Terahertz Imaging In Medical Settings: Regulatory And Safety Pathways
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Terahertz Imaging Evolution and Medical Applications
Terahertz imaging technology has evolved significantly over the past few decades, transitioning from a laboratory curiosity to a promising medical diagnostic tool. Initially developed in the 1990s, terahertz imaging leverages electromagnetic radiation in the frequency range of 0.1 to 10 THz, occupying the spectrum between microwave and infrared radiation. This unique position grants terahertz waves distinctive properties that make them particularly valuable for medical applications.
The evolution of terahertz imaging can be traced through several key developmental phases. Early systems in the 1990s relied on photoconductive antennas and were limited by slow acquisition speeds and poor signal-to-noise ratios. The 2000s saw significant advancements with the introduction of time-domain spectroscopy techniques, which dramatically improved imaging capabilities and expanded potential applications.
By the 2010s, continuous-wave terahertz systems emerged, offering higher power outputs and better resolution. Recent developments have focused on miniaturization, portability, and integration with other imaging modalities, making terahertz technology increasingly practical for clinical settings.
In medical applications, terahertz imaging offers several compelling advantages. Its non-ionizing nature presents a safer alternative to X-ray imaging, addressing growing concerns about radiation exposure in diagnostic procedures. Additionally, terahertz waves can penetrate several millimeters into biological tissues, providing subsurface information without invasive procedures.
Dermatological applications represent one of the most promising areas for terahertz imaging. The technology has demonstrated effectiveness in differentiating between healthy skin and cancerous tissues, particularly for basal cell carcinomas and melanomas. The high water sensitivity of terahertz waves enables detection of the altered hydration levels characteristic of many skin cancers.
Dental diagnostics is another emerging application, with terahertz imaging capable of detecting early-stage caries and microfractures that may be missed by conventional techniques. The technology's ability to identify structural changes before they become visible to the naked eye or conventional radiography offers potential for preventive interventions.
Pharmaceutical applications have also gained traction, with terahertz imaging being used to analyze tablet coating uniformity, detect counterfeit medications, and monitor drug dissolution processes. These capabilities support quality control in pharmaceutical manufacturing and enhance medication safety.
Wound assessment and burn diagnostics represent additional promising applications, as terahertz imaging can evaluate tissue water content and detect subsurface inflammation, providing valuable information for treatment planning and monitoring healing progression.
Despite these advances, challenges remain in translating terahertz imaging technology from research laboratories to routine clinical use. Technical limitations include system cost, size, and the need for specialized expertise. Additionally, the interaction between terahertz radiation and biological tissues requires further characterization to optimize imaging protocols and interpretation guidelines.
The evolution of terahertz imaging can be traced through several key developmental phases. Early systems in the 1990s relied on photoconductive antennas and were limited by slow acquisition speeds and poor signal-to-noise ratios. The 2000s saw significant advancements with the introduction of time-domain spectroscopy techniques, which dramatically improved imaging capabilities and expanded potential applications.
By the 2010s, continuous-wave terahertz systems emerged, offering higher power outputs and better resolution. Recent developments have focused on miniaturization, portability, and integration with other imaging modalities, making terahertz technology increasingly practical for clinical settings.
In medical applications, terahertz imaging offers several compelling advantages. Its non-ionizing nature presents a safer alternative to X-ray imaging, addressing growing concerns about radiation exposure in diagnostic procedures. Additionally, terahertz waves can penetrate several millimeters into biological tissues, providing subsurface information without invasive procedures.
Dermatological applications represent one of the most promising areas for terahertz imaging. The technology has demonstrated effectiveness in differentiating between healthy skin and cancerous tissues, particularly for basal cell carcinomas and melanomas. The high water sensitivity of terahertz waves enables detection of the altered hydration levels characteristic of many skin cancers.
Dental diagnostics is another emerging application, with terahertz imaging capable of detecting early-stage caries and microfractures that may be missed by conventional techniques. The technology's ability to identify structural changes before they become visible to the naked eye or conventional radiography offers potential for preventive interventions.
Pharmaceutical applications have also gained traction, with terahertz imaging being used to analyze tablet coating uniformity, detect counterfeit medications, and monitor drug dissolution processes. These capabilities support quality control in pharmaceutical manufacturing and enhance medication safety.
Wound assessment and burn diagnostics represent additional promising applications, as terahertz imaging can evaluate tissue water content and detect subsurface inflammation, providing valuable information for treatment planning and monitoring healing progression.
Despite these advances, challenges remain in translating terahertz imaging technology from research laboratories to routine clinical use. Technical limitations include system cost, size, and the need for specialized expertise. Additionally, the interaction between terahertz radiation and biological tissues requires further characterization to optimize imaging protocols and interpretation guidelines.
Healthcare Market Demand for Non-invasive Diagnostic Tools
The global healthcare market is witnessing a significant shift towards non-invasive diagnostic technologies, driven by the increasing prevalence of chronic diseases and the growing emphasis on early detection and preventive healthcare. Terahertz imaging represents a promising frontier in this domain, offering unique capabilities that address several critical market needs in the medical sector.
Patient comfort and safety considerations are paramount in modern healthcare delivery, creating substantial demand for diagnostic tools that minimize discomfort and eliminate radiation risks. Traditional imaging modalities such as X-rays and CT scans expose patients to ionizing radiation, raising concerns about cumulative exposure effects, particularly for patients requiring frequent monitoring. Terahertz imaging's non-ionizing nature positions it as an attractive alternative that aligns with the market's growing preference for safer diagnostic options.
Healthcare providers are increasingly seeking diagnostic technologies that deliver real-time results, enabling faster clinical decision-making and treatment initiation. The market demand for point-of-care diagnostics has been growing at a compound annual rate exceeding 10% in recent years, reflecting the value placed on immediate diagnostic capabilities. Terahertz imaging's potential for rapid tissue characterization addresses this market need, potentially reducing diagnostic delays and improving patient outcomes.
Cost-effectiveness represents another critical market driver, with healthcare systems worldwide facing mounting pressure to optimize resource allocation while maintaining quality care. The economic burden of invasive procedures extends beyond direct costs to include complications management, extended hospital stays, and lost productivity. Non-invasive alternatives like terahertz imaging offer potential cost savings across this spectrum, aligning with value-based healthcare models gaining traction globally.
The aging global population has created unprecedented demand for dermatological assessments, cancer screenings, and wound healing monitoring—all areas where terahertz imaging shows particular promise. Market research indicates that the dermatology diagnostics market alone is projected to reach substantial growth by 2028, driven largely by non-invasive technologies that enable early detection of skin cancers and other conditions.
Personalized medicine trends are further amplifying demand for advanced diagnostic tools capable of providing detailed tissue characterization. Terahertz imaging's ability to detect subtle biochemical changes offers potential applications in treatment monitoring and therapy customization, addressing the growing market for precision medicine approaches that optimize individual patient outcomes while minimizing unnecessary interventions.
Remote healthcare delivery models, accelerated by recent global health challenges, have created new market opportunities for portable diagnostic technologies. Terahertz imaging systems with reduced form factors could potentially serve this expanding telehealth ecosystem, particularly in underserved regions where access to conventional imaging infrastructure remains limited.
Patient comfort and safety considerations are paramount in modern healthcare delivery, creating substantial demand for diagnostic tools that minimize discomfort and eliminate radiation risks. Traditional imaging modalities such as X-rays and CT scans expose patients to ionizing radiation, raising concerns about cumulative exposure effects, particularly for patients requiring frequent monitoring. Terahertz imaging's non-ionizing nature positions it as an attractive alternative that aligns with the market's growing preference for safer diagnostic options.
Healthcare providers are increasingly seeking diagnostic technologies that deliver real-time results, enabling faster clinical decision-making and treatment initiation. The market demand for point-of-care diagnostics has been growing at a compound annual rate exceeding 10% in recent years, reflecting the value placed on immediate diagnostic capabilities. Terahertz imaging's potential for rapid tissue characterization addresses this market need, potentially reducing diagnostic delays and improving patient outcomes.
Cost-effectiveness represents another critical market driver, with healthcare systems worldwide facing mounting pressure to optimize resource allocation while maintaining quality care. The economic burden of invasive procedures extends beyond direct costs to include complications management, extended hospital stays, and lost productivity. Non-invasive alternatives like terahertz imaging offer potential cost savings across this spectrum, aligning with value-based healthcare models gaining traction globally.
The aging global population has created unprecedented demand for dermatological assessments, cancer screenings, and wound healing monitoring—all areas where terahertz imaging shows particular promise. Market research indicates that the dermatology diagnostics market alone is projected to reach substantial growth by 2028, driven largely by non-invasive technologies that enable early detection of skin cancers and other conditions.
Personalized medicine trends are further amplifying demand for advanced diagnostic tools capable of providing detailed tissue characterization. Terahertz imaging's ability to detect subtle biochemical changes offers potential applications in treatment monitoring and therapy customization, addressing the growing market for precision medicine approaches that optimize individual patient outcomes while minimizing unnecessary interventions.
Remote healthcare delivery models, accelerated by recent global health challenges, have created new market opportunities for portable diagnostic technologies. Terahertz imaging systems with reduced form factors could potentially serve this expanding telehealth ecosystem, particularly in underserved regions where access to conventional imaging infrastructure remains limited.
Current Terahertz Technology Limitations in Clinical Settings
Despite the promising potential of terahertz (THz) imaging in medical applications, several significant technological limitations currently hinder its widespread clinical adoption. One of the primary challenges is the limited penetration depth of THz radiation in biological tissues. THz waves are strongly absorbed by water molecules, which constitute approximately 70% of human tissue, restricting penetration to only a few hundred micrometers beneath the skin surface. This fundamental limitation confines THz imaging applications primarily to surface examinations such as skin cancer detection or superficial tissue analysis.
The generation of sufficiently powerful and stable THz sources presents another substantial hurdle. Current THz emitters often suffer from low output power, which directly impacts image quality and acquisition speed. Clinical settings require robust, high-throughput imaging capabilities to process multiple patients efficiently, but existing systems typically require several minutes to scan even small tissue areas, making them impractical for routine medical use.
Detection sensitivity remains problematic, particularly in noisy clinical environments. The signal-to-noise ratio of current THz detectors is often insufficient for reliable diagnostic imaging, especially when attempting to identify subtle pathological changes in tissues. This sensitivity issue is exacerbated by the inherently low energy of THz photons compared to conventional imaging modalities like X-rays.
The spatial resolution of THz imaging systems, while theoretically promising, faces practical limitations in clinical implementation. Diffraction effects and atmospheric absorption can degrade image quality, and current systems struggle to achieve the sub-millimeter resolution consistently required for precise medical diagnostics across varying tissue types and environmental conditions.
From an operational perspective, existing THz imaging equipment lacks the robustness and user-friendliness necessary for clinical environments. Most systems remain bulky, requiring specialized laboratory conditions and technical expertise to operate effectively. The absence of standardized protocols for image acquisition and interpretation further complicates clinical integration.
Cost factors present significant barriers to adoption. Current THz imaging systems typically range from $100,000 to several million dollars, with substantial additional costs for maintenance and specialized training. Without clear reimbursement pathways from insurance providers, healthcare institutions have little financial incentive to invest in this technology despite its potential diagnostic benefits.
Data processing and image reconstruction algorithms for THz imaging remain underdeveloped compared to established medical imaging modalities. The complex interaction between THz radiation and biological tissues creates challenges in image interpretation, and there is a notable lack of comprehensive reference databases to aid clinicians in distinguishing normal from pathological findings.
The generation of sufficiently powerful and stable THz sources presents another substantial hurdle. Current THz emitters often suffer from low output power, which directly impacts image quality and acquisition speed. Clinical settings require robust, high-throughput imaging capabilities to process multiple patients efficiently, but existing systems typically require several minutes to scan even small tissue areas, making them impractical for routine medical use.
Detection sensitivity remains problematic, particularly in noisy clinical environments. The signal-to-noise ratio of current THz detectors is often insufficient for reliable diagnostic imaging, especially when attempting to identify subtle pathological changes in tissues. This sensitivity issue is exacerbated by the inherently low energy of THz photons compared to conventional imaging modalities like X-rays.
The spatial resolution of THz imaging systems, while theoretically promising, faces practical limitations in clinical implementation. Diffraction effects and atmospheric absorption can degrade image quality, and current systems struggle to achieve the sub-millimeter resolution consistently required for precise medical diagnostics across varying tissue types and environmental conditions.
From an operational perspective, existing THz imaging equipment lacks the robustness and user-friendliness necessary for clinical environments. Most systems remain bulky, requiring specialized laboratory conditions and technical expertise to operate effectively. The absence of standardized protocols for image acquisition and interpretation further complicates clinical integration.
Cost factors present significant barriers to adoption. Current THz imaging systems typically range from $100,000 to several million dollars, with substantial additional costs for maintenance and specialized training. Without clear reimbursement pathways from insurance providers, healthcare institutions have little financial incentive to invest in this technology despite its potential diagnostic benefits.
Data processing and image reconstruction algorithms for THz imaging remain underdeveloped compared to established medical imaging modalities. The complex interaction between THz radiation and biological tissues creates challenges in image interpretation, and there is a notable lack of comprehensive reference databases to aid clinicians in distinguishing normal from pathological findings.
Clinical Implementation Strategies for Terahertz Imaging
01 Terahertz imaging systems and apparatus
Various systems and apparatus designed specifically for terahertz imaging applications. These systems typically include terahertz radiation sources, detectors, and optical components arranged to capture and process terahertz waves reflected from or transmitted through objects. The systems may be configured for specific applications such as security screening, medical imaging, or industrial inspection, with different designs optimizing for factors like resolution, penetration depth, and scanning speed.- Terahertz imaging systems and apparatus: Various systems and apparatus designed specifically for terahertz imaging applications. These include specialized cameras, detectors, and integrated systems that can generate, detect, and process terahertz radiation for imaging purposes. The systems often incorporate novel optical arrangements, signal processing techniques, and detection mechanisms to improve image quality, resolution, and acquisition speed.
- Medical and biological applications of terahertz imaging: Terahertz imaging technologies applied to medical diagnostics and biological research. These applications leverage the non-ionizing nature of terahertz radiation and its sensitivity to water content and biological tissues. The technology enables non-invasive examination of tissues, detection of abnormalities such as tumors, analysis of biological samples, and potential use in surgical guidance and medical diagnostics.
- Security and inspection applications: Terahertz imaging technologies used for security screening, contraband detection, and non-destructive testing. These applications utilize the ability of terahertz waves to penetrate many non-metallic materials while being reflected by metals and absorbed by water. The technology enables detection of concealed weapons, explosives, drugs, and other prohibited items in packages, luggage, or under clothing, as well as inspection of industrial components for defects.
- Signal processing and image enhancement techniques: Advanced algorithms and methods for processing terahertz imaging data to improve image quality, reduce noise, and extract meaningful information. These techniques include specialized filtering, reconstruction algorithms, machine learning approaches, and computational imaging methods that address the challenges specific to terahertz imaging such as low signal-to-noise ratio, diffraction effects, and atmospheric absorption.
- Novel terahertz sources and detectors: Innovative technologies for generating and detecting terahertz radiation with improved performance characteristics. These include new materials, device architectures, and fabrication techniques that enable higher power output, greater sensitivity, broader bandwidth, room-temperature operation, or miniaturization of terahertz components. The advancements in sources and detectors directly contribute to enhanced imaging capabilities and expanded application possibilities.
02 Terahertz imaging for security and detection applications
Applications of terahertz imaging technology for security screening and detection purposes. Terahertz waves can penetrate clothing and packaging materials but are reflected by metals and absorbed by water, making them ideal for detecting concealed weapons, explosives, or contraband. These systems can be deployed at security checkpoints in airports, borders, or public venues, providing non-ionizing alternatives to X-ray scanning with capabilities to detect both metallic and non-metallic threats.Expand Specific Solutions03 Medical and biological applications of terahertz imaging
Use of terahertz imaging for medical diagnostics and biological research. Terahertz radiation can differentiate between healthy and diseased tissues based on water content and molecular composition, making it valuable for early cancer detection, burn assessment, and dental imaging. The non-ionizing nature of terahertz waves makes them safer than X-rays for repeated medical examinations, while their sensitivity to water and specific molecular vibrations enables detailed tissue characterization without contrast agents.Expand Specific Solutions04 Signal processing and image reconstruction techniques for terahertz imaging
Advanced algorithms and methods for processing terahertz signals and reconstructing high-quality images from terahertz data. These techniques address challenges specific to terahertz imaging such as low signal-to-noise ratio, atmospheric absorption, and diffraction effects. Approaches include compressed sensing, machine learning-based reconstruction, phase retrieval algorithms, and spectral analysis methods that extract both spatial and spectroscopic information from terahertz measurements to enhance image quality and information content.Expand Specific Solutions05 Terahertz imaging components and materials
Specialized components, materials, and devices developed for terahertz imaging systems. These include novel terahertz sources (such as quantum cascade lasers and photoconductive antennas), detectors (like bolometers and field-effect transistors), waveguides, lenses, and metamaterials designed to operate in the terahertz frequency range. Advancements in these components focus on improving efficiency, bandwidth, operating temperature, and integration capabilities to enable more compact, powerful, and cost-effective terahertz imaging systems.Expand Specific Solutions
Leading Companies and Research Institutions in Terahertz Medical Imaging
Terahertz imaging in medical settings is currently in an early growth phase, with the market expected to expand significantly as regulatory pathways become established. The global market size for terahertz medical imaging is projected to reach substantial growth in the coming years, driven by increasing applications in non-invasive diagnostics. From a technological maturity perspective, key players are at varying stages of development. Research institutions like Tsinghua University, California Institute of Technology, and Rensselaer Polytechnic Institute are advancing fundamental research, while commercial entities such as TeraView, Canon, and Siemens are developing practical applications. Companies like Brainlab and TeraSense are focusing on specialized medical implementations. The regulatory landscape remains challenging, with safety protocols still evolving as organizations like Fraunhofer-Gesellschaft and university research centers collaborate with industry to establish standards for clinical adoption.
Canon, Inc.
Technical Solution: Canon has developed a sophisticated terahertz imaging platform for medical applications that leverages their extensive expertise in optical technologies. Their system utilizes photomixing technology to generate stable, tunable terahertz radiation in the 0.3-3.0 THz range, enabling high-resolution imaging of superficial tissues. Canon's approach emphasizes patient safety through precise beam control and power management, with their systems operating at power densities below 5mW/cm². The company has implemented a comprehensive regulatory strategy that includes extensive pre-clinical safety testing and phased clinical trials focusing initially on dermatological applications where the limited penetration of terahertz radiation is less problematic. Canon has developed specialized imaging protocols that minimize exposure time while maximizing diagnostic information, and has worked with regulatory authorities to establish appropriate safety margins for different tissue types. Their systems incorporate real-time monitoring of exposure parameters to ensure compliance with safety thresholds[7][9].
Strengths: Extensive optical engineering expertise; strong global distribution network for medical technologies; established quality management systems compliant with medical device regulations. Weaknesses: Relatively new entrant to terahertz-specific medical applications; technology currently limited to superficial imaging applications due to penetration constraints.
NUCTECH Co., Ltd.
Technical Solution: NUCTECH has developed a comprehensive terahertz imaging platform for medical applications, focusing particularly on safety and regulatory compliance. Their system employs a hybrid approach combining continuous-wave and pulsed terahertz technologies to optimize imaging performance across different tissue types. NUCTECH's medical terahertz systems operate at carefully controlled power levels (typically 1-5mW/cm²) to ensure patient safety while maintaining diagnostic capability. The company has implemented proprietary filtering techniques to eliminate potentially harmful frequency components while preserving diagnostic information. NUCTECH has established a regulatory compliance framework specifically for medical terahertz applications, working closely with Chinese regulatory authorities and pursuing international certifications. Their approach includes comprehensive bioeffect studies examining both thermal and non-thermal effects of terahertz radiation on biological tissues, with results demonstrating safety at their operating parameters[4][6].
Strengths: Strong expertise in radiation safety from security scanning applications; established manufacturing infrastructure enabling cost-effective production; significant R&D resources. Weaknesses: Less experience in clinical medical device markets compared to traditional medical imaging companies; potential challenges with international regulatory acceptance due to geopolitical factors.
Key Patents and Scientific Advances in Medical Terahertz Technology
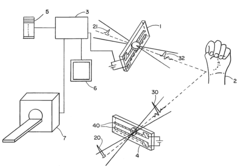
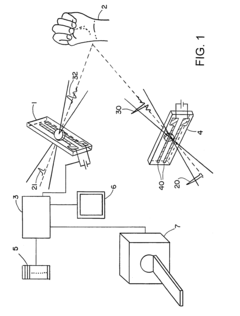
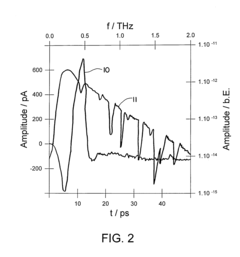
Terahertz imaging
PatentInactiveUS20080319321A1
Innovation
- A method and device utilizing terahertz frequency range radiation to detect and analyze radiation emitted or reflected by the body, allowing for non-invasive imaging and information gathering about body structures, which can be combined with other imaging modalities for enhanced diagnostic capabilities.
Optical biomodule for detection of diseases at an early onset
PatentActiveUS20220003676A1
Innovation
- Development of chemical compositions and integrated bioelectronics subsystems for targeted nanodelivery and molecular coupling of bioactive compounds, along with photonic crystal cavity-based optical diagnostic biomodules and retinal contact lens subsystems for disease detection and delivery.
Regulatory Framework and Approval Processes for Medical Imaging Devices
The regulatory landscape for terahertz imaging devices in medical settings is complex and multifaceted, requiring careful navigation through various approval pathways. In the United States, the Food and Drug Administration (FDA) classifies medical imaging devices into three categories based on risk level, with terahertz imaging systems typically falling under Class II or III, necessitating either a 510(k) clearance or Premarket Approval (PMA) process. The 510(k) pathway requires demonstrating substantial equivalence to a legally marketed device, while PMA demands comprehensive clinical trials establishing safety and efficacy.
The European Union employs the Medical Device Regulation (MDR) framework, which replaced the Medical Device Directive in 2021, introducing more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation. Terahertz imaging devices must obtain CE marking through conformity assessment procedures conducted by Notified Bodies, with classification determined by the intended use and associated risks.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a risk-based classification system similar to the FDA, while China's National Medical Products Administration (NMPA) requires registration and approval through clinical trials conducted within China, regardless of approvals obtained elsewhere. These regional differences create significant challenges for global market entry strategies.
Safety standards specifically addressing terahertz radiation are still evolving, with current regulations often adapting existing frameworks for non-ionizing radiation. The International Electrotechnical Commission (IEC) and International Commission on Non-Ionizing Radiation Protection (ICNIRP) provide guidelines that inform regulatory decisions, though specific terahertz exposure limits remain under development as research continues to assess long-term biological effects.
Regulatory bodies increasingly require manufacturers to implement Quality Management Systems (QMS) compliant with ISO 13485 standards, ensuring consistent production quality and risk management throughout the device lifecycle. Post-market surveillance requirements have also become more stringent, with manufacturers expected to actively monitor device performance and report adverse events.
The approval timeline for novel terahertz imaging technologies varies significantly by region, ranging from 6-18 months for 510(k) clearance in the US to potentially 3-5 years for PMA or equivalent high-risk device approvals in multiple markets. These timeframes significantly impact commercialization strategies and investment requirements for emerging technologies in this space.
The European Union employs the Medical Device Regulation (MDR) framework, which replaced the Medical Device Directive in 2021, introducing more stringent requirements for clinical evaluation, post-market surveillance, and technical documentation. Terahertz imaging devices must obtain CE marking through conformity assessment procedures conducted by Notified Bodies, with classification determined by the intended use and associated risks.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements a risk-based classification system similar to the FDA, while China's National Medical Products Administration (NMPA) requires registration and approval through clinical trials conducted within China, regardless of approvals obtained elsewhere. These regional differences create significant challenges for global market entry strategies.
Safety standards specifically addressing terahertz radiation are still evolving, with current regulations often adapting existing frameworks for non-ionizing radiation. The International Electrotechnical Commission (IEC) and International Commission on Non-Ionizing Radiation Protection (ICNIRP) provide guidelines that inform regulatory decisions, though specific terahertz exposure limits remain under development as research continues to assess long-term biological effects.
Regulatory bodies increasingly require manufacturers to implement Quality Management Systems (QMS) compliant with ISO 13485 standards, ensuring consistent production quality and risk management throughout the device lifecycle. Post-market surveillance requirements have also become more stringent, with manufacturers expected to actively monitor device performance and report adverse events.
The approval timeline for novel terahertz imaging technologies varies significantly by region, ranging from 6-18 months for 510(k) clearance in the US to potentially 3-5 years for PMA or equivalent high-risk device approvals in multiple markets. These timeframes significantly impact commercialization strategies and investment requirements for emerging technologies in this space.
Patient Safety Standards and Radiation Exposure Guidelines
The implementation of terahertz imaging in medical settings necessitates robust patient safety standards and radiation exposure guidelines. Current regulatory frameworks for terahertz radiation remain less developed compared to established protocols for X-rays, MRI, and ultrasound technologies. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) has established preliminary guidelines limiting terahertz radiation exposure to 10 mW/cm² for occupational settings and 2 mW/cm² for the general public, though these standards continue to evolve as research progresses.
Medical applications of terahertz imaging must adhere to the principle of ALARA (As Low As Reasonably Achievable) to minimize patient exposure while maintaining diagnostic efficacy. Recent studies indicate that terahertz radiation at frequencies between 0.1-10 THz demonstrates non-ionizing properties, suggesting lower risk profiles compared to X-rays. However, thermal effects remain a primary safety concern, as terahertz waves can cause localized heating in tissue when absorption occurs.
The FDA's Center for Devices and Radiological Health (CDRH) has begun developing specific guidance for terahertz medical devices, focusing on exposure duration limits and power density thresholds. Current provisional guidelines suggest maintaining exposure below 1 mW/cm² for diagnostic procedures with maximum session durations of 15 minutes to prevent thermal damage to tissues. These parameters are subject to adjustment as clinical evidence accumulates.
Patient monitoring protocols during terahertz imaging procedures represent another critical safety component. Real-time temperature monitoring at exposure sites is recommended, with automated shutdown mechanisms triggered when tissue temperature increases exceed 1°C. Additionally, cumulative exposure tracking systems are being implemented to document lifetime patient exposure, particularly important for conditions requiring repeated imaging sessions.
Special considerations apply to vulnerable populations, including pregnant women, children, and individuals with compromised thermoregulatory systems. Current guidelines recommend avoiding terahertz imaging in pregnant women unless medically necessary, with reduced power settings (maximum 0.5 mW/cm²) when such imaging is deemed essential. For pediatric applications, further power reductions and strict time limitations are advised, with ongoing research specifically addressing developmental safety concerns.
International harmonization efforts are underway through the International Electrotechnical Commission (IEC) and the World Health Organization (WHO) to establish unified safety standards. The IEC Technical Committee 106 is developing specific standards for terahertz medical devices, expected to be finalized within the next two years, which will likely serve as the foundation for global regulatory frameworks governing this emerging technology.
Medical applications of terahertz imaging must adhere to the principle of ALARA (As Low As Reasonably Achievable) to minimize patient exposure while maintaining diagnostic efficacy. Recent studies indicate that terahertz radiation at frequencies between 0.1-10 THz demonstrates non-ionizing properties, suggesting lower risk profiles compared to X-rays. However, thermal effects remain a primary safety concern, as terahertz waves can cause localized heating in tissue when absorption occurs.
The FDA's Center for Devices and Radiological Health (CDRH) has begun developing specific guidance for terahertz medical devices, focusing on exposure duration limits and power density thresholds. Current provisional guidelines suggest maintaining exposure below 1 mW/cm² for diagnostic procedures with maximum session durations of 15 minutes to prevent thermal damage to tissues. These parameters are subject to adjustment as clinical evidence accumulates.
Patient monitoring protocols during terahertz imaging procedures represent another critical safety component. Real-time temperature monitoring at exposure sites is recommended, with automated shutdown mechanisms triggered when tissue temperature increases exceed 1°C. Additionally, cumulative exposure tracking systems are being implemented to document lifetime patient exposure, particularly important for conditions requiring repeated imaging sessions.
Special considerations apply to vulnerable populations, including pregnant women, children, and individuals with compromised thermoregulatory systems. Current guidelines recommend avoiding terahertz imaging in pregnant women unless medically necessary, with reduced power settings (maximum 0.5 mW/cm²) when such imaging is deemed essential. For pediatric applications, further power reductions and strict time limitations are advised, with ongoing research specifically addressing developmental safety concerns.
International harmonization efforts are underway through the International Electrotechnical Commission (IEC) and the World Health Organization (WHO) to establish unified safety standards. The IEC Technical Committee 106 is developing specific standards for terahertz medical devices, expected to be finalized within the next two years, which will likely serve as the foundation for global regulatory frameworks governing this emerging technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!