of isolated metal atoms, which tend to aggregate into nanoparticles or clusters during synthesis, especially at higher concentrations.
The strong metal-support interaction (SMSI) necessary for stabilizing single atoms becomes increasingly difficult to maintain as metal concentration increases. Current synthetic approaches often rely on defect engineering of support materials to create anchoring sites, but these defects are limited in number and can become saturated, imposing an upper threshold on achievable metal loading.
Wet chemistry methods, including impregnation and co-precipitation, suffer from uneven metal distribution and poor control over the metal species' coordination environment. During thermal treatments, which are essential for activating catalysts, metal migration and aggregation frequently occur, further reducing the single-atom content in the final product.
Another significant limitation is the characterization challenge associated with verifying true single-atom dispersion at higher loadings. Conventional techniques like X-ray absorption spectroscopy (XAS) and aberration-corrected electron microscopy have resolution limitations when metal concentrations increase, making it difficult to definitively confirm the atomic dispersion state.
Scalability presents an additional hurdle, as laboratory-scale synthesis methods that successfully achieve high single-atom loading often fail when scaled up to industrial production levels. The precise control of synthesis parameters becomes exponentially more difficult in larger reaction vessels, leading to inconsistent results and reduced single-atom yield.
The economic constraints further complicate matters, as many current high-loading synthesis approaches require expensive precursors, complex equipment, or energy-intensive processes. This creates a significant barrier to commercial adoption, even when technical challenges are overcome in laboratory settings.
Environmental considerations also pose limitations, as some promising synthesis routes involve toxic reagents or generate hazardous waste streams. Developing green synthesis protocols that maintain high single-atom loading while minimizing environmental impact represents an ongoing challenge that has yet to be adequately addressed by the research community.
The strong metal-support interaction (SMSI) necessary for stabilizing single atoms becomes increasingly difficult to maintain as metal concentration increases. Current synthetic approaches often rely on defect engineering of support materials to create anchoring sites, but these defects are limited in number and can become saturated, imposing an upper threshold on achievable metal loading.
Wet chemistry methods, including impregnation and co-precipitation, suffer from uneven metal distribution and poor control over the metal species' coordination environment. During thermal treatments, which are essential for activating catalysts, metal migration and aggregation frequently occur, further reducing the single-atom content in the final product.
Another significant limitation is the characterization challenge associated with verifying true single-atom dispersion at higher loadings. Conventional techniques like X-ray absorption spectroscopy (XAS) and aberration-corrected electron microscopy have resolution limitations when metal concentrations increase, making it difficult to definitively confirm the atomic dispersion state.
Scalability presents an additional hurdle, as laboratory-scale synthesis methods that successfully achieve high single-atom loading often fail when scaled up to industrial production levels. The precise control of synthesis parameters becomes exponentially more difficult in larger reaction vessels, leading to inconsistent results and reduced single-atom yield.
The economic constraints further complicate matters, as many current high-loading synthesis approaches require expensive precursors, complex equipment, or energy-intensive processes. This creates a significant barrier to commercial adoption, even when technical challenges are overcome in laboratory settings.
Environmental considerations also pose limitations, as some promising synthesis routes involve toxic reagents or generate hazardous waste streams. Developing green synthesis protocols that maintain high single-atom loading while minimizing environmental impact represents an ongoing challenge that has yet to be adequately addressed by the research community.
Current Synthesis Strategies for High-Loading SACs
01 Preparation methods for single-atom catalysts
Various methods can be employed to prepare single-atom catalysts with controlled atom loading. These include wet chemical synthesis, atomic layer deposition, and electrochemical deposition techniques. The preparation method significantly influences the dispersion, stability, and loading density of the atomic catalysts on the support material, which in turn affects catalytic performance. Optimized synthesis routes enable precise control over the atomic loading while preventing aggregation into nanoparticles.- Preparation methods for single-atom catalysts: Various methods can be employed to prepare single-atom catalysts with controlled atom loading. These include wet chemical approaches, atomic layer deposition, and thermal treatments that enable precise distribution of metal atoms on support materials. These preparation techniques are critical for achieving optimal dispersion of catalytic atoms and preventing aggregation, which directly impacts catalytic performance and efficiency.
- Support materials for single-atom catalysts: The choice of support material significantly influences the loading capacity and stability of single-atom catalysts. Materials such as carbon-based supports, metal oxides, and 2D materials provide different anchoring sites for metal atoms. The interaction between the support and the metal atoms determines the maximum achievable atom loading while maintaining the single-atom state, preventing clustering into nanoparticles.
- Loading optimization techniques: Optimization techniques for atom loading include controlled precursor introduction, surface functionalization of supports, and post-synthesis treatments. These approaches aim to maximize the metal atom loading while maintaining the single-atom dispersion state. Advanced characterization methods are employed to verify the atomic dispersion and quantify the actual loading achieved in the catalyst system.
- Applications of high-loading single-atom catalysts: High-loading single-atom catalysts find applications in various fields including energy conversion, environmental remediation, and chemical synthesis. The increased density of active sites in high-loading catalysts enhances catalytic activity while maintaining the advantages of single-atom catalysis such as high atom efficiency and unique selectivity patterns. These catalysts show particular promise in electrochemical reactions and hydrogenation processes.
- Stability enhancement of high-loading single-atom catalysts: Maintaining stability at high atom loadings presents significant challenges as metal atoms tend to aggregate under reaction conditions. Various strategies have been developed to enhance stability, including strong metal-support interactions, confinement approaches, and the use of coordination environments that anchor the metal atoms. These methods enable the development of single-atom catalysts with both high loading and excellent operational stability.
02 Support materials for single-atom catalysts
The choice of support material is crucial for achieving optimal atom loading in single-atom catalysts. Various supports including carbon-based materials (graphene, carbon nanotubes), metal oxides, and MOFs (Metal-Organic Frameworks) provide different anchoring sites for single atoms. The support's surface chemistry, porosity, and defect structure determine the maximum achievable atom loading and prevent atom migration and aggregation during catalytic reactions. Engineered supports with tailored binding sites can significantly increase the loading capacity while maintaining the single-atom state.Expand Specific Solutions03 Metal-atom loading optimization techniques
Techniques for optimizing metal-atom loading in single-atom catalysts include controlled precursor introduction, stepwise loading processes, and post-synthesis treatments. The metal loading can be precisely tuned by adjusting precursor concentration, deposition time, and reaction conditions. Advanced characterization methods such as XAFS, STEM, and XPS are employed to confirm the single-atom nature and quantify the loading. Optimization strategies focus on maximizing the number of catalytically active single atoms while preventing their aggregation into clusters or nanoparticles.Expand Specific Solutions04 Stabilization strategies for high atom loading
Achieving high atom loading in single-atom catalysts requires effective stabilization strategies to prevent aggregation. These include the use of strong metal-support interactions, coordination with specific functional groups, confinement in porous structures, and electronic stabilization through charge transfer. Advanced stabilization approaches involve the creation of isolated atomic sites with specific coordination environments that can accommodate higher metal loadings while maintaining the single-atom state. These strategies enable the development of single-atom catalysts with enhanced stability under reaction conditions.Expand Specific Solutions05 Performance correlation with atom loading
The catalytic performance of single-atom catalysts shows strong correlation with atom loading levels. While increasing atom loading generally enhances activity due to more available active sites, there exists an optimal loading threshold beyond which performance may decline due to atom aggregation or altered electronic properties. The relationship between atom loading and selectivity, stability, and activity is complex and depends on the specific reaction and catalyst system. Understanding these correlations enables the rational design of single-atom catalysts with optimized atom loading for targeted applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
The field of "Synthesis Optimization For High Single-Atom Loading" is currently in an early growth phase, characterized by intensive research and emerging commercial applications. The global market for single-atom catalysts is expanding rapidly, projected to reach significant value due to applications in clean energy, environmental remediation, and chemical manufacturing. Technologically, academic institutions like Chinese Academy of Science Institute of Chemistry, University of Science & Technology of China, and Central South University are leading fundamental research, while companies including Pacific Biosciences, SK Innovation, and Industrial Technology Research Institute are advancing practical applications. The technology remains in development with challenges in scalability and stability, though recent breakthroughs in synthesis methods are accelerating progress toward commercial viability.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed advanced synthesis optimization techniques for high single-atom loading that focus on precise control of atomic dispersion. Their approach utilizes controlled pyrolysis methods with temperature-programmed reduction to achieve uniform distribution of metal atoms on various supports. They've pioneered the use of coordination chemistry principles to create stable metal-nitrogen-carbon (M-N-C) structures with single-atom catalytic sites. Their research demonstrates achievement of single-atom loadings up to 10 wt% while maintaining atomic dispersion, significantly higher than conventional methods that typically achieve only 1-2 wt%. The institute has also developed in-situ characterization techniques using X-ray absorption spectroscopy and aberration-corrected electron microscopy to monitor and verify single-atom formation during synthesis processes.
Strengths: Achieves exceptionally high metal loadings (up to 10 wt%) while maintaining atomic dispersion; advanced characterization capabilities for atomic-level verification. Weaknesses: Complex synthesis procedures may limit industrial scalability; some techniques require specialized equipment and highly controlled environments that increase production costs.
University of Science & Technology of China
Technical Solution: The University of Science & Technology of China has developed innovative approaches to single-atom catalyst synthesis optimization focusing on spatial confinement strategies. Their technology employs metal-organic frameworks (MOFs) as precursors and templates to achieve high single-atom loading while preventing aggregation. The research team has pioneered a "double-confinement" strategy that utilizes both physical barriers and chemical coordination to stabilize isolated metal atoms during high-temperature treatments. This approach has demonstrated successful synthesis of single-atom catalysts with loadings reaching 5-7 wt% for various transition metals including Fe, Co, and Ni. Their methodology incorporates precise control of defect engineering in carbon-based supports to create abundant anchoring sites for metal atoms. The university has also developed novel characterization protocols combining XAFS, HAADF-STEM, and in-situ spectroscopic techniques to verify atomic dispersion and coordination environments.
Strengths: Double-confinement strategy effectively prevents metal aggregation during high-temperature treatments; versatile approach applicable to multiple transition metals. Weaknesses: MOF-based precursors can be expensive for large-scale production; some synthesis routes require complex multi-step procedures that may limit commercial viability.
Key Patents and Breakthroughs in SAC Synthesis
Single-atom catalysts and method of manufacture thereof
PatentPendingUS20230366111A1
Innovation
- The synthesis of single-atom catalysts (SACs) comprising nanofibers with uniformly dispersed single-atom metal sites, particularly Pt, Ru, and Pd, anchored on conductive polymers like polyaniline (PANI), which are produced through an electrochemical method that avoids the formation of metal clusters and nanoparticles, thereby maximizing active site exposure and catalytic efficiency.
Characterization Techniques for Single-Atom Catalysts
Characterization of single-atom catalysts (SACs) represents a critical component in the optimization of synthesis methods for high single-atom loading. Advanced microscopy techniques, particularly aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), have emerged as the gold standard for direct visualization of individual metal atoms dispersed on support materials. This technique provides atomic-resolution imaging with Z-contrast capability, allowing researchers to distinguish between single atoms and small clusters based on brightness differences.
X-ray absorption spectroscopy (XAS), including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offers complementary information about the electronic structure and coordination environment of single atoms. EXAFS analysis is particularly valuable for determining metal-support interactions, coordination numbers, and bond distances, which directly correlate with synthesis optimization parameters.
X-ray photoelectron spectroscopy (XPS) provides essential information about the oxidation states of single atoms and their electronic interactions with support materials. This technique has proven invaluable for understanding how different synthesis approaches affect the chemical state of metal atoms, which ultimately influences catalytic performance and stability.
Advanced synchrotron-based techniques such as resonant inelastic X-ray scattering (RIXS) and X-ray emission spectroscopy (XES) are increasingly being employed to probe the electronic structure of single atoms with unprecedented detail. These techniques can reveal subtle electronic changes during catalyst activation and reaction processes, providing insights for synthesis refinement.
Computational methods, particularly density functional theory (DFT), have become indispensable for interpreting experimental characterization data and predicting optimal synthesis conditions. DFT calculations can model metal-support interactions, adsorption energies, and reaction pathways, guiding experimental efforts toward higher single-atom loading.
In situ and operando characterization techniques represent the frontier of SAC research, allowing real-time observation of structural changes during synthesis, activation, and catalytic reactions. These approaches include environmental TEM, in situ XAS, and ambient-pressure XPS, which provide dynamic information about single-atom evolution under realistic conditions.
Correlative multi-technique approaches that combine microscopic and spectroscopic methods are increasingly recognized as essential for comprehensive characterization of SACs. By integrating data from multiple techniques, researchers can develop more accurate structure-property relationships that inform synthesis optimization strategies for achieving higher single-atom loading while maintaining stability and performance.
X-ray absorption spectroscopy (XAS), including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offers complementary information about the electronic structure and coordination environment of single atoms. EXAFS analysis is particularly valuable for determining metal-support interactions, coordination numbers, and bond distances, which directly correlate with synthesis optimization parameters.
X-ray photoelectron spectroscopy (XPS) provides essential information about the oxidation states of single atoms and their electronic interactions with support materials. This technique has proven invaluable for understanding how different synthesis approaches affect the chemical state of metal atoms, which ultimately influences catalytic performance and stability.
Advanced synchrotron-based techniques such as resonant inelastic X-ray scattering (RIXS) and X-ray emission spectroscopy (XES) are increasingly being employed to probe the electronic structure of single atoms with unprecedented detail. These techniques can reveal subtle electronic changes during catalyst activation and reaction processes, providing insights for synthesis refinement.
Computational methods, particularly density functional theory (DFT), have become indispensable for interpreting experimental characterization data and predicting optimal synthesis conditions. DFT calculations can model metal-support interactions, adsorption energies, and reaction pathways, guiding experimental efforts toward higher single-atom loading.
In situ and operando characterization techniques represent the frontier of SAC research, allowing real-time observation of structural changes during synthesis, activation, and catalytic reactions. These approaches include environmental TEM, in situ XAS, and ambient-pressure XPS, which provide dynamic information about single-atom evolution under realistic conditions.
Correlative multi-technique approaches that combine microscopic and spectroscopic methods are increasingly recognized as essential for comprehensive characterization of SACs. By integrating data from multiple techniques, researchers can develop more accurate structure-property relationships that inform synthesis optimization strategies for achieving higher single-atom loading while maintaining stability and performance.
Scalability and Industrial Implementation Considerations
Scaling up single-atom catalyst (SAC) synthesis from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Current laboratory-scale synthesis methods typically produce gram-level quantities, whereas industrial applications require kilogram to ton-scale production capabilities. The transition demands careful consideration of process parameters to maintain the high dispersion and stability of single atoms while increasing production volume.
Equipment modification represents a primary consideration for industrial implementation. Conventional laboratory equipment such as small batch reactors must be redesigned for continuous flow processes that enable higher throughput without compromising the precise control needed for single-atom loading. Specialized reactor designs incorporating uniform heating, controlled atmosphere systems, and precise dosing mechanisms are essential for maintaining synthesis quality at scale.
Raw material sourcing becomes increasingly critical at industrial scales. The purity and consistency of precursors directly impact the final catalyst performance. Establishing reliable supply chains for high-purity metal precursors and support materials is necessary, with particular attention to batch-to-batch consistency. Cost considerations may necessitate the development of recycling processes for precious metals used in SAC production.
Quality control protocols must evolve significantly for industrial implementation. In-line monitoring techniques such as real-time spectroscopic analysis can provide immediate feedback on single-atom loading and dispersion during production. Statistical process control methods should be implemented to identify and correct deviations before they affect product quality, with automated sampling systems ensuring representative quality assessment.
Environmental and safety considerations gain prominence at industrial scale. The handling of metal precursors, particularly those containing toxic elements, requires robust containment systems and waste treatment protocols. Energy efficiency becomes economically significant, necessitating heat recovery systems and process optimization to reduce the carbon footprint of production.
Economic viability ultimately determines industrial implementation success. While SACs offer superior atom efficiency compared to conventional catalysts, their production costs must be justified by performance benefits. Techno-economic analysis should consider not only production costs but also catalyst lifetime, activity maintenance, and potential for regeneration. Strategic partnerships between academic institutions and industry can accelerate the development of commercially viable production methods through shared expertise and resources.
Equipment modification represents a primary consideration for industrial implementation. Conventional laboratory equipment such as small batch reactors must be redesigned for continuous flow processes that enable higher throughput without compromising the precise control needed for single-atom loading. Specialized reactor designs incorporating uniform heating, controlled atmosphere systems, and precise dosing mechanisms are essential for maintaining synthesis quality at scale.
Raw material sourcing becomes increasingly critical at industrial scales. The purity and consistency of precursors directly impact the final catalyst performance. Establishing reliable supply chains for high-purity metal precursors and support materials is necessary, with particular attention to batch-to-batch consistency. Cost considerations may necessitate the development of recycling processes for precious metals used in SAC production.
Quality control protocols must evolve significantly for industrial implementation. In-line monitoring techniques such as real-time spectroscopic analysis can provide immediate feedback on single-atom loading and dispersion during production. Statistical process control methods should be implemented to identify and correct deviations before they affect product quality, with automated sampling systems ensuring representative quality assessment.
Environmental and safety considerations gain prominence at industrial scale. The handling of metal precursors, particularly those containing toxic elements, requires robust containment systems and waste treatment protocols. Energy efficiency becomes economically significant, necessitating heat recovery systems and process optimization to reduce the carbon footprint of production.
Economic viability ultimately determines industrial implementation success. While SACs offer superior atom efficiency compared to conventional catalysts, their production costs must be justified by performance benefits. Techno-economic analysis should consider not only production costs but also catalyst lifetime, activity maintenance, and potential for regeneration. Strategic partnerships between academic institutions and industry can accelerate the development of commercially viable production methods through shared expertise and resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!