Use of Sodium Percarbonate in Environmental Restoration Projects
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Background and Objectives
Sodium percarbonate, a compound formed by the combination of sodium carbonate and hydrogen peroxide, has emerged as a promising agent in environmental restoration projects. This eco-friendly oxidizing agent has gained attention due to its ability to break down organic pollutants and its potential for soil and water remediation. The use of sodium percarbonate in environmental restoration represents a significant shift towards more sustainable and less harmful cleaning methods.
The development of sodium percarbonate can be traced back to the early 20th century, but its application in environmental restoration is a more recent phenomenon. As global awareness of environmental issues has grown, there has been an increasing demand for effective yet environmentally benign remediation techniques. Sodium percarbonate has stepped into this role, offering a balance between cleaning efficacy and ecological responsibility.
The primary objective in utilizing sodium percarbonate for environmental restoration is to harness its oxidizing properties to degrade contaminants while minimizing negative impacts on ecosystems. When dissolved in water, sodium percarbonate releases hydrogen peroxide, which then breaks down into water and oxygen. This process can effectively oxidize a wide range of organic pollutants, including petroleum hydrocarbons, pesticides, and various industrial chemicals.
Another key goal in the application of sodium percarbonate is to develop cost-effective and scalable remediation solutions. Environmental restoration projects often face budget constraints, and the relatively low cost and ease of handling of sodium percarbonate make it an attractive option for large-scale applications. Researchers and environmental engineers are working to optimize its use in different environmental matrices, including soil, groundwater, and surface water bodies.
The technology surrounding sodium percarbonate in environmental restoration is evolving rapidly. Current research focuses on enhancing its effectiveness through various activation methods, such as the use of catalysts or combining it with other remediation techniques. The aim is to broaden its applicability to a wider range of contaminants and environmental conditions, potentially revolutionizing the field of in-situ chemical oxidation for environmental cleanup.
As environmental regulations become more stringent worldwide, the demand for green remediation technologies is expected to grow. Sodium percarbonate, with its biodegradable nature and non-toxic byproducts, aligns well with this trend. The ongoing research and development in this field are likely to uncover new applications and improve existing methodologies, further cementing the role of sodium percarbonate in environmental restoration efforts.
The development of sodium percarbonate can be traced back to the early 20th century, but its application in environmental restoration is a more recent phenomenon. As global awareness of environmental issues has grown, there has been an increasing demand for effective yet environmentally benign remediation techniques. Sodium percarbonate has stepped into this role, offering a balance between cleaning efficacy and ecological responsibility.
The primary objective in utilizing sodium percarbonate for environmental restoration is to harness its oxidizing properties to degrade contaminants while minimizing negative impacts on ecosystems. When dissolved in water, sodium percarbonate releases hydrogen peroxide, which then breaks down into water and oxygen. This process can effectively oxidize a wide range of organic pollutants, including petroleum hydrocarbons, pesticides, and various industrial chemicals.
Another key goal in the application of sodium percarbonate is to develop cost-effective and scalable remediation solutions. Environmental restoration projects often face budget constraints, and the relatively low cost and ease of handling of sodium percarbonate make it an attractive option for large-scale applications. Researchers and environmental engineers are working to optimize its use in different environmental matrices, including soil, groundwater, and surface water bodies.
The technology surrounding sodium percarbonate in environmental restoration is evolving rapidly. Current research focuses on enhancing its effectiveness through various activation methods, such as the use of catalysts or combining it with other remediation techniques. The aim is to broaden its applicability to a wider range of contaminants and environmental conditions, potentially revolutionizing the field of in-situ chemical oxidation for environmental cleanup.
As environmental regulations become more stringent worldwide, the demand for green remediation technologies is expected to grow. Sodium percarbonate, with its biodegradable nature and non-toxic byproducts, aligns well with this trend. The ongoing research and development in this field are likely to uncover new applications and improve existing methodologies, further cementing the role of sodium percarbonate in environmental restoration efforts.
Environmental Restoration Market Analysis
The environmental restoration market has experienced significant growth in recent years, driven by increasing awareness of environmental issues and stricter regulations. The global market for environmental remediation technologies was valued at $85.5 billion in 2020 and is projected to reach $152.8 billion by 2027, growing at a CAGR of 7.5% during the forecast period. This growth is attributed to the rising need for cleaning up contaminated sites, restoring ecosystems, and mitigating the impacts of industrial activities on the environment.
The use of sodium percarbonate in environmental restoration projects represents a niche but growing segment within this market. Sodium percarbonate, an adduct of sodium carbonate and hydrogen peroxide, is gaining traction due to its eco-friendly nature and effectiveness in various remediation applications. The compound's ability to release oxygen and act as a powerful oxidizing agent makes it particularly useful in soil and water treatment processes.
In the soil remediation sector, which accounts for approximately 40% of the environmental restoration market, sodium percarbonate is increasingly being used for in-situ chemical oxidation (ISCO) treatments. This application is particularly effective in addressing organic contaminants such as petroleum hydrocarbons, chlorinated solvents, and pesticides. The market for ISCO technologies is expected to grow at a CAGR of 9.2% from 2021 to 2028, with sodium percarbonate-based solutions playing a significant role in this expansion.
Water treatment applications, including groundwater remediation and wastewater treatment, represent another key area where sodium percarbonate is gaining market share. The compound's ability to effectively remove organic pollutants, reduce chemical oxygen demand (COD), and improve water clarity has led to its increased adoption in both municipal and industrial water treatment processes. The global water treatment chemicals market, valued at $30.6 billion in 2019, is projected to reach $47.5 billion by 2027, with oxidizing agents like sodium percarbonate contributing to this growth.
Geographically, North America and Europe currently dominate the environmental restoration market, accounting for over 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing environmental concerns. This regional shift is likely to create new opportunities for sodium percarbonate applications in environmental restoration projects across emerging economies.
The market for sodium percarbonate in environmental restoration is characterized by a mix of established chemical manufacturers and specialized environmental technology companies. Key players in this space are investing in research and development to enhance the efficiency and applicability of sodium percarbonate-based solutions, focusing on factors such as controlled release mechanisms, synergistic formulations, and application-specific optimizations.
The use of sodium percarbonate in environmental restoration projects represents a niche but growing segment within this market. Sodium percarbonate, an adduct of sodium carbonate and hydrogen peroxide, is gaining traction due to its eco-friendly nature and effectiveness in various remediation applications. The compound's ability to release oxygen and act as a powerful oxidizing agent makes it particularly useful in soil and water treatment processes.
In the soil remediation sector, which accounts for approximately 40% of the environmental restoration market, sodium percarbonate is increasingly being used for in-situ chemical oxidation (ISCO) treatments. This application is particularly effective in addressing organic contaminants such as petroleum hydrocarbons, chlorinated solvents, and pesticides. The market for ISCO technologies is expected to grow at a CAGR of 9.2% from 2021 to 2028, with sodium percarbonate-based solutions playing a significant role in this expansion.
Water treatment applications, including groundwater remediation and wastewater treatment, represent another key area where sodium percarbonate is gaining market share. The compound's ability to effectively remove organic pollutants, reduce chemical oxygen demand (COD), and improve water clarity has led to its increased adoption in both municipal and industrial water treatment processes. The global water treatment chemicals market, valued at $30.6 billion in 2019, is projected to reach $47.5 billion by 2027, with oxidizing agents like sodium percarbonate contributing to this growth.
Geographically, North America and Europe currently dominate the environmental restoration market, accounting for over 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing environmental concerns. This regional shift is likely to create new opportunities for sodium percarbonate applications in environmental restoration projects across emerging economies.
The market for sodium percarbonate in environmental restoration is characterized by a mix of established chemical manufacturers and specialized environmental technology companies. Key players in this space are investing in research and development to enhance the efficiency and applicability of sodium percarbonate-based solutions, focusing on factors such as controlled release mechanisms, synergistic formulations, and application-specific optimizations.
Current Applications and Challenges
Sodium percarbonate has gained significant attention in environmental restoration projects due to its effectiveness as an oxidizing agent and its eco-friendly nature. Currently, it is widely applied in soil and groundwater remediation, particularly for the treatment of organic contaminants. In soil remediation, sodium percarbonate is used to break down persistent organic pollutants, such as petroleum hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), and chlorinated solvents. Its ability to release hydrogen peroxide upon dissolution makes it an excellent in-situ chemical oxidation (ISCO) agent.
In groundwater treatment, sodium percarbonate is employed to address a range of contaminants, including BTEX compounds (benzene, toluene, ethylbenzene, and xylenes), methyl tertiary-butyl ether (MTBE), and various industrial solvents. The compound's high solubility allows for effective distribution throughout the contaminated aquifer, promoting rapid oxidation of pollutants. Additionally, sodium percarbonate has shown promise in the remediation of mining-impacted waters, where it can help neutralize acidic conditions and precipitate dissolved metals.
Despite its widespread use, several challenges persist in the application of sodium percarbonate for environmental restoration. One primary concern is the potential for uncontrolled reactions and heat generation when the compound comes into contact with certain organic materials or metals. This can lead to safety hazards and reduced effectiveness if not properly managed. Furthermore, the rapid decomposition of sodium percarbonate in aqueous environments can limit its longevity and treatment radius, necessitating multiple applications or innovative delivery methods to achieve desired results.
Another challenge lies in the optimization of sodium percarbonate dosage and application techniques. Overdosing can lead to unnecessary costs and potential negative impacts on soil microbial communities, while underdosing may result in incomplete contaminant degradation. Achieving the right balance requires careful site characterization and treatability studies. Moreover, the alkaline nature of sodium percarbonate solutions can temporarily alter soil and groundwater pH, potentially affecting ecosystem dynamics and contaminant mobility.
The presence of naturally occurring organic matter and inorganic species in soil and groundwater can also interfere with the oxidation process, reducing the efficiency of sodium percarbonate treatment. These scavenging reactions consume the active oxidant species, diminishing their availability for contaminant degradation. Additionally, the formation of carbonate precipitates during treatment can lead to reduced soil permeability, potentially limiting the distribution of the oxidant and treated water flow.
As environmental regulations become more stringent, there is an increasing need to develop more targeted and controlled release formulations of sodium percarbonate. This would help address issues related to its rapid decomposition and improve its overall effectiveness in various environmental matrices. Furthermore, research is ongoing to better understand and mitigate the potential impacts of sodium percarbonate on soil and aquatic ecosystems, ensuring that its use in environmental restoration projects remains sustainable and environmentally sound.
In groundwater treatment, sodium percarbonate is employed to address a range of contaminants, including BTEX compounds (benzene, toluene, ethylbenzene, and xylenes), methyl tertiary-butyl ether (MTBE), and various industrial solvents. The compound's high solubility allows for effective distribution throughout the contaminated aquifer, promoting rapid oxidation of pollutants. Additionally, sodium percarbonate has shown promise in the remediation of mining-impacted waters, where it can help neutralize acidic conditions and precipitate dissolved metals.
Despite its widespread use, several challenges persist in the application of sodium percarbonate for environmental restoration. One primary concern is the potential for uncontrolled reactions and heat generation when the compound comes into contact with certain organic materials or metals. This can lead to safety hazards and reduced effectiveness if not properly managed. Furthermore, the rapid decomposition of sodium percarbonate in aqueous environments can limit its longevity and treatment radius, necessitating multiple applications or innovative delivery methods to achieve desired results.
Another challenge lies in the optimization of sodium percarbonate dosage and application techniques. Overdosing can lead to unnecessary costs and potential negative impacts on soil microbial communities, while underdosing may result in incomplete contaminant degradation. Achieving the right balance requires careful site characterization and treatability studies. Moreover, the alkaline nature of sodium percarbonate solutions can temporarily alter soil and groundwater pH, potentially affecting ecosystem dynamics and contaminant mobility.
The presence of naturally occurring organic matter and inorganic species in soil and groundwater can also interfere with the oxidation process, reducing the efficiency of sodium percarbonate treatment. These scavenging reactions consume the active oxidant species, diminishing their availability for contaminant degradation. Additionally, the formation of carbonate precipitates during treatment can lead to reduced soil permeability, potentially limiting the distribution of the oxidant and treated water flow.
As environmental regulations become more stringent, there is an increasing need to develop more targeted and controlled release formulations of sodium percarbonate. This would help address issues related to its rapid decomposition and improve its overall effectiveness in various environmental matrices. Furthermore, research is ongoing to better understand and mitigate the potential impacts of sodium percarbonate on soil and aquatic ecosystems, ensuring that its use in environmental restoration projects remains sustainable and environmentally sound.
Existing Environmental Restoration Solutions
01 Production methods of sodium percarbonate
Various methods for producing sodium percarbonate are described, including crystallization processes, spray drying techniques, and fluidized bed methods. These processes aim to improve the stability, purity, and particle characteristics of the final product.- Synthesis and production of sodium percarbonate: Various methods for synthesizing and producing sodium percarbonate are described. These methods involve the reaction of sodium carbonate with hydrogen peroxide under specific conditions to form stable sodium percarbonate crystals. The processes may include steps such as crystallization, drying, and stabilization to improve the quality and stability of the final product.
- Stabilization of sodium percarbonate: Techniques for stabilizing sodium percarbonate to improve its shelf life and performance are discussed. These may include coating the particles with stabilizing agents, incorporating additives, or modifying the crystal structure. The goal is to prevent decomposition and maintain the active oxygen content during storage and use.
- Applications in cleaning and bleaching products: Sodium percarbonate is widely used in cleaning and bleaching formulations. It serves as an effective oxygen-based bleaching agent in laundry detergents, dishwashing products, and other household cleaners. The compound releases hydrogen peroxide when dissolved in water, providing stain removal and disinfecting properties.
- Environmental and safety considerations: The environmental impact and safety aspects of sodium percarbonate are addressed. As an oxygen-based compound, it is considered more environmentally friendly compared to chlorine-based bleaches. Safety measures for handling, storage, and disposal are discussed to minimize risks associated with its use in various applications.
- Formulation with other ingredients: Methods for combining sodium percarbonate with other ingredients in various formulations are explored. This includes its incorporation into detergent compositions, bleaching agents, and other cleaning products. Compatibility with surfactants, enzymes, and other active ingredients is considered to optimize performance and stability.
02 Stabilization of sodium percarbonate
Techniques for enhancing the stability of sodium percarbonate are discussed, including the use of coating materials, additives, and specific processing conditions. These methods aim to improve the shelf life and performance of sodium percarbonate in various applications.Expand Specific Solutions03 Applications in cleaning and bleaching
Sodium percarbonate is widely used in cleaning and bleaching formulations. Its applications include laundry detergents, dishwashing products, and general-purpose cleaners. The compound's ability to release hydrogen peroxide in aqueous solutions makes it an effective oxidizing agent for stain removal and disinfection.Expand Specific Solutions04 Formulation with other ingredients
Sodium percarbonate is often combined with other ingredients to enhance its performance or create specialized products. These formulations may include surfactants, enzymes, builders, and other active ingredients to improve cleaning efficiency, stability, or specific functionalities.Expand Specific Solutions05 Environmental and safety considerations
The use of sodium percarbonate in various applications takes into account environmental and safety factors. Its biodegradability, low toxicity, and ability to decompose into harmless byproducts make it an eco-friendly alternative to other bleaching agents. Safety measures for handling and storage are also considered in product development.Expand Specific Solutions
Key Industry Players and Competitors
The use of sodium percarbonate in environmental restoration projects is in a growth phase, with increasing market size due to rising environmental concerns and stricter regulations. The technology's maturity is advancing, as evidenced by the involvement of major chemical companies like Solvay SA, Henkel AG & Co. KGaA, and Kemira Oyj. These firms are leveraging their expertise in chemical production and water treatment to develop innovative applications. Academic institutions such as Chengdu University of Technology and Central South University are contributing to research and development, indicating a collaborative approach between industry and academia. The competitive landscape is diverse, with both established players and specialized environmental remediation companies like Envirogen Technologies and ToxSorb entering the market.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate formulations for environmental restoration projects. Their technology focuses on controlled-release mechanisms, allowing for sustained oxidation of contaminants in soil and water. The company's approach involves encapsulating sodium percarbonate particles with biodegradable polymers, which gradually dissolve to release the active compound over time[1]. This method ensures a more efficient and long-lasting treatment process, particularly in groundwater remediation applications. Solvay's sodium percarbonate products are engineered to maintain stability in various pH conditions, enhancing their effectiveness across different environmental scenarios[3].
Strengths: Controlled-release technology for sustained treatment; Stable across various pH levels; Biodegradable encapsulation. Weaknesses: Potentially higher cost due to advanced formulation; May require specialized application methods.
Henkel AG & Co. KGaA
Technical Solution: Henkel has innovated in the use of sodium percarbonate for environmental restoration, particularly focusing on its application in wastewater treatment and soil remediation. Their approach combines sodium percarbonate with proprietary surfactant technologies to enhance penetration and distribution in contaminated media[2]. This synergistic formulation allows for improved contact between the oxidizing agent and pollutants, leading to more efficient degradation of organic contaminants. Henkel's technology also incorporates pH buffers to optimize the oxidation reaction conditions, ensuring maximum effectiveness of the sodium percarbonate in various environmental matrices[4].
Strengths: Enhanced penetration and distribution in contaminated media; Optimized pH conditions for oxidation reactions. Weaknesses: May be less effective in highly alkaline environments; Potential for increased cost due to additional formulation components.
Innovative Sodium Percarbonate Applications
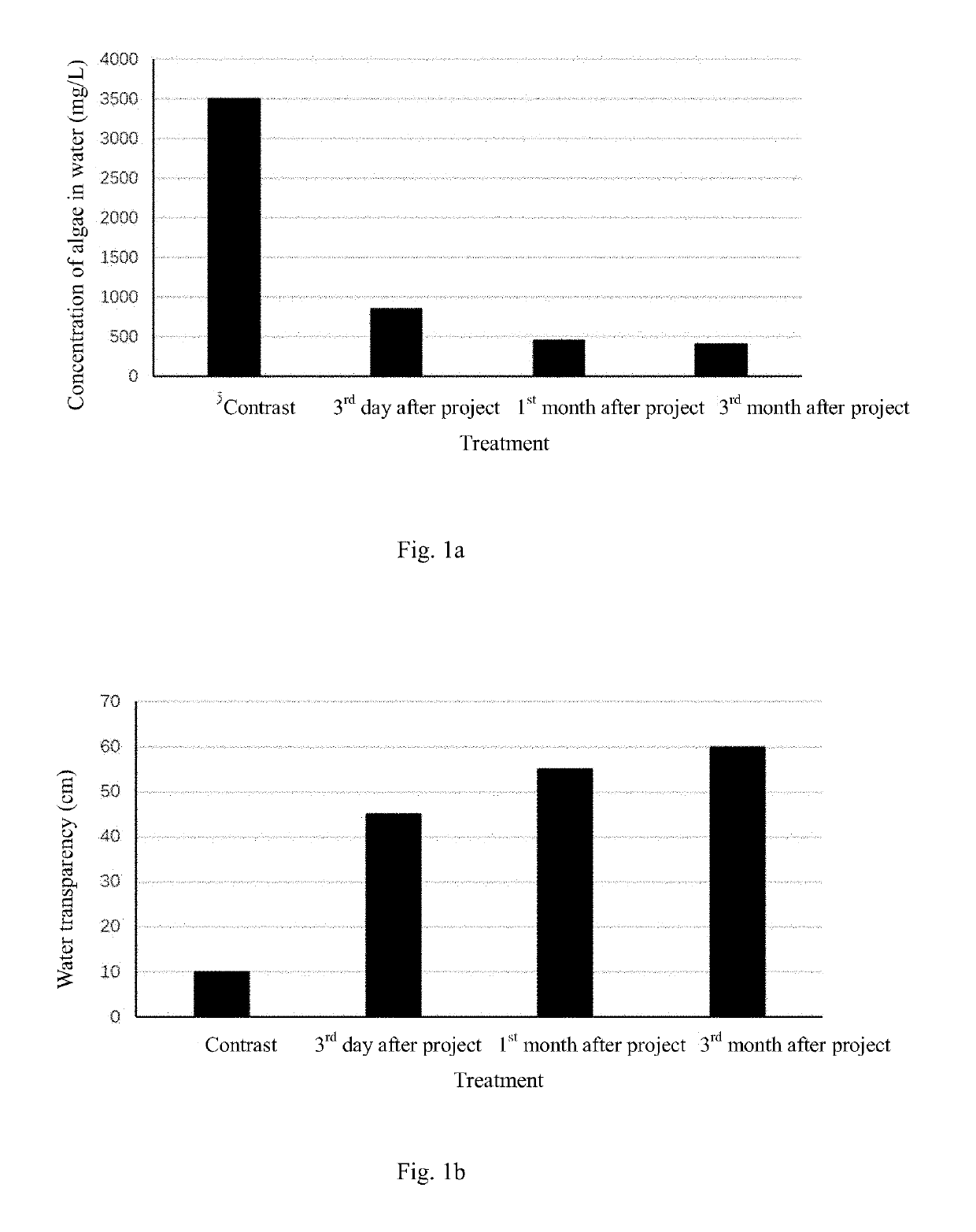
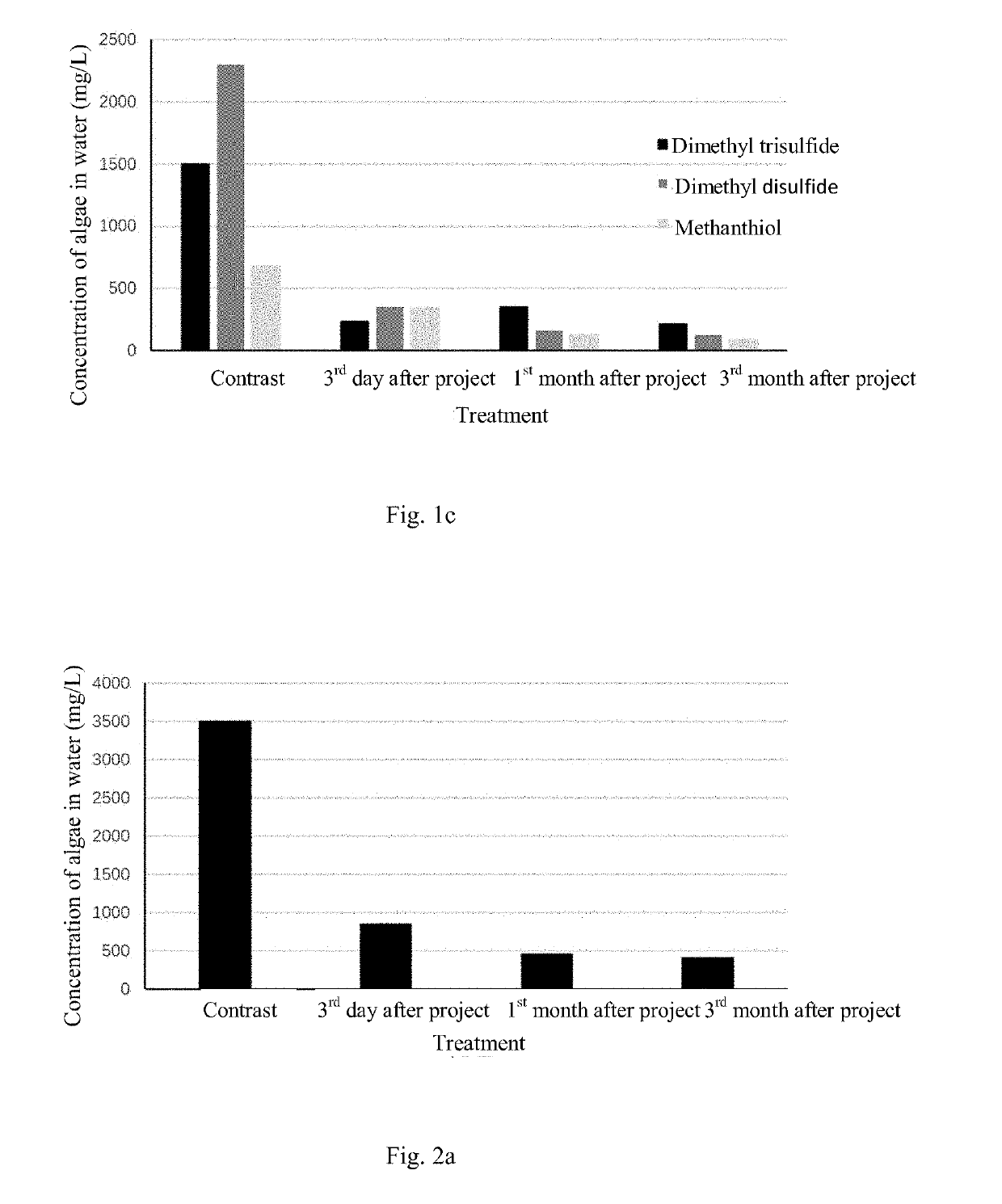
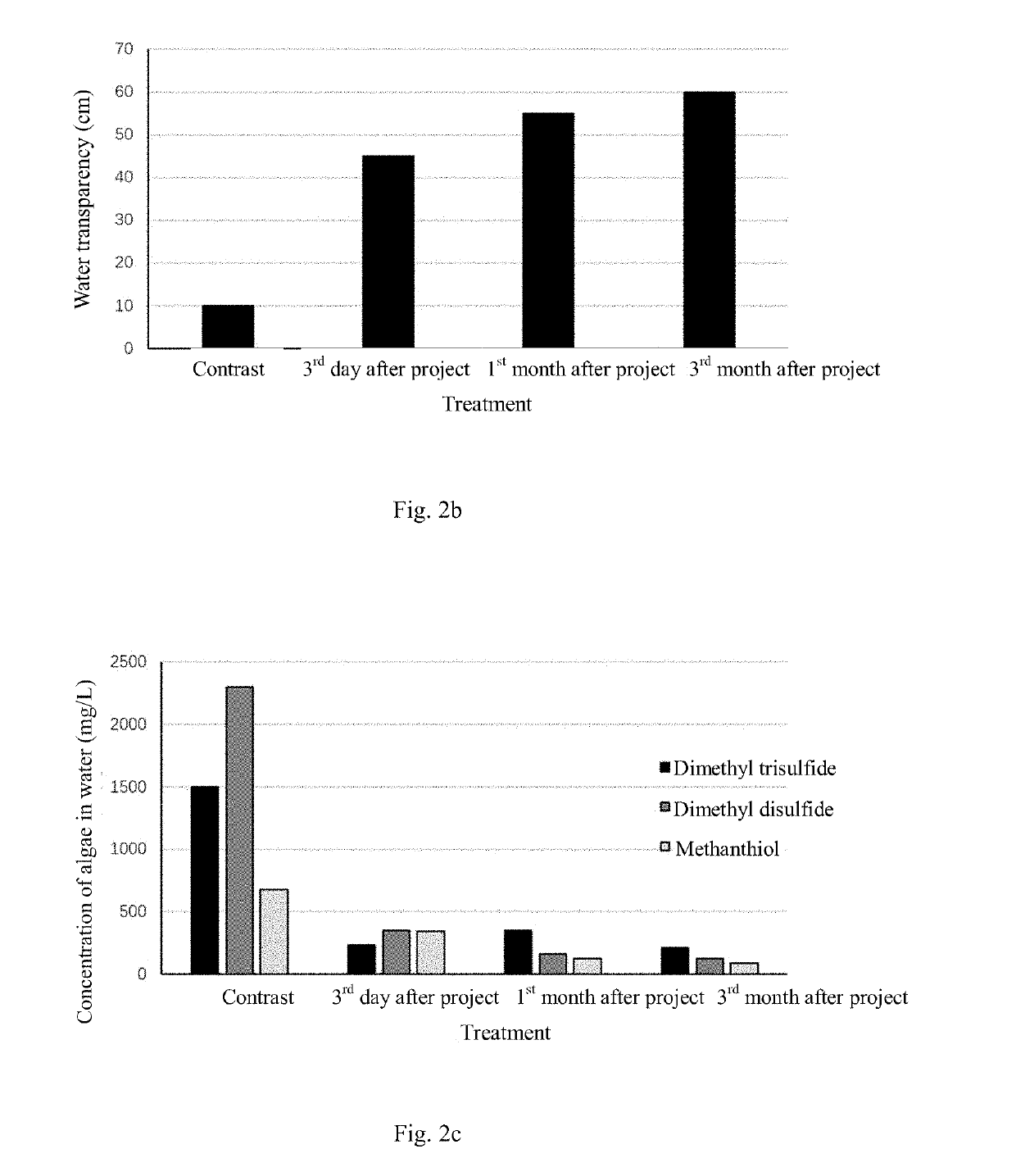
Method for in-situ harmless treatment of cyanophycean accumulation and suspended solids in lakeside wetlands
PatentActiveUS20190194044A1
Innovation
- A method involving the use of a modified starch and sand flocculant for sedimentation, followed by stabilization and reoxygenation using quicklime and indigenous plant species to manage cyanophycean accumulation, reducing malodorous substance release and promoting ecological restoration.
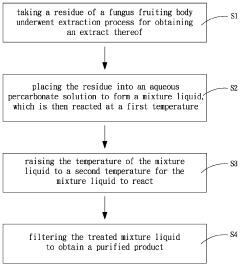
Purification method of fungal cell wall composition
PatentActiveUS20230310529A1
Innovation
- An aqueous percarbonate solution is used for decolorization and digestion, replacing the two-stage treatment with sodium hydroxide and hypochlorite or hydrogen peroxide, allowing for simultaneous decolorization and decomposition at controlled temperatures, reducing waste burden and increasing recovery rates.
Regulatory Framework for Chemical Use
The regulatory framework for chemical use in environmental restoration projects involving sodium percarbonate is complex and multifaceted. At the federal level in the United States, the Environmental Protection Agency (EPA) plays a crucial role in overseeing the use of chemicals in environmental applications. The EPA's Toxic Substances Control Act (TSCA) regulates the production, importation, use, and disposal of chemical substances, including sodium percarbonate.
Under the Clean Water Act (CWA), the use of sodium percarbonate in aquatic environments may require permits, especially if the project involves discharging chemicals into water bodies. The National Pollutant Discharge Elimination System (NPDES) permit program is particularly relevant in this context, as it regulates point source pollution discharges into U.S. waters.
State-level regulations also play a significant role in governing the use of chemicals like sodium percarbonate in environmental restoration. Many states have their own environmental protection agencies that may impose additional requirements or restrictions on chemical use. These state-level regulations often address specific local environmental concerns and may be more stringent than federal standards.
The Occupational Safety and Health Administration (OSHA) sets standards for the safe handling and use of chemicals in workplace settings, including environmental restoration sites. OSHA's Hazard Communication Standard requires proper labeling, safety data sheets, and training for workers handling sodium percarbonate and other chemicals.
International regulations must also be considered for projects spanning multiple countries or in areas with shared water resources. The Stockholm Convention on Persistent Organic Pollutants and the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade are examples of international agreements that may impact the use of chemicals in environmental restoration.
Environmental impact assessments (EIAs) are often required before initiating restoration projects involving chemical use. These assessments evaluate the potential environmental consequences of the proposed activities and help ensure compliance with relevant regulations. The National Environmental Policy Act (NEPA) in the United States mandates federal agencies to assess the environmental effects of their proposed actions, including the use of chemicals in restoration projects.
Monitoring and reporting requirements are typically integral to the regulatory framework. Project managers must often document the quantities of chemicals used, their environmental fate, and any observed impacts on ecosystems. This data is crucial for regulatory compliance and for informing future policy decisions regarding chemical use in environmental restoration.
Under the Clean Water Act (CWA), the use of sodium percarbonate in aquatic environments may require permits, especially if the project involves discharging chemicals into water bodies. The National Pollutant Discharge Elimination System (NPDES) permit program is particularly relevant in this context, as it regulates point source pollution discharges into U.S. waters.
State-level regulations also play a significant role in governing the use of chemicals like sodium percarbonate in environmental restoration. Many states have their own environmental protection agencies that may impose additional requirements or restrictions on chemical use. These state-level regulations often address specific local environmental concerns and may be more stringent than federal standards.
The Occupational Safety and Health Administration (OSHA) sets standards for the safe handling and use of chemicals in workplace settings, including environmental restoration sites. OSHA's Hazard Communication Standard requires proper labeling, safety data sheets, and training for workers handling sodium percarbonate and other chemicals.
International regulations must also be considered for projects spanning multiple countries or in areas with shared water resources. The Stockholm Convention on Persistent Organic Pollutants and the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade are examples of international agreements that may impact the use of chemicals in environmental restoration.
Environmental impact assessments (EIAs) are often required before initiating restoration projects involving chemical use. These assessments evaluate the potential environmental consequences of the proposed activities and help ensure compliance with relevant regulations. The National Environmental Policy Act (NEPA) in the United States mandates federal agencies to assess the environmental effects of their proposed actions, including the use of chemicals in restoration projects.
Monitoring and reporting requirements are typically integral to the regulatory framework. Project managers must often document the quantities of chemicals used, their environmental fate, and any observed impacts on ecosystems. This data is crucial for regulatory compliance and for informing future policy decisions regarding chemical use in environmental restoration.
Environmental Impact Assessment
The use of sodium percarbonate in environmental restoration projects necessitates a comprehensive environmental impact assessment to ensure its safe and effective application. This assessment primarily focuses on the chemical's interaction with various ecosystem components and its potential long-term effects on the environment.
Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate. The hydrogen peroxide component acts as a powerful oxidizing agent, which is the primary mechanism for its environmental remediation capabilities. However, this oxidizing property also requires careful evaluation of its impact on non-target organisms and ecosystems.
In aquatic environments, the introduction of sodium percarbonate can lead to temporary increases in dissolved oxygen levels. While this can be beneficial for addressing hypoxic conditions, it may also cause stress to aquatic organisms adapted to lower oxygen concentrations. The assessment must consider the rate of oxygen release and its distribution throughout the water column to minimize potential negative impacts on aquatic life.
The decomposition of sodium percarbonate results in the formation of sodium carbonate, which can affect water pH levels. This pH alteration may have implications for aquatic ecosystems, particularly in poorly buffered water bodies. The assessment should include modeling of pH changes over time and space, considering factors such as water volume, flow rates, and existing buffer capacity.
Soil applications of sodium percarbonate require evaluation of its effects on soil chemistry and microbial communities. The oxidizing nature of the compound may temporarily disrupt soil microbial populations, which play crucial roles in nutrient cycling and soil health. Long-term studies are necessary to understand the recovery and potential shifts in microbial community composition following treatment.
The assessment must also consider the fate and transport of sodium percarbonate and its breakdown products in the environment. This includes evaluating the potential for groundwater contamination, especially in areas with high water tables or permeable soils. Additionally, the assessment should examine any potential for bioaccumulation of sodium or other byproducts in the food chain.
Ecotoxicological studies form a critical component of the environmental impact assessment. These studies should encompass a range of organisms representative of the ecosystem, including plants, invertebrates, fish, and potentially higher-order consumers. Acute and chronic toxicity tests, as well as behavioral studies, can provide insights into the potential risks associated with sodium percarbonate use.
Finally, the assessment must consider the broader ecological implications of using sodium percarbonate in restoration projects. This includes evaluating its effectiveness in achieving restoration goals, potential synergistic or antagonistic effects with other environmental factors, and the overall resilience of the ecosystem to the introduced changes. Long-term monitoring plans should be developed to track ecosystem recovery and identify any unforeseen consequences of the treatment.
Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate. The hydrogen peroxide component acts as a powerful oxidizing agent, which is the primary mechanism for its environmental remediation capabilities. However, this oxidizing property also requires careful evaluation of its impact on non-target organisms and ecosystems.
In aquatic environments, the introduction of sodium percarbonate can lead to temporary increases in dissolved oxygen levels. While this can be beneficial for addressing hypoxic conditions, it may also cause stress to aquatic organisms adapted to lower oxygen concentrations. The assessment must consider the rate of oxygen release and its distribution throughout the water column to minimize potential negative impacts on aquatic life.
The decomposition of sodium percarbonate results in the formation of sodium carbonate, which can affect water pH levels. This pH alteration may have implications for aquatic ecosystems, particularly in poorly buffered water bodies. The assessment should include modeling of pH changes over time and space, considering factors such as water volume, flow rates, and existing buffer capacity.
Soil applications of sodium percarbonate require evaluation of its effects on soil chemistry and microbial communities. The oxidizing nature of the compound may temporarily disrupt soil microbial populations, which play crucial roles in nutrient cycling and soil health. Long-term studies are necessary to understand the recovery and potential shifts in microbial community composition following treatment.
The assessment must also consider the fate and transport of sodium percarbonate and its breakdown products in the environment. This includes evaluating the potential for groundwater contamination, especially in areas with high water tables or permeable soils. Additionally, the assessment should examine any potential for bioaccumulation of sodium or other byproducts in the food chain.
Ecotoxicological studies form a critical component of the environmental impact assessment. These studies should encompass a range of organisms representative of the ecosystem, including plants, invertebrates, fish, and potentially higher-order consumers. Acute and chronic toxicity tests, as well as behavioral studies, can provide insights into the potential risks associated with sodium percarbonate use.
Finally, the assessment must consider the broader ecological implications of using sodium percarbonate in restoration projects. This includes evaluating its effectiveness in achieving restoration goals, potential synergistic or antagonistic effects with other environmental factors, and the overall resilience of the ecosystem to the introduced changes. Long-term monitoring plans should be developed to track ecosystem recovery and identify any unforeseen consequences of the treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!