Terahertz-Based Breath Analysis For Non-Invasive Diagnostics
AUG 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Terahertz Breath Analysis Background and Objectives
Terahertz (THz) technology represents a significant frontier in electromagnetic spectrum applications, occupying the frequency range between microwave and infrared radiation (0.1-10 THz). This spectral region has remained relatively unexplored until recent decades due to technological limitations in generating and detecting THz radiation. The evolution of THz technology has accelerated since the 1990s with the development of time-domain spectroscopy systems, quantum cascade lasers, and improved detector technologies.
The application of THz technology to breath analysis emerges from the unique interaction between THz radiation and biological molecules. Human breath contains numerous volatile organic compounds (VOCs) and biomarkers that can indicate various physiological conditions and pathological states. Traditional breath analysis methods often require complex sample preparation, expensive equipment, or invasive procedures, limiting their widespread clinical adoption.
THz spectroscopy offers distinct advantages for breath analysis due to its non-ionizing nature, high sensitivity to molecular rotational and vibrational modes, and ability to detect subtle changes in molecular composition. The technology can potentially identify disease-specific biomarkers in exhaled breath without requiring blood samples or tissue biopsies, representing a paradigm shift in diagnostic approaches.
The historical trajectory of breath analysis dates back to ancient Greek physicians who recognized distinct breath odors associated with certain diseases. Modern scientific breath analysis began in the 1970s with gas chromatography-mass spectrometry techniques. THz technology represents the next evolutionary step in this field, offering potentially higher sensitivity and specificity for biomarker detection.
The primary objectives of THz-based breath analysis research include developing reliable, portable, and cost-effective diagnostic systems capable of real-time analysis of breath samples. These systems aim to detect specific biomarkers associated with conditions such as diabetes, various cancers, respiratory diseases, and metabolic disorders. The technology seeks to achieve high sensitivity (detecting biomarkers at parts-per-billion concentrations) while maintaining specificity to avoid false positives.
Additional objectives include miniaturization of THz systems for point-of-care applications, development of standardized breath collection protocols, creation of comprehensive biomarker databases, and establishment of clinical validation methodologies. The ultimate goal is to transition THz breath analysis from laboratory research to practical clinical applications that can improve early disease detection, enable non-invasive monitoring of treatment efficacy, and potentially revolutionize preventive healthcare through accessible screening methods.
The convergence of advances in THz technology, biomarker research, and data analytics creates a promising foundation for achieving these objectives, though significant technical and clinical validation challenges remain to be addressed.
The application of THz technology to breath analysis emerges from the unique interaction between THz radiation and biological molecules. Human breath contains numerous volatile organic compounds (VOCs) and biomarkers that can indicate various physiological conditions and pathological states. Traditional breath analysis methods often require complex sample preparation, expensive equipment, or invasive procedures, limiting their widespread clinical adoption.
THz spectroscopy offers distinct advantages for breath analysis due to its non-ionizing nature, high sensitivity to molecular rotational and vibrational modes, and ability to detect subtle changes in molecular composition. The technology can potentially identify disease-specific biomarkers in exhaled breath without requiring blood samples or tissue biopsies, representing a paradigm shift in diagnostic approaches.
The historical trajectory of breath analysis dates back to ancient Greek physicians who recognized distinct breath odors associated with certain diseases. Modern scientific breath analysis began in the 1970s with gas chromatography-mass spectrometry techniques. THz technology represents the next evolutionary step in this field, offering potentially higher sensitivity and specificity for biomarker detection.
The primary objectives of THz-based breath analysis research include developing reliable, portable, and cost-effective diagnostic systems capable of real-time analysis of breath samples. These systems aim to detect specific biomarkers associated with conditions such as diabetes, various cancers, respiratory diseases, and metabolic disorders. The technology seeks to achieve high sensitivity (detecting biomarkers at parts-per-billion concentrations) while maintaining specificity to avoid false positives.
Additional objectives include miniaturization of THz systems for point-of-care applications, development of standardized breath collection protocols, creation of comprehensive biomarker databases, and establishment of clinical validation methodologies. The ultimate goal is to transition THz breath analysis from laboratory research to practical clinical applications that can improve early disease detection, enable non-invasive monitoring of treatment efficacy, and potentially revolutionize preventive healthcare through accessible screening methods.
The convergence of advances in THz technology, biomarker research, and data analytics creates a promising foundation for achieving these objectives, though significant technical and clinical validation challenges remain to be addressed.
Market Demand for Non-Invasive Diagnostic Solutions
The global market for non-invasive diagnostic solutions has been experiencing substantial growth, driven by increasing healthcare costs, patient preference for less intrusive procedures, and the need for rapid diagnostic capabilities. The non-invasive diagnostics market was valued at approximately $12 billion in 2022 and is projected to reach $20 billion by 2028, representing a compound annual growth rate of 8.9%.
Terahertz-based breath analysis represents a particularly promising segment within this market. Traditional diagnostic methods often involve invasive procedures such as blood draws, tissue biopsies, or endoscopies, which can cause patient discomfort, carry infection risks, and require specialized medical facilities. The demand for alternatives has created a significant market opportunity for breath analysis technologies.
Healthcare providers are increasingly seeking diagnostic tools that can provide real-time results while minimizing patient discomfort. Terahertz spectroscopy offers unique advantages in this context, as it can detect molecular biomarkers in exhaled breath with high sensitivity and specificity. This capability addresses the growing demand for point-of-care testing solutions that enable faster clinical decision-making and reduce hospital readmissions.
The aging global population is another key driver for non-invasive diagnostics. With more than 727 million people aged 65 or older worldwide as of 2020, and this number expected to double by 2050, there is increasing pressure on healthcare systems to adopt cost-effective diagnostic solutions suitable for chronic disease management. Terahertz breath analysis could significantly reduce the burden of regular testing for conditions such as diabetes, COPD, and various cancers.
Consumer preferences are also shifting toward greater involvement in personal health monitoring. The wearable health technology market exceeded $18 billion in 2021, demonstrating strong consumer interest in non-invasive health tracking. This trend creates favorable conditions for the adoption of breath analysis technologies that could eventually be integrated into portable or home-use devices.
Regulatory bodies worldwide are increasingly recognizing the value of non-invasive diagnostics. The FDA has established specific pathways for breath-based diagnostic devices, while the European Medicines Agency has included breath analysis in its innovation task force priorities. These regulatory developments are expected to accelerate market access for terahertz-based breath analysis technologies.
Industry analysts predict that respiratory disease diagnosis will represent the largest application segment for breath analysis technologies, followed by diabetes monitoring and cancer screening. The COVID-19 pandemic has further heightened interest in respiratory diagnostics, creating additional market pull for technologies that can rapidly screen for respiratory infections without invasive procedures.
Terahertz-based breath analysis represents a particularly promising segment within this market. Traditional diagnostic methods often involve invasive procedures such as blood draws, tissue biopsies, or endoscopies, which can cause patient discomfort, carry infection risks, and require specialized medical facilities. The demand for alternatives has created a significant market opportunity for breath analysis technologies.
Healthcare providers are increasingly seeking diagnostic tools that can provide real-time results while minimizing patient discomfort. Terahertz spectroscopy offers unique advantages in this context, as it can detect molecular biomarkers in exhaled breath with high sensitivity and specificity. This capability addresses the growing demand for point-of-care testing solutions that enable faster clinical decision-making and reduce hospital readmissions.
The aging global population is another key driver for non-invasive diagnostics. With more than 727 million people aged 65 or older worldwide as of 2020, and this number expected to double by 2050, there is increasing pressure on healthcare systems to adopt cost-effective diagnostic solutions suitable for chronic disease management. Terahertz breath analysis could significantly reduce the burden of regular testing for conditions such as diabetes, COPD, and various cancers.
Consumer preferences are also shifting toward greater involvement in personal health monitoring. The wearable health technology market exceeded $18 billion in 2021, demonstrating strong consumer interest in non-invasive health tracking. This trend creates favorable conditions for the adoption of breath analysis technologies that could eventually be integrated into portable or home-use devices.
Regulatory bodies worldwide are increasingly recognizing the value of non-invasive diagnostics. The FDA has established specific pathways for breath-based diagnostic devices, while the European Medicines Agency has included breath analysis in its innovation task force priorities. These regulatory developments are expected to accelerate market access for terahertz-based breath analysis technologies.
Industry analysts predict that respiratory disease diagnosis will represent the largest application segment for breath analysis technologies, followed by diabetes monitoring and cancer screening. The COVID-19 pandemic has further heightened interest in respiratory diagnostics, creating additional market pull for technologies that can rapidly screen for respiratory infections without invasive procedures.
Current State and Challenges in Terahertz Sensing Technology
Terahertz (THz) sensing technology has experienced significant advancements in recent years, particularly in the domain of non-invasive breath analysis diagnostics. Currently, the global research landscape shows varying levels of development across regions, with North America, Europe, and East Asia leading in both fundamental research and commercial applications. The United States maintains a strong position in system integration and clinical validation, while European institutions excel in spectroscopic analysis techniques. East Asian countries, particularly China and Japan, have made remarkable progress in miniaturization and cost reduction of THz systems.
The current state of THz breath analysis technology demonstrates promising capabilities in detecting volatile organic compounds (VOCs) and biomarkers associated with various diseases. Laboratory-scale systems have successfully identified markers for conditions including diabetes, certain cancers, and respiratory infections with sensitivity levels approaching parts-per-billion. Recent advancements in quantum cascade lasers and photoconductive antennas have significantly improved signal-to-noise ratios, enabling more reliable detection of trace compounds in exhaled breath.
Despite these achievements, several critical challenges impede widespread adoption of THz-based breath analysis. The foremost technical limitation remains the relatively low sensitivity compared to established techniques such as gas chromatography-mass spectrometry (GC-MS). THz systems struggle to detect compounds at concentrations below parts-per-billion, which is insufficient for certain early-stage disease biomarkers that exist at parts-per-trillion levels.
Environmental interference presents another significant obstacle. Water vapor absorption in the THz range creates substantial background noise, complicating the detection of target compounds in humid breath samples. Current solutions involving desiccants or reference chambers add complexity and reduce the technology's practicality for point-of-care applications.
Standardization issues further hinder progress, as variations in sampling protocols, reference databases, and analysis algorithms make cross-study comparisons difficult. The lack of comprehensive biomarker libraries specifically for THz spectral signatures limits diagnostic capabilities and clinical validation efforts.
From an engineering perspective, existing THz systems remain bulky, expensive, and power-intensive. While laboratory demonstrations show promising results, transitioning to portable, cost-effective devices suitable for clinical settings requires significant engineering breakthroughs in component miniaturization and power efficiency.
Regulatory hurdles also present formidable challenges. The novelty of THz sensing technology means that clear regulatory pathways for medical device approval are still evolving, creating uncertainty for commercial development and clinical implementation. This regulatory landscape varies considerably across different regions, further complicating global deployment strategies.
The current state of THz breath analysis technology demonstrates promising capabilities in detecting volatile organic compounds (VOCs) and biomarkers associated with various diseases. Laboratory-scale systems have successfully identified markers for conditions including diabetes, certain cancers, and respiratory infections with sensitivity levels approaching parts-per-billion. Recent advancements in quantum cascade lasers and photoconductive antennas have significantly improved signal-to-noise ratios, enabling more reliable detection of trace compounds in exhaled breath.
Despite these achievements, several critical challenges impede widespread adoption of THz-based breath analysis. The foremost technical limitation remains the relatively low sensitivity compared to established techniques such as gas chromatography-mass spectrometry (GC-MS). THz systems struggle to detect compounds at concentrations below parts-per-billion, which is insufficient for certain early-stage disease biomarkers that exist at parts-per-trillion levels.
Environmental interference presents another significant obstacle. Water vapor absorption in the THz range creates substantial background noise, complicating the detection of target compounds in humid breath samples. Current solutions involving desiccants or reference chambers add complexity and reduce the technology's practicality for point-of-care applications.
Standardization issues further hinder progress, as variations in sampling protocols, reference databases, and analysis algorithms make cross-study comparisons difficult. The lack of comprehensive biomarker libraries specifically for THz spectral signatures limits diagnostic capabilities and clinical validation efforts.
From an engineering perspective, existing THz systems remain bulky, expensive, and power-intensive. While laboratory demonstrations show promising results, transitioning to portable, cost-effective devices suitable for clinical settings requires significant engineering breakthroughs in component miniaturization and power efficiency.
Regulatory hurdles also present formidable challenges. The novelty of THz sensing technology means that clear regulatory pathways for medical device approval are still evolving, creating uncertainty for commercial development and clinical implementation. This regulatory landscape varies considerably across different regions, further complicating global deployment strategies.
Existing Terahertz Breath Analysis Technical Solutions
01 Terahertz spectroscopy for breath biomarker detection
Terahertz spectroscopy techniques are used to detect and analyze biomarkers in exhaled breath for non-invasive disease diagnosis. These systems can identify specific molecular signatures in breath samples by measuring the absorption, reflection, or transmission of terahertz radiation. The technology enables detection of various disease indicators without requiring blood samples or invasive procedures, making it suitable for early disease screening and monitoring.- Terahertz spectroscopy for breath biomarker detection: Terahertz spectroscopy technology can be used to detect and analyze biomarkers in exhaled breath for non-invasive disease diagnosis. This approach utilizes the unique spectral fingerprints of molecules in the terahertz frequency range to identify specific biomarkers associated with various health conditions. The technology enables real-time, sensitive detection of volatile organic compounds and other breath components that may indicate disease states without requiring invasive procedures.
- Portable terahertz breath analysis devices: Compact and portable terahertz-based devices have been developed for point-of-care breath analysis. These devices integrate miniaturized terahertz sources, detectors, and signal processing components to enable breath analysis outside of laboratory settings. The portable nature of these systems allows for widespread deployment in clinical environments, emergency settings, or even home use, making non-invasive diagnostics more accessible while maintaining high sensitivity and specificity.
- AI and machine learning integration with terahertz breath analysis: Advanced artificial intelligence and machine learning algorithms are being integrated with terahertz breath analysis systems to improve diagnostic accuracy. These computational approaches can identify complex patterns in terahertz spectral data that may not be apparent through conventional analysis methods. By training on large datasets of breath samples from both healthy individuals and those with specific conditions, these systems can detect subtle biomarker signatures indicative of various diseases, enhancing the sensitivity and specificity of non-invasive breath diagnostics.
- Disease-specific breath biomarker identification using terahertz technology: Terahertz spectroscopy enables the identification of disease-specific biomarkers in exhaled breath for conditions such as diabetes, cancer, respiratory diseases, and infections. By analyzing the unique spectral patterns associated with particular pathological states, terahertz-based systems can detect early signs of disease before clinical symptoms appear. This approach allows for targeted screening and monitoring of specific conditions through non-invasive breath sampling, potentially enabling earlier intervention and improved patient outcomes.
- Multi-modal sensing approaches combining terahertz with other technologies: Hybrid diagnostic systems that combine terahertz spectroscopy with complementary sensing technologies provide enhanced breath analysis capabilities. These multi-modal approaches may integrate terahertz sensing with infrared spectroscopy, mass spectrometry, or electrochemical sensors to capture a more comprehensive profile of breath biomarkers. By leveraging the strengths of different analytical methods, these systems can overcome the limitations of individual technologies, resulting in more robust and accurate non-invasive diagnostic tools for complex health conditions.
02 Portable breath analysis devices using terahertz technology
Compact and portable terahertz-based breath analysis systems have been developed for point-of-care diagnostics. These devices integrate miniaturized terahertz sources, detectors, and breath collection mechanisms into handheld or portable units. The portability enables real-time breath analysis in various settings including clinics, homes, and remote locations, facilitating widespread access to non-invasive diagnostic capabilities.Expand Specific Solutions03 AI and machine learning integration with terahertz breath analysis
Advanced artificial intelligence and machine learning algorithms are integrated with terahertz breath analysis systems to improve diagnostic accuracy. These computational methods analyze complex spectral data from breath samples to identify patterns associated with specific diseases. The AI systems can detect subtle changes in breath composition that might indicate early-stage diseases, enabling more precise and earlier diagnosis than conventional methods.Expand Specific Solutions04 Disease-specific breath biomarker identification using terahertz technology
Terahertz-based systems are developed to target specific diseases by identifying unique breath biomarkers associated with particular conditions. These systems can detect biomarkers for respiratory diseases, metabolic disorders, cancer, and infectious diseases. The technology enables differentiation between similar conditions based on subtle variations in breath composition, allowing for more accurate differential diagnosis through non-invasive means.Expand Specific Solutions05 Real-time monitoring and continuous breath analysis systems
Continuous monitoring systems using terahertz technology enable real-time analysis of breath for ongoing health assessment. These systems can track changes in breath composition over time, allowing for the monitoring of disease progression, treatment effectiveness, and metabolic changes. The continuous nature of these systems provides dynamic health information rather than static snapshots, improving the management of chronic conditions and therapeutic interventions.Expand Specific Solutions
Key Industry Players in Terahertz Medical Applications
Terahertz-based breath analysis for non-invasive diagnostics is emerging as a promising field in the early growth stage, with a projected market size reaching $2.5 billion by 2028. The technology is advancing from experimental to early commercial applications, with varying degrees of maturity across players. Leading companies like Samsung Electronics and Koninklijke Philips are investing heavily in clinical validation, while specialized firms such as Zeteo Tech and ENDO Medical focus on specific diagnostic applications. Academic institutions including University of Florida and China University of Mining & Technology are driving fundamental research. Canon and Senseair are developing sensor technologies, while healthcare-focused entities like NMI and Cardiac Pacemakers are exploring integration into medical devices, creating a diverse competitive landscape spanning technology development to clinical implementation.
University of Florida
Technical Solution: The University of Florida has developed an innovative terahertz breath analysis platform called "THz-Breath" that utilizes a unique combination of terahertz time-domain spectroscopy and advanced computational methods for non-invasive disease detection. Their system employs a femtosecond laser-based terahertz source that generates broadband terahertz radiation (0.1-5 THz), providing comprehensive spectral coverage for detecting a wide range of biomarkers. The university's approach incorporates a patented breath collection system that uses selective filtering to concentrate biomarkers of interest while reducing interference from common breath components. Their technology leverages advanced machine learning algorithms, specifically deep neural networks trained on extensive clinical datasets, to identify subtle spectral patterns associated with various disease states. The system has demonstrated particular success in detecting early-stage lung cancer biomarkers with sensitivity comparable to more invasive diagnostic procedures. The university has also pioneered methods to account for individual metabolic variations, improving diagnostic accuracy across diverse patient populations.
Strengths: Exceptional spectral range allowing detection of diverse biomarkers; sophisticated AI algorithms enhancing diagnostic accuracy; strong research foundation with extensive clinical validation studies. Weaknesses: Currently more research-oriented than commercially ready; requires specialized technical expertise to operate effectively; higher initial equipment costs compared to conventional screening methods.
Zeteo Tech, Inc.
Technical Solution: Zeteo Tech has developed a proprietary terahertz-based breath analysis platform called "BreathScan" that utilizes terahertz spectroscopy to detect and identify biomarkers in exhaled breath for non-invasive disease diagnostics. Their technology employs a compact terahertz source and detector system that can identify molecular signatures of various compounds in breath samples with high specificity. The system incorporates advanced signal processing algorithms to filter out environmental contaminants and focus on disease-specific biomarkers. Zeteo's approach involves capturing breath samples in specialized containers that maintain sample integrity while allowing terahertz radiation to penetrate and interact with the breath compounds. Their technology can detect concentrations of biomarkers in the parts-per-billion range, making it suitable for early disease detection before symptoms become apparent.
Strengths: Highly sensitive detection capabilities allowing for early disease identification; portable system design enabling point-of-care applications; non-invasive methodology improving patient compliance. Weaknesses: Requires careful calibration to account for environmental variables; relatively new technology with limited clinical validation compared to established diagnostic methods; higher initial equipment costs compared to some traditional screening methods.
Core Terahertz Spectroscopy Innovations for Biomarker Detection
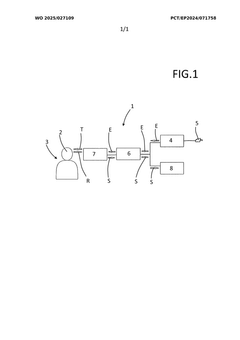
Arrangement for characterising cerebrospinal fluid
PatentWO2025027109A1
Innovation
- A non-invasive arrangement using Terahertz radiation to examine cerebrospinal fluid by emitting and receiving electromagnetic radiation through the skull, with a sensor head and evaluation circuit to determine substance concentrations without penetrating the skin, allowing for low-risk and precise analysis.
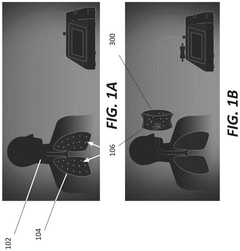
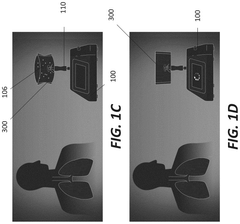
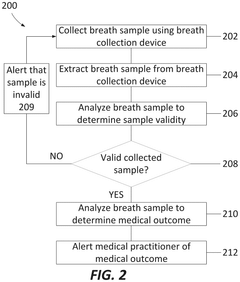
Breath analysis system
PatentPendingUS20240324894A1
Innovation
- A breath collection system featuring a flexible bag with a valve device that opens when the patient bites on a teeth receptacle area, a mouth flange to prevent external airflow, and a microreactor cassette for processing the breath sample, coupled with a CO2 sensor for real-time validation of sample quality, ensuring that only valid breath samples are analyzed.
Clinical Validation and Regulatory Pathway
The clinical validation of terahertz-based breath analysis for non-invasive diagnostics represents a critical pathway toward mainstream medical adoption. Current validation efforts focus on establishing correlations between specific breath biomarkers detected by terahertz technology and confirmed disease states through traditional diagnostic methods. Multi-center clinical trials are underway at institutions including Johns Hopkins Medicine, Mayo Clinic, and Shanghai Respiratory Research Institute, involving patient cohorts with conditions ranging from lung cancer to COVID-19.
These validation studies typically follow a three-phase approach. Initial proof-of-concept studies with small patient populations (n=50-100) establish preliminary sensitivity and specificity metrics. Mid-scale validation studies (n=200-500) then refine these metrics across diverse patient demographics. Finally, large-scale clinical trials (n>1000) provide definitive performance data required for regulatory submission.
The regulatory pathway for terahertz breath analyzers varies by jurisdiction. In the United States, these devices typically fall under FDA Class II medical devices, requiring 510(k) clearance with substantial equivalence to predicate devices. However, novel biomarker detection may necessitate the more rigorous De Novo classification pathway. The FDA's Breakthrough Devices Program offers potential acceleration for technologies demonstrating significant advantages over existing diagnostic methods.
In Europe, the Medical Device Regulation (MDR) classifies these devices as Class IIa or IIb, requiring conformity assessment through a Notified Body. China's NMPA follows a similar classification system but demands local clinical data. Japan's PMDA has established a specific pathway for breath-based diagnostic technologies under its Sakigake designation for innovative medical devices.
Key regulatory challenges include standardization of breath collection protocols, demonstration of analytical validity across environmental conditions, and establishment of clinical decision thresholds. Manufacturers must address concerns regarding potential confounding factors such as diet, medication, and environmental exposures that may affect breath composition.
Recent regulatory milestones include the FDA's 2022 draft guidance on breath-based diagnostic technologies and the European Commission's harmonized standards for breath sampling devices. Industry consortia like the International Association of Breath Research are working with regulatory bodies to establish consensus standards for validation methodologies and performance metrics specific to terahertz breath analysis technologies.
These validation studies typically follow a three-phase approach. Initial proof-of-concept studies with small patient populations (n=50-100) establish preliminary sensitivity and specificity metrics. Mid-scale validation studies (n=200-500) then refine these metrics across diverse patient demographics. Finally, large-scale clinical trials (n>1000) provide definitive performance data required for regulatory submission.
The regulatory pathway for terahertz breath analyzers varies by jurisdiction. In the United States, these devices typically fall under FDA Class II medical devices, requiring 510(k) clearance with substantial equivalence to predicate devices. However, novel biomarker detection may necessitate the more rigorous De Novo classification pathway. The FDA's Breakthrough Devices Program offers potential acceleration for technologies demonstrating significant advantages over existing diagnostic methods.
In Europe, the Medical Device Regulation (MDR) classifies these devices as Class IIa or IIb, requiring conformity assessment through a Notified Body. China's NMPA follows a similar classification system but demands local clinical data. Japan's PMDA has established a specific pathway for breath-based diagnostic technologies under its Sakigake designation for innovative medical devices.
Key regulatory challenges include standardization of breath collection protocols, demonstration of analytical validity across environmental conditions, and establishment of clinical decision thresholds. Manufacturers must address concerns regarding potential confounding factors such as diet, medication, and environmental exposures that may affect breath composition.
Recent regulatory milestones include the FDA's 2022 draft guidance on breath-based diagnostic technologies and the European Commission's harmonized standards for breath sampling devices. Industry consortia like the International Association of Breath Research are working with regulatory bodies to establish consensus standards for validation methodologies and performance metrics specific to terahertz breath analysis technologies.
Bioethical Implications of Breath-Based Disease Screening
The rapid advancement of terahertz-based breath analysis for non-invasive diagnostics raises significant bioethical considerations that must be addressed before widespread implementation. As this technology enables detection of various diseases through simple breath samples, it creates a new paradigm in medical screening that challenges existing ethical frameworks.
Privacy concerns stand at the forefront of these implications. Breath analysis can potentially reveal sensitive health information without requiring traditional invasive procedures. This raises questions about data ownership, storage security, and potential unauthorized access to health information. Unlike blood samples that require explicit consent for collection, breath samples might be collected more casually, potentially blurring informed consent boundaries.
The accessibility of breath-based screening introduces concerns about equitable healthcare distribution. While the technology promises more affordable and widespread disease detection, disparities may emerge if implementation favors certain populations or geographic regions. The potential for creating new healthcare divides based on technological access requires careful consideration in deployment strategies.
False positives and negatives present another critical ethical dimension. Terahertz breath analysis, while promising, still faces technical challenges in accuracy. False positives may trigger unnecessary psychological distress and follow-up procedures, while false negatives could provide dangerous false reassurance. The psychological impact of screening results demands robust counseling frameworks to accompany technological implementation.
Consent and autonomy issues emerge uniquely with breath-based diagnostics. The non-invasive nature might lead to casual implementation without proper informed consent protocols. Additionally, questions arise about screening in public spaces or workplaces, where individuals might face implicit pressure to participate without full understanding of implications.
The potential for discrimination based on breath biomarkers represents another significant concern. Employers, insurers, or other institutions might use breath analysis results for purposes beyond healthcare, potentially leading to discriminatory practices. Regulatory frameworks must evolve to prevent such misuse while enabling beneficial applications.
Cultural and religious perspectives on breath sampling also warrant consideration, as breath carries spiritual significance in many traditions. Implementation strategies must respect diverse cultural viewpoints to ensure ethical deployment across different communities.
As this technology advances toward clinical application, developing comprehensive ethical guidelines that balance innovation with protection of individual rights becomes imperative for responsible integration into healthcare systems.
Privacy concerns stand at the forefront of these implications. Breath analysis can potentially reveal sensitive health information without requiring traditional invasive procedures. This raises questions about data ownership, storage security, and potential unauthorized access to health information. Unlike blood samples that require explicit consent for collection, breath samples might be collected more casually, potentially blurring informed consent boundaries.
The accessibility of breath-based screening introduces concerns about equitable healthcare distribution. While the technology promises more affordable and widespread disease detection, disparities may emerge if implementation favors certain populations or geographic regions. The potential for creating new healthcare divides based on technological access requires careful consideration in deployment strategies.
False positives and negatives present another critical ethical dimension. Terahertz breath analysis, while promising, still faces technical challenges in accuracy. False positives may trigger unnecessary psychological distress and follow-up procedures, while false negatives could provide dangerous false reassurance. The psychological impact of screening results demands robust counseling frameworks to accompany technological implementation.
Consent and autonomy issues emerge uniquely with breath-based diagnostics. The non-invasive nature might lead to casual implementation without proper informed consent protocols. Additionally, questions arise about screening in public spaces or workplaces, where individuals might face implicit pressure to participate without full understanding of implications.
The potential for discrimination based on breath biomarkers represents another significant concern. Employers, insurers, or other institutions might use breath analysis results for purposes beyond healthcare, potentially leading to discriminatory practices. Regulatory frameworks must evolve to prevent such misuse while enabling beneficial applications.
Cultural and religious perspectives on breath sampling also warrant consideration, as breath carries spiritual significance in many traditions. Implementation strategies must respect diverse cultural viewpoints to ensure ethical deployment across different communities.
As this technology advances toward clinical application, developing comprehensive ethical guidelines that balance innovation with protection of individual rights becomes imperative for responsible integration into healthcare systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!