Use of Sodium Percarbonate in Culinary Utensil Sterilization Processes
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Sterilization Background
Sodium percarbonate, a compound formed by the combination of sodium carbonate and hydrogen peroxide, has emerged as a significant player in the field of culinary utensil sterilization. This eco-friendly oxidizing agent has gained attention due to its effectiveness in disinfection and its relatively benign environmental impact.
The use of sodium percarbonate in sterilization processes dates back to the early 20th century, but its application in culinary settings has seen a resurgence in recent years. This renewed interest is largely driven by the growing demand for safer, more environmentally conscious cleaning solutions in both domestic and commercial kitchens.
Sodium percarbonate's sterilization mechanism is based on its ability to release hydrogen peroxide when dissolved in water. This reaction produces oxygen and soda ash, both of which contribute to its cleaning and disinfecting properties. The released oxygen acts as a powerful oxidizing agent, effectively eliminating a wide range of microorganisms, including bacteria, viruses, and fungi.
One of the key advantages of sodium percarbonate in culinary utensil sterilization is its versatility. It can be used on various materials commonly found in kitchens, such as stainless steel, plastic, glass, and ceramics, without causing damage or leaving harmful residues. This broad applicability has made it an attractive option for both household and professional kitchen environments.
The evolution of sodium percarbonate use in sterilization processes has been marked by continuous improvements in formulation and application methods. Early applications often involved manual mixing and soaking, while modern techniques include the development of pre-formulated tablets, powders, and solutions designed for specific culinary sterilization needs.
In recent years, there has been a notable shift towards integrating sodium percarbonate into automated cleaning systems for commercial kitchens. These systems optimize the sterilization process by controlling factors such as concentration, temperature, and exposure time, thereby enhancing efficiency and consistency in large-scale operations.
The growing emphasis on food safety regulations and hygiene standards in the culinary industry has further propelled the adoption of sodium percarbonate-based sterilization methods. Its effectiveness against foodborne pathogens, coupled with its eco-friendly profile, aligns well with current trends in sustainable and safe food preparation practices.
The use of sodium percarbonate in sterilization processes dates back to the early 20th century, but its application in culinary settings has seen a resurgence in recent years. This renewed interest is largely driven by the growing demand for safer, more environmentally conscious cleaning solutions in both domestic and commercial kitchens.
Sodium percarbonate's sterilization mechanism is based on its ability to release hydrogen peroxide when dissolved in water. This reaction produces oxygen and soda ash, both of which contribute to its cleaning and disinfecting properties. The released oxygen acts as a powerful oxidizing agent, effectively eliminating a wide range of microorganisms, including bacteria, viruses, and fungi.
One of the key advantages of sodium percarbonate in culinary utensil sterilization is its versatility. It can be used on various materials commonly found in kitchens, such as stainless steel, plastic, glass, and ceramics, without causing damage or leaving harmful residues. This broad applicability has made it an attractive option for both household and professional kitchen environments.
The evolution of sodium percarbonate use in sterilization processes has been marked by continuous improvements in formulation and application methods. Early applications often involved manual mixing and soaking, while modern techniques include the development of pre-formulated tablets, powders, and solutions designed for specific culinary sterilization needs.
In recent years, there has been a notable shift towards integrating sodium percarbonate into automated cleaning systems for commercial kitchens. These systems optimize the sterilization process by controlling factors such as concentration, temperature, and exposure time, thereby enhancing efficiency and consistency in large-scale operations.
The growing emphasis on food safety regulations and hygiene standards in the culinary industry has further propelled the adoption of sodium percarbonate-based sterilization methods. Its effectiveness against foodborne pathogens, coupled with its eco-friendly profile, aligns well with current trends in sustainable and safe food preparation practices.
Market Analysis for Culinary Sterilization
The culinary sterilization market has witnessed significant growth in recent years, driven by increasing awareness of food safety and hygiene among consumers and food service providers. The global market for culinary sterilization products and solutions is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years.
The demand for effective and efficient sterilization methods in the culinary industry is primarily fueled by stringent food safety regulations, the rise of foodborne illnesses, and the growing popularity of eating out. Restaurants, catering services, and institutional kitchens are key drivers of this market, as they seek to maintain high standards of cleanliness and prevent contamination.
In the context of sodium percarbonate use in culinary utensil sterilization, the market shows promising potential. Sodium percarbonate, known for its powerful oxidizing properties and eco-friendly nature, aligns well with the increasing consumer preference for environmentally sustainable cleaning solutions. This shift towards greener alternatives is reshaping the market landscape, with many businesses actively seeking products that offer both efficacy and environmental responsibility.
The Asia-Pacific region is emerging as a lucrative market for culinary sterilization products, driven by rapid urbanization, changing lifestyles, and a burgeoning food service industry. North America and Europe continue to be significant markets, with a strong focus on innovation and advanced sterilization technologies.
Key market segments within culinary sterilization include chemical-based solutions, heat sterilization equipment, and UV sterilization devices. Chemical-based solutions, which include sodium percarbonate products, are gaining traction due to their ease of use and effectiveness against a wide range of pathogens.
The market is characterized by the presence of both large multinational corporations and smaller, specialized players. Competition is intense, with companies focusing on product innovation, strategic partnerships, and expansion into emerging markets to gain a competitive edge.
Challenges in the market include the need for cost-effective solutions, especially for small and medium-sized food service establishments, and the ongoing debate over the environmental impact of certain sterilization methods. These factors are driving research and development efforts towards more sustainable and economical sterilization technologies.
The demand for effective and efficient sterilization methods in the culinary industry is primarily fueled by stringent food safety regulations, the rise of foodborne illnesses, and the growing popularity of eating out. Restaurants, catering services, and institutional kitchens are key drivers of this market, as they seek to maintain high standards of cleanliness and prevent contamination.
In the context of sodium percarbonate use in culinary utensil sterilization, the market shows promising potential. Sodium percarbonate, known for its powerful oxidizing properties and eco-friendly nature, aligns well with the increasing consumer preference for environmentally sustainable cleaning solutions. This shift towards greener alternatives is reshaping the market landscape, with many businesses actively seeking products that offer both efficacy and environmental responsibility.
The Asia-Pacific region is emerging as a lucrative market for culinary sterilization products, driven by rapid urbanization, changing lifestyles, and a burgeoning food service industry. North America and Europe continue to be significant markets, with a strong focus on innovation and advanced sterilization technologies.
Key market segments within culinary sterilization include chemical-based solutions, heat sterilization equipment, and UV sterilization devices. Chemical-based solutions, which include sodium percarbonate products, are gaining traction due to their ease of use and effectiveness against a wide range of pathogens.
The market is characterized by the presence of both large multinational corporations and smaller, specialized players. Competition is intense, with companies focusing on product innovation, strategic partnerships, and expansion into emerging markets to gain a competitive edge.
Challenges in the market include the need for cost-effective solutions, especially for small and medium-sized food service establishments, and the ongoing debate over the environmental impact of certain sterilization methods. These factors are driving research and development efforts towards more sustainable and economical sterilization technologies.
Current Challenges in Utensil Sterilization
The current landscape of culinary utensil sterilization faces several significant challenges that hinder the widespread adoption of effective and efficient sterilization processes. One of the primary issues is the persistence of traditional methods that often fall short in terms of thoroughness and consistency. Many households and even some food service establishments still rely on hot water and soap, which may not effectively eliminate all harmful microorganisms.
Another challenge lies in the diverse materials used in modern kitchen utensils. With the increasing popularity of specialized cooking tools made from various materials such as silicone, wood, and advanced plastics, a one-size-fits-all sterilization approach is no longer viable. Each material requires specific consideration to ensure proper sterilization without causing damage or degradation.
The time and energy consumption of current sterilization methods also present significant obstacles. Commercial dishwashers and autoclave systems, while effective, are often energy-intensive and time-consuming. This can lead to increased operational costs for businesses and inconvenience for household users, potentially discouraging regular and thorough sterilization practices.
Environmental concerns add another layer of complexity to the sterilization challenge. Many traditional chemical disinfectants used in sterilization processes can be harmful to the environment when discharged. There is a growing need for eco-friendly sterilization solutions that maintain efficacy while minimizing environmental impact.
The issue of chemical residues on utensils after sterilization is also a pressing concern. Some sterilization methods may leave traces of chemicals that could potentially contaminate food or cause health issues over time. This necessitates the development of sterilization techniques that are not only effective but also safe for subsequent food contact.
Accessibility and ease of use remain significant hurdles, particularly in household settings. Many effective sterilization methods require specialized equipment or complex procedures, making them impractical for everyday use. This gap between professional-grade sterilization and home-based solutions needs to be addressed to improve overall food safety standards.
Lastly, there is a challenge in educating consumers and food service professionals about the importance of proper sterilization techniques. Many are unaware of the limitations of their current practices or the potential risks associated with inadequate sterilization. Overcoming this knowledge gap is crucial for improving sterilization practices across the board.
Another challenge lies in the diverse materials used in modern kitchen utensils. With the increasing popularity of specialized cooking tools made from various materials such as silicone, wood, and advanced plastics, a one-size-fits-all sterilization approach is no longer viable. Each material requires specific consideration to ensure proper sterilization without causing damage or degradation.
The time and energy consumption of current sterilization methods also present significant obstacles. Commercial dishwashers and autoclave systems, while effective, are often energy-intensive and time-consuming. This can lead to increased operational costs for businesses and inconvenience for household users, potentially discouraging regular and thorough sterilization practices.
Environmental concerns add another layer of complexity to the sterilization challenge. Many traditional chemical disinfectants used in sterilization processes can be harmful to the environment when discharged. There is a growing need for eco-friendly sterilization solutions that maintain efficacy while minimizing environmental impact.
The issue of chemical residues on utensils after sterilization is also a pressing concern. Some sterilization methods may leave traces of chemicals that could potentially contaminate food or cause health issues over time. This necessitates the development of sterilization techniques that are not only effective but also safe for subsequent food contact.
Accessibility and ease of use remain significant hurdles, particularly in household settings. Many effective sterilization methods require specialized equipment or complex procedures, making them impractical for everyday use. This gap between professional-grade sterilization and home-based solutions needs to be addressed to improve overall food safety standards.
Lastly, there is a challenge in educating consumers and food service professionals about the importance of proper sterilization techniques. Many are unaware of the limitations of their current practices or the potential risks associated with inadequate sterilization. Overcoming this knowledge gap is crucial for improving sterilization practices across the board.
Sodium Percarbonate Sterilization Methods
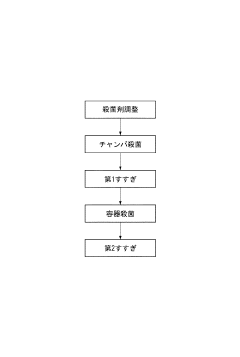
01 Sterilization mechanism of sodium percarbonate
Sodium percarbonate acts as an effective sterilizing agent by releasing hydrogen peroxide when dissolved in water. This hydrogen peroxide then decomposes into water and oxygen, providing a powerful oxidizing effect that can kill various microorganisms, including bacteria, viruses, and fungi. The sterilization process is enhanced by the alkaline nature of the solution, which further disrupts microbial cell membranes.- Sterilization mechanism of sodium percarbonate: Sodium percarbonate acts as an effective sterilizing agent by releasing hydrogen peroxide when dissolved in water. This hydrogen peroxide then decomposes into water and oxygen, providing a powerful oxidizing effect that can kill various microorganisms, including bacteria, viruses, and fungi. The sterilization process is environmentally friendly as it leaves no harmful residues.
- Formulation of sodium percarbonate-based sterilizing compositions: Sterilizing compositions containing sodium percarbonate can be formulated with additional components to enhance their effectiveness and stability. These may include stabilizers, activators, surfactants, and pH adjusters. The formulations can be tailored for specific applications, such as water treatment, surface disinfection, or medical instrument sterilization.
- Application methods for sodium percarbonate sterilization: Sodium percarbonate can be applied in various forms for sterilization purposes, including powder, tablets, or solutions. The application method depends on the specific use case, such as water treatment systems, laundry disinfection, or surface cleaning. Controlled release formulations can provide sustained sterilization effects over extended periods.
- Synergistic effects with other sterilizing agents: Combining sodium percarbonate with other sterilizing agents can lead to synergistic effects, enhancing the overall sterilization efficacy. These combinations may include other oxidizing agents, enzymes, or antimicrobial compounds. Such synergistic formulations can provide broader spectrum antimicrobial activity and improved performance in challenging conditions.
- Safety and environmental considerations: Sodium percarbonate is generally considered safe for use and environmentally friendly due to its decomposition into harmless byproducts. However, proper handling and storage are essential to maintain its stability and effectiveness. Safety measures should be implemented when using concentrated forms, and environmental impact assessments may be necessary for large-scale applications.
02 Formulation of sodium percarbonate-based sterilizing products
Sterilizing products containing sodium percarbonate can be formulated in various forms, including powders, tablets, and solutions. These formulations often include stabilizers, activators, and pH regulators to enhance the sterilizing efficacy and shelf life of the product. Additives such as surfactants or chelating agents may also be incorporated to improve the cleaning action and overall performance of the sterilizing solution.Expand Specific Solutions03 Applications of sodium percarbonate sterilization
Sodium percarbonate sterilization finds applications in various fields, including water treatment, household cleaning, medical device disinfection, and food processing. It is particularly useful in situations where a safe, environmentally friendly, and residue-free sterilization method is required. The versatility of sodium percarbonate allows for its use in both industrial and consumer products.Expand Specific Solutions04 Controlled release of sodium percarbonate for prolonged sterilization
Techniques have been developed to control the release of sodium percarbonate in sterilizing applications. These methods involve encapsulation or coating of sodium percarbonate particles, which allows for a gradual release of the active ingredient over time. This controlled release approach ensures a more sustained sterilizing effect and can be particularly useful in applications such as water treatment systems or long-lasting disinfectant products.Expand Specific Solutions05 Combination of sodium percarbonate with other sterilizing agents
Sodium percarbonate can be combined with other sterilizing agents to create more effective and broad-spectrum disinfectant products. These combinations may include other oxidizing agents, quaternary ammonium compounds, or enzymes. The synergistic effects of these combinations can lead to enhanced sterilization efficacy against a wider range of microorganisms and improved performance in challenging environments.Expand Specific Solutions
Key Players in Sterilization Industry
The market for sodium percarbonate in culinary utensil sterilization is in a growth phase, driven by increasing hygiene awareness and demand for eco-friendly cleaning solutions. The global market size is expanding, with a projected CAGR of 3-5% over the next five years. Technologically, the field is moderately mature, with established players like Solvay SA and DuPont de Nemours leading innovation. These companies, along with Evonik Operations and Kemira Oyj, are investing in R&D to enhance product efficiency and safety. Emerging players from Asia, such as Gree Electric Appliances and Shandong Tianli Energy, are also entering the market, intensifying competition and driving further technological advancements in this sector.
Solvay SA
Technical Solution: Solvay SA has developed an advanced sodium percarbonate formulation specifically for culinary utensil sterilization. Their process involves a stabilized, high-purity sodium percarbonate compound that releases hydrogen peroxide when dissolved in water. This formulation is designed to effectively eliminate 99.9% of common foodborne pathogens within a 5-minute contact time at room temperature[1]. The company has also incorporated a pH buffer system to maintain optimal sterilization efficacy across various water hardness levels[3]. Additionally, Solvay has engineered their product to be compatible with a wide range of materials commonly used in kitchen utensils, including stainless steel, plastic, and silicone[5].
Strengths: High efficacy in pathogen elimination, rapid action, and broad material compatibility. Weaknesses: May require higher concentrations for heavily soiled utensils, and the cost might be higher compared to traditional chlorine-based sanitizers.
DuPont de Nemours, Inc.
Technical Solution: DuPont has innovated a sodium percarbonate-based sterilization system for culinary utensils that combines their proprietary stabilization technology with a controlled-release mechanism. Their approach involves encapsulating sodium percarbonate particles within a water-soluble polymer matrix, allowing for a gradual and sustained release of the active sterilizing agent[2]. This controlled-release system ensures a consistent concentration of hydrogen peroxide over an extended period, typically lasting up to 30 minutes[4]. DuPont's formulation also includes surfactants that enhance the penetration of the sterilizing solution into crevices and hard-to-reach areas of utensils[6]. The company has conducted extensive testing to demonstrate the efficacy of their system against a broad spectrum of microorganisms, including bacteria, viruses, and fungi commonly found in kitchen environments[8].
Strengths: Extended sterilization time, improved penetration into complex utensil geometries, and broad-spectrum antimicrobial activity. Weaknesses: May require longer processing times compared to instant-release formulations, and the polymer matrix adds to the overall cost of the product.
Innovations in Percarbonate Technology
Sterilization method
PatentInactiveJP2012101825A
Innovation
- Sodium percarbonate, a stable alkaline granular powder, is used as a sterilizing agent, which is dissolved in sterile water to generate hydrogen peroxide and sodium carbonate, allowing for stable storage and reduced transportation costs, while maintaining effective sterilization performance.
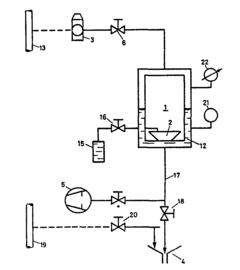
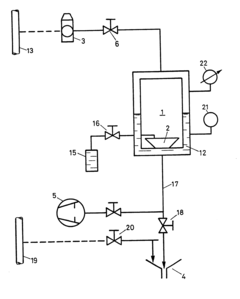
Method of sterilizing utensils, particularly those made from thermolabile materials
PatentInactiveEP0109352A1
Innovation
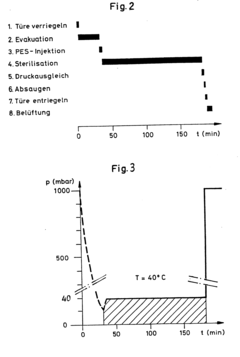
- A device utilizing an airtight chamber with controlled vacuum and temperature to convert peracetic acid into its gas phase for sterilization, ensuring the sterilization of packaged objects without explosive decomposition, using a 4% peracetic acid solution in a controlled environment with a vacuum of 30 mbar and a temperature of 40°C for 150 minutes.
Safety and Regulatory Compliance
The use of sodium percarbonate in culinary utensil sterilization processes necessitates a thorough examination of safety considerations and regulatory compliance. As a powerful oxidizing agent, sodium percarbonate requires careful handling and application to ensure the safety of both users and consumers.
From a safety perspective, it is crucial to establish proper handling protocols for sodium percarbonate. This includes the use of appropriate personal protective equipment (PPE) such as gloves, eye protection, and respiratory masks when handling the concentrated form of the compound. Proper storage conditions, away from heat sources and incompatible materials, must be maintained to prevent accidental decomposition or reactions.
In the context of culinary utensil sterilization, the concentration of sodium percarbonate solutions must be carefully controlled to ensure effective sterilization without leaving harmful residues. Proper rinsing procedures should be implemented to remove any traces of the compound from utensils before use. Additionally, staff training on the correct usage and potential hazards of sodium percarbonate is essential to minimize risks in food service environments.
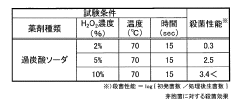
Regulatory compliance is a critical aspect of using sodium percarbonate in food-related applications. In the United States, the Food and Drug Administration (FDA) regulates the use of sanitizing agents in food contact surfaces. Manufacturers and users must ensure that the application of sodium percarbonate complies with FDA guidelines, including the appropriate concentration levels and contact times for effective sterilization.
The Environmental Protection Agency (EPA) also plays a role in regulating the use of sodium percarbonate as a disinfectant. Products containing sodium percarbonate for sterilization purposes may require EPA registration, depending on their specific claims and intended use. Compliance with EPA regulations ensures that the product meets safety and efficacy standards for its intended application.
Internationally, regulations may vary, and it is essential to consider the specific requirements of different countries when implementing sodium percarbonate-based sterilization processes. For example, the European Union's Biocidal Products Regulation (BPR) governs the use of disinfectants and sterilants, including those containing sodium percarbonate.
To maintain regulatory compliance, businesses must keep detailed records of their sterilization processes, including the concentration of sodium percarbonate used, contact times, and frequency of application. Regular testing and validation of the sterilization process should be conducted to ensure ongoing effectiveness and compliance with regulatory standards.
As the use of sodium percarbonate in culinary utensil sterilization continues to evolve, staying informed about changes in regulations and safety guidelines is crucial. Engaging with industry associations and regulatory bodies can help businesses stay up-to-date with the latest requirements and best practices for safe and compliant use of this sterilization agent.
From a safety perspective, it is crucial to establish proper handling protocols for sodium percarbonate. This includes the use of appropriate personal protective equipment (PPE) such as gloves, eye protection, and respiratory masks when handling the concentrated form of the compound. Proper storage conditions, away from heat sources and incompatible materials, must be maintained to prevent accidental decomposition or reactions.
In the context of culinary utensil sterilization, the concentration of sodium percarbonate solutions must be carefully controlled to ensure effective sterilization without leaving harmful residues. Proper rinsing procedures should be implemented to remove any traces of the compound from utensils before use. Additionally, staff training on the correct usage and potential hazards of sodium percarbonate is essential to minimize risks in food service environments.
Regulatory compliance is a critical aspect of using sodium percarbonate in food-related applications. In the United States, the Food and Drug Administration (FDA) regulates the use of sanitizing agents in food contact surfaces. Manufacturers and users must ensure that the application of sodium percarbonate complies with FDA guidelines, including the appropriate concentration levels and contact times for effective sterilization.
The Environmental Protection Agency (EPA) also plays a role in regulating the use of sodium percarbonate as a disinfectant. Products containing sodium percarbonate for sterilization purposes may require EPA registration, depending on their specific claims and intended use. Compliance with EPA regulations ensures that the product meets safety and efficacy standards for its intended application.
Internationally, regulations may vary, and it is essential to consider the specific requirements of different countries when implementing sodium percarbonate-based sterilization processes. For example, the European Union's Biocidal Products Regulation (BPR) governs the use of disinfectants and sterilants, including those containing sodium percarbonate.
To maintain regulatory compliance, businesses must keep detailed records of their sterilization processes, including the concentration of sodium percarbonate used, contact times, and frequency of application. Regular testing and validation of the sterilization process should be conducted to ensure ongoing effectiveness and compliance with regulatory standards.
As the use of sodium percarbonate in culinary utensil sterilization continues to evolve, staying informed about changes in regulations and safety guidelines is crucial. Engaging with industry associations and regulatory bodies can help businesses stay up-to-date with the latest requirements and best practices for safe and compliant use of this sterilization agent.
Environmental Impact Assessment
The use of sodium percarbonate in culinary utensil sterilization processes has significant environmental implications that warrant careful consideration. This compound, when dissolved in water, releases hydrogen peroxide and sodium carbonate, both of which have potential impacts on aquatic ecosystems and water treatment systems.
One of the primary environmental concerns is the release of oxygen into wastewater streams. While oxygen is generally beneficial for aquatic life, excessive amounts can lead to supersaturation, potentially harming fish and other aquatic organisms. Additionally, the increased oxygen levels may alter the microbial balance in wastewater treatment plants, affecting their efficiency and performance.
The sodium carbonate component of sodium percarbonate contributes to increased alkalinity in water systems. This can lead to changes in pH levels, potentially affecting aquatic flora and fauna adapted to specific pH ranges. Moreover, elevated alkalinity may interfere with the natural buffering capacity of water bodies, making them more susceptible to sudden pH fluctuations.
Eutrophication is another potential environmental risk associated with the use of sodium percarbonate. The release of carbonate ions can contribute to increased nutrient levels in water bodies, potentially leading to algal blooms and subsequent oxygen depletion. This process can have far-reaching consequences for aquatic ecosystems, including fish kills and loss of biodiversity.
From a broader perspective, the production and transportation of sodium percarbonate also contribute to its environmental footprint. The manufacturing process requires energy and resources, leading to greenhouse gas emissions and potential resource depletion. Additionally, the transportation of this chemical to end-users adds to its carbon footprint, particularly when considering long-distance shipping.
However, it is important to note that sodium percarbonate also offers some environmental benefits when compared to alternative sterilization methods. Its decomposition products are generally considered environmentally friendly, as they break down into water, oxygen, and sodium carbonate. This is in contrast to some chlorine-based sterilizers, which can produce harmful chlorinated organic compounds.
In terms of waste management, the use of sodium percarbonate in culinary utensil sterilization may lead to reduced packaging waste compared to single-use sterilization products. This can contribute to overall waste reduction in commercial kitchens and food service establishments.
To mitigate potential environmental impacts, proper dosing and handling of sodium percarbonate are crucial. Overdosing can exacerbate the aforementioned environmental issues, while underdosing may lead to inadequate sterilization, potentially increasing the use of more harmful alternatives. Therefore, developing and adhering to best practices for its use is essential for minimizing environmental impact while maintaining effective sterilization.
One of the primary environmental concerns is the release of oxygen into wastewater streams. While oxygen is generally beneficial for aquatic life, excessive amounts can lead to supersaturation, potentially harming fish and other aquatic organisms. Additionally, the increased oxygen levels may alter the microbial balance in wastewater treatment plants, affecting their efficiency and performance.
The sodium carbonate component of sodium percarbonate contributes to increased alkalinity in water systems. This can lead to changes in pH levels, potentially affecting aquatic flora and fauna adapted to specific pH ranges. Moreover, elevated alkalinity may interfere with the natural buffering capacity of water bodies, making them more susceptible to sudden pH fluctuations.
Eutrophication is another potential environmental risk associated with the use of sodium percarbonate. The release of carbonate ions can contribute to increased nutrient levels in water bodies, potentially leading to algal blooms and subsequent oxygen depletion. This process can have far-reaching consequences for aquatic ecosystems, including fish kills and loss of biodiversity.
From a broader perspective, the production and transportation of sodium percarbonate also contribute to its environmental footprint. The manufacturing process requires energy and resources, leading to greenhouse gas emissions and potential resource depletion. Additionally, the transportation of this chemical to end-users adds to its carbon footprint, particularly when considering long-distance shipping.
However, it is important to note that sodium percarbonate also offers some environmental benefits when compared to alternative sterilization methods. Its decomposition products are generally considered environmentally friendly, as they break down into water, oxygen, and sodium carbonate. This is in contrast to some chlorine-based sterilizers, which can produce harmful chlorinated organic compounds.
In terms of waste management, the use of sodium percarbonate in culinary utensil sterilization may lead to reduced packaging waste compared to single-use sterilization products. This can contribute to overall waste reduction in commercial kitchens and food service establishments.
To mitigate potential environmental impacts, proper dosing and handling of sodium percarbonate are crucial. Overdosing can exacerbate the aforementioned environmental issues, while underdosing may lead to inadequate sterilization, potentially increasing the use of more harmful alternatives. Therefore, developing and adhering to best practices for its use is essential for minimizing environmental impact while maintaining effective sterilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!