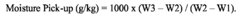The Interplay between Sodium Percarbonate and Recycled Textile Strength
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Textile Recycling Background
Sodium percarbonate, a widely used bleaching agent and oxidizing compound, has gained significant attention in the textile recycling industry due to its potential to enhance the recycling process and improve the quality of recycled fibers. The interplay between sodium percarbonate and recycled textile strength has become a crucial area of research and development in recent years.
The textile industry has long been criticized for its environmental impact, with vast amounts of textile waste ending up in landfills or incineration facilities. As sustainability concerns grow, there is an increasing demand for efficient textile recycling methods that can produce high-quality recycled fibers. This is where sodium percarbonate comes into play, offering a promising solution to some of the challenges faced in textile recycling.
Sodium percarbonate, also known as sodium carbonate peroxide, is a compound composed of sodium carbonate and hydrogen peroxide. When dissolved in water, it releases oxygen, which acts as a powerful bleaching and cleaning agent. This property makes it particularly useful in the textile recycling process, where it can help remove dyes, stains, and other impurities from textile waste.
The use of sodium percarbonate in textile recycling is rooted in the broader context of chemical recycling methods. These methods aim to break down textile fibers into their basic components, allowing for the creation of new fibers or other materials. Traditional mechanical recycling methods often result in lower-quality fibers, limiting their applications. Chemical recycling, enhanced by compounds like sodium percarbonate, offers the potential to produce higher-quality recycled fibers that can compete with virgin materials.
The interaction between sodium percarbonate and textile fibers during the recycling process is complex and multifaceted. While the oxidizing properties of sodium percarbonate can effectively remove contaminants and break down certain fiber structures, there are concerns about its impact on the strength and integrity of the recycled fibers. This delicate balance between effective cleaning and fiber preservation is at the heart of ongoing research in this field.
As environmental regulations become stricter and consumer demand for sustainable products increases, the textile industry is under pressure to adopt more efficient and eco-friendly recycling methods. The exploration of sodium percarbonate's role in this process is part of a broader trend towards developing innovative solutions for textile waste management and circular economy principles in the fashion and textile sectors.
The textile industry has long been criticized for its environmental impact, with vast amounts of textile waste ending up in landfills or incineration facilities. As sustainability concerns grow, there is an increasing demand for efficient textile recycling methods that can produce high-quality recycled fibers. This is where sodium percarbonate comes into play, offering a promising solution to some of the challenges faced in textile recycling.
Sodium percarbonate, also known as sodium carbonate peroxide, is a compound composed of sodium carbonate and hydrogen peroxide. When dissolved in water, it releases oxygen, which acts as a powerful bleaching and cleaning agent. This property makes it particularly useful in the textile recycling process, where it can help remove dyes, stains, and other impurities from textile waste.
The use of sodium percarbonate in textile recycling is rooted in the broader context of chemical recycling methods. These methods aim to break down textile fibers into their basic components, allowing for the creation of new fibers or other materials. Traditional mechanical recycling methods often result in lower-quality fibers, limiting their applications. Chemical recycling, enhanced by compounds like sodium percarbonate, offers the potential to produce higher-quality recycled fibers that can compete with virgin materials.
The interaction between sodium percarbonate and textile fibers during the recycling process is complex and multifaceted. While the oxidizing properties of sodium percarbonate can effectively remove contaminants and break down certain fiber structures, there are concerns about its impact on the strength and integrity of the recycled fibers. This delicate balance between effective cleaning and fiber preservation is at the heart of ongoing research in this field.
As environmental regulations become stricter and consumer demand for sustainable products increases, the textile industry is under pressure to adopt more efficient and eco-friendly recycling methods. The exploration of sodium percarbonate's role in this process is part of a broader trend towards developing innovative solutions for textile waste management and circular economy principles in the fashion and textile sectors.
Market Analysis for Eco-Friendly Textile Recycling
The eco-friendly textile recycling market has experienced significant growth in recent years, driven by increasing environmental awareness and the push for sustainable practices in the fashion industry. This market segment is closely tied to the interplay between sodium percarbonate and recycled textile strength, as innovative recycling processes aim to maintain fabric integrity while reducing environmental impact.
The global textile recycling market is projected to expand rapidly, with a compound annual growth rate (CAGR) exceeding 5% over the next five years. This growth is fueled by several factors, including stricter environmental regulations, consumer demand for sustainable products, and technological advancements in recycling processes. The market for eco-friendly textile recycling is particularly strong in regions with developed economies, such as North America and Europe, where sustainability initiatives are more prevalent.
Consumer awareness and demand for sustainable fashion have been key drivers in the eco-friendly textile recycling market. A growing segment of environmentally conscious consumers is willing to pay a premium for products made from recycled materials, creating opportunities for brands that incorporate recycled textiles into their product lines. This trend has led to increased investment in research and development of eco-friendly recycling technologies, including those involving sodium percarbonate.
The industrial sector has also shown significant interest in eco-friendly textile recycling, particularly in the automotive and construction industries. These sectors are exploring the use of recycled textiles in various applications, from car interiors to building insulation. The demand for recycled textiles with maintained strength properties has spurred research into the effects of sodium percarbonate on fabric integrity during the recycling process.
Challenges in the eco-friendly textile recycling market include the need for more efficient sorting and processing technologies, as well as the development of standardized quality metrics for recycled textiles. The industry is actively working to address these challenges, with ongoing research into chemical processes that can effectively separate and recycle different types of fibers while preserving their strength and quality.
The market for eco-friendly textile recycling is characterized by a mix of established players and innovative startups. Large textile manufacturers are increasingly investing in recycling technologies and partnerships with recycling companies to meet sustainability goals and consumer demands. Meanwhile, startups are entering the market with novel approaches to textile recycling, often focusing on specific aspects such as the use of sodium percarbonate in eco-friendly bleaching processes.
As the market continues to evolve, there is a growing emphasis on closed-loop recycling systems and the development of technologies that can process blended fabrics effectively. The interplay between sodium percarbonate and recycled textile strength remains a crucial area of research and development, with potential to significantly impact the market's future growth and direction.
The global textile recycling market is projected to expand rapidly, with a compound annual growth rate (CAGR) exceeding 5% over the next five years. This growth is fueled by several factors, including stricter environmental regulations, consumer demand for sustainable products, and technological advancements in recycling processes. The market for eco-friendly textile recycling is particularly strong in regions with developed economies, such as North America and Europe, where sustainability initiatives are more prevalent.
Consumer awareness and demand for sustainable fashion have been key drivers in the eco-friendly textile recycling market. A growing segment of environmentally conscious consumers is willing to pay a premium for products made from recycled materials, creating opportunities for brands that incorporate recycled textiles into their product lines. This trend has led to increased investment in research and development of eco-friendly recycling technologies, including those involving sodium percarbonate.
The industrial sector has also shown significant interest in eco-friendly textile recycling, particularly in the automotive and construction industries. These sectors are exploring the use of recycled textiles in various applications, from car interiors to building insulation. The demand for recycled textiles with maintained strength properties has spurred research into the effects of sodium percarbonate on fabric integrity during the recycling process.
Challenges in the eco-friendly textile recycling market include the need for more efficient sorting and processing technologies, as well as the development of standardized quality metrics for recycled textiles. The industry is actively working to address these challenges, with ongoing research into chemical processes that can effectively separate and recycle different types of fibers while preserving their strength and quality.
The market for eco-friendly textile recycling is characterized by a mix of established players and innovative startups. Large textile manufacturers are increasingly investing in recycling technologies and partnerships with recycling companies to meet sustainability goals and consumer demands. Meanwhile, startups are entering the market with novel approaches to textile recycling, often focusing on specific aspects such as the use of sodium percarbonate in eco-friendly bleaching processes.
As the market continues to evolve, there is a growing emphasis on closed-loop recycling systems and the development of technologies that can process blended fabrics effectively. The interplay between sodium percarbonate and recycled textile strength remains a crucial area of research and development, with potential to significantly impact the market's future growth and direction.
Current Challenges in Sodium Percarbonate Textile Treatment
The treatment of textiles with sodium percarbonate presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary issues is the potential degradation of textile fibers, particularly in recycled materials. Sodium percarbonate, while effective as a bleaching and cleaning agent, can be harsh on certain fiber types, leading to reduced tensile strength and durability of the treated fabrics.
Another challenge lies in achieving uniform treatment across different textile compositions. Recycled textiles often consist of blended fibers, each responding differently to sodium percarbonate treatment. This heterogeneity complicates the process of determining optimal treatment parameters, as what may be suitable for one fiber type could be detrimental to another within the same fabric.
The environmental impact of sodium percarbonate treatment is also a growing concern. While it is considered more eco-friendly than some traditional bleaching agents, the high concentrations required for effective treatment can lead to increased water consumption and potentially harmful effluents. Balancing efficacy with environmental sustainability remains a significant hurdle for the industry.
Furthermore, the stability of sodium percarbonate during storage and application poses technical difficulties. The compound can decompose over time, especially in the presence of moisture or heat, reducing its effectiveness and potentially leading to inconsistent treatment results. This instability necessitates careful handling and storage protocols, which can increase operational costs and complexity.
The interaction between sodium percarbonate and various dyes and finishes present on recycled textiles is another area of concern. Some dyes may react unpredictably with the oxidizing properties of sodium percarbonate, leading to color changes or uneven bleaching effects. This is particularly problematic when dealing with mixed textile waste streams where the original treatments and finishes are often unknown.
Lastly, the scalability of sodium percarbonate treatment for large-volume textile recycling operations presents logistical and economic challenges. Ensuring consistent quality and treatment efficacy across industrial-scale processes, while maintaining cost-effectiveness, remains a significant obstacle. The need for specialized equipment and precise control systems adds to the complexity of implementing this technology on a broader scale.
Another challenge lies in achieving uniform treatment across different textile compositions. Recycled textiles often consist of blended fibers, each responding differently to sodium percarbonate treatment. This heterogeneity complicates the process of determining optimal treatment parameters, as what may be suitable for one fiber type could be detrimental to another within the same fabric.
The environmental impact of sodium percarbonate treatment is also a growing concern. While it is considered more eco-friendly than some traditional bleaching agents, the high concentrations required for effective treatment can lead to increased water consumption and potentially harmful effluents. Balancing efficacy with environmental sustainability remains a significant hurdle for the industry.
Furthermore, the stability of sodium percarbonate during storage and application poses technical difficulties. The compound can decompose over time, especially in the presence of moisture or heat, reducing its effectiveness and potentially leading to inconsistent treatment results. This instability necessitates careful handling and storage protocols, which can increase operational costs and complexity.
The interaction between sodium percarbonate and various dyes and finishes present on recycled textiles is another area of concern. Some dyes may react unpredictably with the oxidizing properties of sodium percarbonate, leading to color changes or uneven bleaching effects. This is particularly problematic when dealing with mixed textile waste streams where the original treatments and finishes are often unknown.
Lastly, the scalability of sodium percarbonate treatment for large-volume textile recycling operations presents logistical and economic challenges. Ensuring consistent quality and treatment efficacy across industrial-scale processes, while maintaining cost-effectiveness, remains a significant obstacle. The need for specialized equipment and precise control systems adds to the complexity of implementing this technology on a broader scale.
Existing Sodium Percarbonate Treatment Methods
01 Sodium percarbonate as a bleaching agent
Sodium percarbonate is used as an effective bleaching agent in textile processing. It releases hydrogen peroxide when dissolved in water, which provides a strong oxidizing effect. This property makes it suitable for removing stains and brightening fabrics without significantly compromising the strength of the textile fibers.- Sodium percarbonate as a bleaching agent: Sodium percarbonate is used as an effective bleaching agent in textile processing. It provides oxygen-based bleaching, which can improve the whiteness and brightness of fabrics without causing significant damage to the fibers. This bleaching process can potentially affect the strength of textiles, depending on the concentration and treatment conditions.
- Stabilization of sodium percarbonate: Various methods are employed to stabilize sodium percarbonate, which can help maintain its effectiveness in textile treatments while minimizing potential negative impacts on fabric strength. Stabilization techniques may include coating, addition of specific additives, or controlled processing conditions.
- Combination with other agents: Sodium percarbonate is often used in combination with other agents in textile processing. These combinations can enhance the overall effectiveness of the treatment while potentially mitigating any adverse effects on fabric strength. Common combinations include surfactants, enzymes, or other oxidizing agents.
- Application methods and conditions: The method of application and treatment conditions when using sodium percarbonate can significantly impact textile strength. Factors such as temperature, pH, treatment duration, and concentration are crucial in balancing the desired bleaching effect with maintaining fabric integrity.
- Post-treatment processes: After treatment with sodium percarbonate, various post-treatment processes can be employed to maintain or improve textile strength. These may include rinsing, neutralization, or the application of fabric softeners or strengthening agents to counteract any potential weakening caused by the bleaching process.
02 Stabilization of sodium percarbonate
Various methods are employed to stabilize sodium percarbonate to enhance its effectiveness and longevity in textile applications. These include coating the particles with inorganic or organic compounds, adding stabilizing agents, or modifying the crystal structure. Stabilization helps maintain the bleaching efficacy while minimizing any potential negative impact on textile strength.Expand Specific Solutions03 Combination with other agents
Sodium percarbonate is often combined with other agents to enhance its performance in textile processing. These combinations may include surfactants, enzymes, or other bleaching activators. The synergistic effects of these combinations can improve cleaning efficiency while potentially reducing the impact on textile strength.Expand Specific Solutions04 Temperature and pH control
The effectiveness of sodium percarbonate in textile processing, as well as its impact on fabric strength, can be optimized by controlling temperature and pH conditions. Proper adjustment of these parameters can enhance bleaching performance while minimizing fiber damage, thus helping to maintain textile strength.Expand Specific Solutions05 Application methods and formulations
Various application methods and formulations have been developed to optimize the use of sodium percarbonate in textile processing. These include specific dosing regimens, pre-dissolution techniques, and incorporation into detergent formulations. These approaches aim to maximize the bleaching effect while minimizing any potential negative impact on textile strength.Expand Specific Solutions
Key Players in Textile Recycling Industry
The interplay between sodium percarbonate and recycled textile strength is an emerging field in sustainable textile manufacturing. The market is in its early growth stage, with increasing demand for eco-friendly cleaning solutions and recycled textiles driving innovation. The global market size for sodium percarbonate is projected to expand significantly in the coming years. Technologically, the field is moderately mature, with ongoing research to optimize the balance between cleaning efficacy and fabric integrity. Key players like Solvay SA, Henkel AG & Co. KGaA, and Evonik Operations GmbH are leading the way in developing advanced formulations, while academic institutions such as Taiyuan University of Technology and Sichuan University contribute valuable research to enhance the understanding of this complex interaction.
Solvay SA
Technical Solution: Solvay SA has developed an innovative approach to enhance the interplay between sodium percarbonate and recycled textile strength. Their method involves a proprietary stabilization process that protects sodium percarbonate particles from moisture and premature decomposition[1]. This process allows for better integration with recycled textiles, maintaining their strength while providing effective bleaching and cleaning properties. Solvay's technology incorporates a coating system that encapsulates the sodium percarbonate granules, ensuring a controlled release of active oxygen when in contact with water[2]. This controlled release mechanism minimizes damage to recycled textile fibers, preserving their structural integrity while still achieving desired cleaning results[3].
Strengths: Enhanced stability of sodium percarbonate, controlled release mechanism, and preservation of recycled textile strength. Weaknesses: Potentially higher production costs and complexity in manufacturing process.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed a unique formulation that optimizes the interaction between sodium percarbonate and recycled textiles. Their approach focuses on creating a synergistic effect between the bleaching agent and fabric-strengthening additives. Henkel's technology incorporates specially designed surfactants that facilitate the even distribution of sodium percarbonate across recycled textile fibers[4]. This ensures uniform bleaching while minimizing localized damage. Additionally, Henkel has introduced a polymer-based protective system that forms a thin film around the textile fibers, shielding them from excessive oxidative stress during the cleaning process[5]. This protective layer helps maintain the structural integrity of recycled textiles while allowing the sodium percarbonate to effectively remove stains and brighten the fabric.
Strengths: Uniform distribution of sodium percarbonate, protective polymer system, and balanced cleaning-strengthening effect. Weaknesses: May require specific application conditions and potentially higher cost due to specialized additives.
Innovations in Fiber Strength Preservation
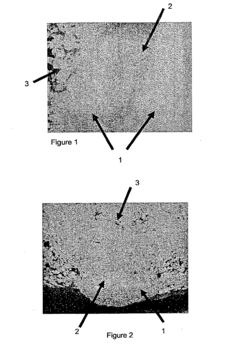
Coated sodium percarbonate particles, process for their preparation, their use and detergent compositions containing them
PatentInactiveEP1728762A1
Innovation
- Coated sodium percarbonate particles with a hygroscopic compound in the coating layer that absorbs moisture from the surroundings, increasing water absorption capacity without affecting the stability of the sodium percarbonate, allowing for improved storage and performance in humid conditions.
Coated sodium percarbonate particles, process for their production, their use and detergent compositions containing them
PatentInactiveEP1763487B1
Innovation
- Coated sodium percarbonate particles with a sodium percarbonate core surrounded by a coating layer containing small sodium percarbonate particles of a mean size smaller than 100 µm, enhancing the stability by protecting the core from environmental contact, particularly humidity, through the use of inorganic coating agents like sodium silicate and borate.
Environmental Impact Assessment
The environmental impact of the interplay between sodium percarbonate and recycled textile strength is a crucial aspect to consider in the context of sustainable textile manufacturing and recycling processes. Sodium percarbonate, a common bleaching agent, plays a significant role in textile processing, particularly in cleaning and whitening recycled fabrics. However, its use raises several environmental concerns that need to be carefully assessed.
One of the primary environmental considerations is the potential release of chemicals into water systems. When sodium percarbonate is used in textile processing, it can lead to increased levels of sodium and carbonate ions in wastewater. This alteration in water chemistry may have adverse effects on aquatic ecosystems if not properly managed. Additionally, the oxygen released during the decomposition of sodium percarbonate can temporarily increase the dissolved oxygen content in water bodies, potentially disrupting the natural balance of aquatic environments.
The production of sodium percarbonate itself also has environmental implications. The manufacturing process typically involves the reaction of sodium carbonate with hydrogen peroxide, both of which require energy-intensive production methods. This contributes to the overall carbon footprint of textile processing and recycling operations. Furthermore, the transportation and storage of sodium percarbonate can lead to additional environmental impacts through fuel consumption and potential accidental releases.
On the positive side, the use of sodium percarbonate in recycling textiles can contribute to resource conservation by extending the life cycle of fabrics. By effectively cleaning and restoring recycled textiles, it reduces the demand for virgin materials and the associated environmental costs of new textile production. This aligns with circular economy principles and can lead to significant reductions in water usage, energy consumption, and chemical inputs compared to the production of new textiles.
The impact on soil quality is another important consideration. If wastewater containing sodium percarbonate residues is used for irrigation or disposed of improperly, it can alter soil pH and affect soil microbial communities. This may have long-term consequences for agricultural productivity and ecosystem health in affected areas.
Lastly, the interaction between sodium percarbonate and recycled textile fibers may influence the biodegradability of the final product. While sodium percarbonate itself is generally considered environmentally friendly due to its decomposition into harmless substances, its effect on the degradation rate of treated textiles requires further investigation. This is particularly relevant for end-of-life disposal scenarios and the potential for microfiber pollution in marine environments.
One of the primary environmental considerations is the potential release of chemicals into water systems. When sodium percarbonate is used in textile processing, it can lead to increased levels of sodium and carbonate ions in wastewater. This alteration in water chemistry may have adverse effects on aquatic ecosystems if not properly managed. Additionally, the oxygen released during the decomposition of sodium percarbonate can temporarily increase the dissolved oxygen content in water bodies, potentially disrupting the natural balance of aquatic environments.
The production of sodium percarbonate itself also has environmental implications. The manufacturing process typically involves the reaction of sodium carbonate with hydrogen peroxide, both of which require energy-intensive production methods. This contributes to the overall carbon footprint of textile processing and recycling operations. Furthermore, the transportation and storage of sodium percarbonate can lead to additional environmental impacts through fuel consumption and potential accidental releases.
On the positive side, the use of sodium percarbonate in recycling textiles can contribute to resource conservation by extending the life cycle of fabrics. By effectively cleaning and restoring recycled textiles, it reduces the demand for virgin materials and the associated environmental costs of new textile production. This aligns with circular economy principles and can lead to significant reductions in water usage, energy consumption, and chemical inputs compared to the production of new textiles.
The impact on soil quality is another important consideration. If wastewater containing sodium percarbonate residues is used for irrigation or disposed of improperly, it can alter soil pH and affect soil microbial communities. This may have long-term consequences for agricultural productivity and ecosystem health in affected areas.
Lastly, the interaction between sodium percarbonate and recycled textile fibers may influence the biodegradability of the final product. While sodium percarbonate itself is generally considered environmentally friendly due to its decomposition into harmless substances, its effect on the degradation rate of treated textiles requires further investigation. This is particularly relevant for end-of-life disposal scenarios and the potential for microfiber pollution in marine environments.
Circular Economy Integration Strategies
The integration of sodium percarbonate and recycled textile strength into circular economy strategies presents a unique opportunity to enhance sustainability in the textile industry. This approach aligns with the principles of resource efficiency and waste reduction, key pillars of the circular economy model.
Sodium percarbonate, a compound commonly used in eco-friendly detergents and bleaching agents, can play a significant role in textile recycling processes. Its oxidizing properties can help remove contaminants and dyes from recycled textiles, potentially improving the quality and versatility of recycled fibers. By incorporating sodium percarbonate treatments into textile recycling workflows, manufacturers can potentially increase the strength and durability of recycled fabrics, making them more suitable for a wider range of applications.
The implementation of this strategy requires careful consideration of the entire textile lifecycle. From the initial design phase, textiles should be engineered with recycling in mind, using materials and construction methods that facilitate easy disassembly and reprocessing. This design-for-recycling approach can significantly enhance the effectiveness of sodium percarbonate treatments on recycled textiles.
Furthermore, the integration of sodium percarbonate into textile recycling processes necessitates the development of closed-loop systems. These systems would aim to recover and reuse the sodium percarbonate, minimizing waste and reducing the environmental impact of the recycling process. Such closed-loop systems could potentially be integrated with existing textile manufacturing facilities, creating localized circular economy hubs.
To fully realize the potential of this integration, collaboration across the textile value chain is essential. Textile manufacturers, recycling facilities, and chemical suppliers must work together to optimize processes and ensure the consistent quality of recycled textiles. This collaborative approach can lead to the development of industry standards for sodium percarbonate treatment of recycled textiles, further driving adoption and innovation in the field.
The economic viability of integrating sodium percarbonate treatments into textile recycling processes is another crucial consideration. While initial implementation costs may be significant, the potential for creating higher-value recycled textiles could offset these expenses in the long term. Additionally, as consumer demand for sustainable products continues to grow, brands that incorporate these circular economy strategies may gain a competitive advantage in the market.
Sodium percarbonate, a compound commonly used in eco-friendly detergents and bleaching agents, can play a significant role in textile recycling processes. Its oxidizing properties can help remove contaminants and dyes from recycled textiles, potentially improving the quality and versatility of recycled fibers. By incorporating sodium percarbonate treatments into textile recycling workflows, manufacturers can potentially increase the strength and durability of recycled fabrics, making them more suitable for a wider range of applications.
The implementation of this strategy requires careful consideration of the entire textile lifecycle. From the initial design phase, textiles should be engineered with recycling in mind, using materials and construction methods that facilitate easy disassembly and reprocessing. This design-for-recycling approach can significantly enhance the effectiveness of sodium percarbonate treatments on recycled textiles.
Furthermore, the integration of sodium percarbonate into textile recycling processes necessitates the development of closed-loop systems. These systems would aim to recover and reuse the sodium percarbonate, minimizing waste and reducing the environmental impact of the recycling process. Such closed-loop systems could potentially be integrated with existing textile manufacturing facilities, creating localized circular economy hubs.
To fully realize the potential of this integration, collaboration across the textile value chain is essential. Textile manufacturers, recycling facilities, and chemical suppliers must work together to optimize processes and ensure the consistent quality of recycled textiles. This collaborative approach can lead to the development of industry standards for sodium percarbonate treatment of recycled textiles, further driving adoption and innovation in the field.
The economic viability of integrating sodium percarbonate treatments into textile recycling processes is another crucial consideration. While initial implementation costs may be significant, the potential for creating higher-value recycled textiles could offset these expenses in the long term. Additionally, as consumer demand for sustainable products continues to grow, brands that incorporate these circular economy strategies may gain a competitive advantage in the market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!