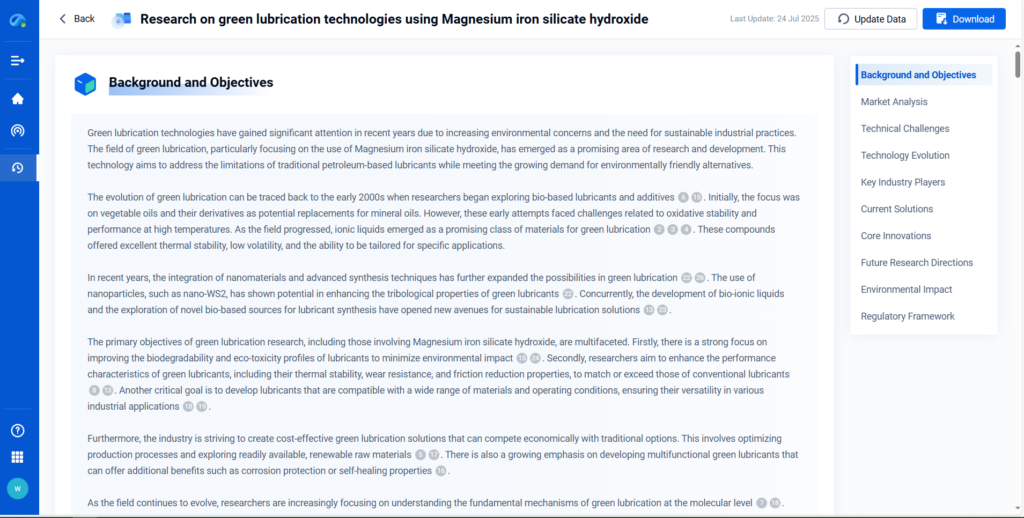
Magnesium Iron Silicate Hydroxide (MSH) is emerging as a highly versatile material bridging tribology, environmental remediation, geoscience, and advanced material research. Its unique layered structure, tunable surface chemistry, and stability under extreme conditions make it indispensable in both industrial and scientific domains. This article delves into MSH’s multifaceted role across several sectors, supported by real-world studies and technical research through PatSnap Eureka AI Agent.
Composition & Structural Properties
MSH is a phyllosilicate mineral composed of magnesium, iron, silicon, oxygen, and hydroxyl groups. Its lamellar morphology allows interlayer ion exchange, adsorption, and excellent dispersibility in both aqueous and non-aqueous systems. These properties underpin its utility in various composite materials, lubricants, and adsorbent structures.
Comparative Advantages & Limitations
Advantages
- Multi-domain Functionality
Magnesium Iron Silicate Hydroxide (MSH) demonstrates remarkable versatility across diverse fields including tribology, environmental remediation, and geosciences. Its layered lamellar structure and chemical composition enable it to function effectively as a friction reducer in lubrication systems, a high-capacity adsorbent for heavy metals in water treatment, and a stable mineral phase under extreme geological pressures. This broad applicability makes MSH a highly attractive multifunctional material. - Thermal and Chemical Stability
MSH retains its structural integrity and performance under a wide range of harsh conditions, including elevated temperatures, high pressure, and chemically aggressive environments. This robustness allows it to maintain lubricating properties in extreme mechanical systems, resist degradation during prolonged water treatment cycles, and remain stable in deep Earth mantle simulations, supporting its use in both industrial and scientific applications. - Environmental Sustainability
As concerns about ecological impact rise, MSH offers a greener alternative to traditional lubricant additives and adsorbents. It can replace toxic or non-biodegradable compounds, reducing environmental pollution. Its capability to adsorb heavy metals from wastewater without releasing secondary contaminants aligns with sustainable water purification goals. Additionally, MSH-based lubricants contribute to energy savings through friction reduction, supporting carbon footprint minimization in mechanical systems. - Nanostructurability and Synergistic Integration
Advances in nanotechnology enable MSH to be engineered at the nanoscale, enhancing its surface area and reactivity. This facilitates synergistic integration with other materials such as MoS₂, graphene, or polymer matrices, resulting in composite materials with superior mechanical, thermal, or adsorptive properties.

Limitations
- Dispersibility Issues in Non-Polar Mediums
Despite excellent dispersibility in aqueous systems, MSH faces challenges in achieving stable dispersion in non-polar oils and lubricants without surface modification or the use of surfactants. This can limit its direct applicability in certain lubricant formulations or hydrophobic composite matrices, requiring additional processing steps. - Synthesis Scalability and Functionalization Challenges
Producing functionally enhanced or nano-engineered MSH variants at industrial scale remains complex and cost-intensive. Achieving consistent particle size, surface chemistry, and structural properties requires precise control over synthesis parameters. Scaling lab-based functionalization methods for commercial applications continues to be an area for development. - Limited Long-Term Bio-Environmental Impact Data
While MSH is generally regarded as environmentally benign, comprehensive studies on its long-term bioaccumulation, biodegradation, and ecological effects are limited. Understanding its behavior over extended periods in natural ecosystems is essential to ensure safe deployment, particularly for large-scale environmental remediation projects. - Potential Mechanical Limitations Under Extreme Wear
Although MSH improves wear resistance, under extreme mechanical stresses or abrasive conditions, the material’s performance may degrade faster than some advanced synthetic additives. Research into composite formulations and protective coatings is ongoing to address these limitations.
Application Domains
1. Tribology & Lubrication
MSH’s low shear strength, chemical stability, and nanostructured morphology enable remarkable friction reduction and wear resistance in both high-pressure and high-temperature lubrication environments.
Related Reports:
- Experimental studies on Magnesium iron silicate hydroxide frictional properties
- High-temperature superlubricity with Magnesium iron silicate composites
- Lubrication efficiency in high-pressure environments with MSH
- Surface characteristics alteration by Magnesium iron silicate hydroxide
- Reducing friction coefficient using Magnesium iron silicate hydroxide
- Green lubrication technologies with Magnesium iron silicate hydroxide
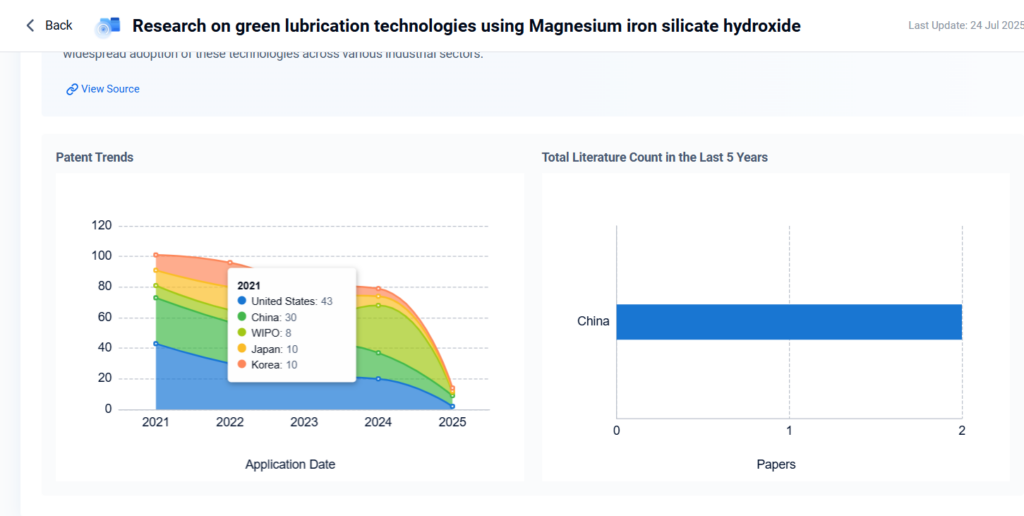
2. Environmental Applications
MSH demonstrates strong affinity for heavy metals and organic pollutants due to its high surface area and ion-exchange capacity, making it an effective adsorbent for wastewater purification.
Related Reports:
- Cadmium ion removal using Magnesium iron silicate hydroxide
- Comparative evaluation of MSH versus traditional adsorbents
- Nanocomposite design using MSH for pollutant capture
- MSH-assisted heavy metal extraction from aqueous solutions
- Performance metrics for MSH-based adsorbents
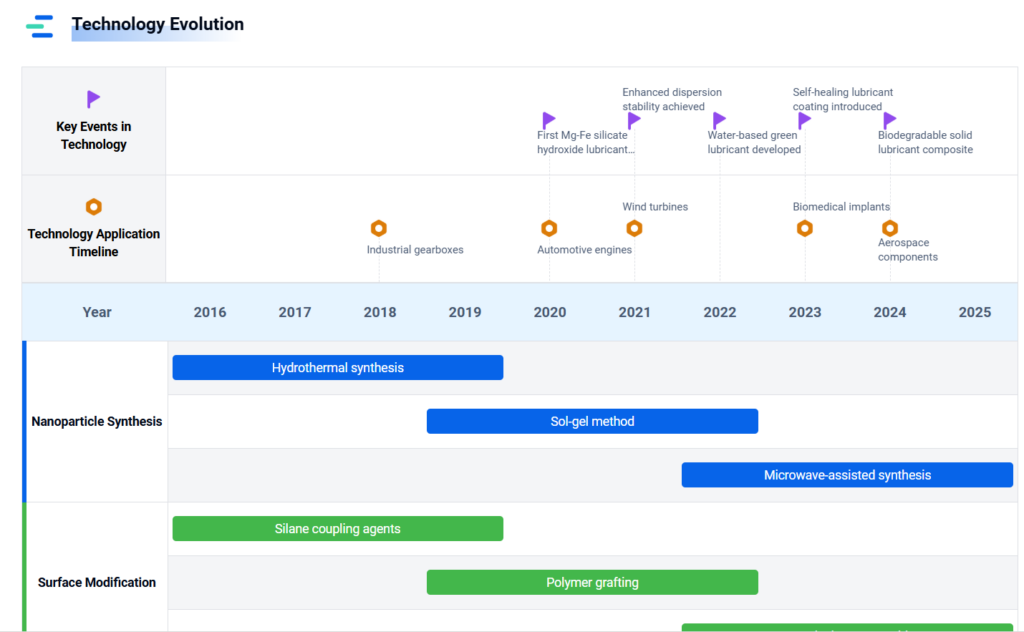
3. Geology & High-Pressure Earth Sciences
In geosciences, MSH offers insights into mantle processes, phase transitions, and mineral stability under extreme pressure. It plays a role in understanding superionic water storage and geochemical cycling.
Related Reports:
- Geological distribution and occurrence of Magnesium iron silicate hydroxide
- High-pressure crystallography of Magnesium iron silicate hydroxide
- Water storage dynamics of MSH under GPa conditions
- MSH’s contribution to deep Earth water reservoirs
- Geological modeling of MSH’s effect on pressure zones

4. Material Synthesis & Advanced Applications
MSH is gaining traction in composite engineering, nanomedicine, and electronics. Its integration enhances material resilience, self-healing properties, and structural integrity across sectors.
Related Reports:
- Synthesis techniques for Magnesium iron silicate hydroxide composites
- Current innovations in MSH solar photovoltaic research
- Exploring semiconductor applications of Magnesium iron silicate hydroxide
5. Interdisciplinary & Frontier Research
Beyond conventional uses, MSH is influencing AI-driven materials design, space exploration, quantum modelling, and nanorobotics, showcasing its potential in next-gen technologies.
Related Reports:
- Quantum studies on MSH high-pressure properties
- Cyber-physical applications derived from MSH materials
- Future prospects in MSH-based geotechnical surveillance
Future Prospects & Research Frontiers
Magnesium Iron Silicate Hydroxide (MSH) stands at the forefront of multiple cutting-edge research domains, with several promising directions emerging:
- Nanostructured MSH Composites
Advances in nanotechnology are enabling precise control over MSH particle size, morphology, and surface chemistry. These nano-engineered composites exhibit enhanced tribological properties, superior adsorption capacity, and improved mechanical strength. Future research will likely focus on tailoring nanoscale features to optimize interactions with polymers, lubricants, and pollutants, broadening the application spectrum. - Energy-Efficient Tribological Systems
The drive for sustainability in mechanical systems has directed attention toward MSH-based lubricants that reduce friction and wear at lower energy costs. Integrating MSH into smart lubrication formulations and coatings promises to extend equipment life and reduce energy consumption, contributing to green manufacturing and transportation technologies. - AI-Assisted Material Design
Machine learning and AI models are increasingly employed to predict and optimize MSH structural properties and functional performance. AI-driven simulations can accelerate discovery of novel MSH formulations with targeted characteristics for specific industrial or environmental applications, shortening R&D cycles and enhancing material innovation. - Quantum Modelling and Deep Earth Simulation
MSH’s unique behavior under extreme pressure and temperature makes it a valuable proxy for investigating superionic phases and water storage in Earth’s mantle. Quantum computational methods are being applied to unravel its high-pressure crystallography and bonding, offering insights into geophysical processes and the planet’s water cycle, with implications for earth sciences and planetary geology. - Sustainable and Scalable Synthesis Approaches
To meet industrial demand, future work must develop scalable, eco-friendly synthesis and functionalization routes that maintain quality and performance. Green chemistry principles will guide this development to ensure minimal environmental impact.
Overall, these frontiers suggest that MSH will play a pivotal role in the next generation of advanced materials, bridging fundamental science and practical applications across diverse sectors.
Conclusion
Magnesium Iron Silicate Hydroxide represents a rare convergence of physical, chemical, and functional traits suited for cross-industry innovation. As research deepens, its integration into sustainable technologies, geological simulations, and material intelligence systems is poised to accelerate.
Ready to uncover more about MSH applications in advanced materials and industrial innovations?
Use PatSnap Eureka AI Agent to explore real-time reports, patent data, and R&D trends powering the future of magnesium silicate technologies.
👉 Book a Eureka demo and unlock the MSH advantage for your next R&D challenge.