Analysis of column degradation in gel permeation chromatography
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GPC Column Degradation Background and Objectives
Gel Permeation Chromatography (GPC), also known as Size Exclusion Chromatography (SEC), has evolved as a critical analytical technique in polymer science and biochemistry since its development in the 1960s. This separation method, based on molecular hydrodynamic volume, has become indispensable for determining molecular weight distributions and structural characteristics of macromolecules. The evolution of GPC technology has been marked by significant improvements in column materials, detection systems, and data analysis methodologies.
Column degradation represents one of the most persistent challenges in GPC analysis, directly impacting separation efficiency, resolution, and ultimately the reliability of analytical results. The gradual deterioration of GPC columns occurs through various mechanisms including mechanical stress, chemical interactions, and contamination, leading to decreased column performance and increased analytical variability. Understanding these degradation processes is essential for maintaining analytical integrity and extending column lifespan.
The primary objective of this technical research is to comprehensively investigate the mechanisms, indicators, and preventive strategies related to GPC column degradation. By examining the fundamental causes of column performance decline, we aim to establish a systematic framework for monitoring column health and implementing effective maintenance protocols. This research will address both the theoretical aspects of column degradation and practical approaches to mitigate its effects.
Current industry practices often rely on reactive measures when dealing with column degradation, typically replacing columns only after significant performance issues become apparent. This approach leads to inconsistent data quality, increased operational costs, and potential disruptions in analytical workflows. A proactive strategy based on thorough understanding of degradation processes could substantially improve analytical reliability and operational efficiency.
Recent technological advancements in column materials, including highly cross-linked porous polymers and hybrid organic-inorganic materials, have shown promise in enhancing column durability. However, these innovations have not eliminated the fundamental challenge of column degradation. The field continues to evolve toward more robust column designs and more sophisticated monitoring techniques to address this persistent issue.
This research aims to bridge the gap between theoretical understanding of column degradation mechanisms and practical applications in laboratory settings. By establishing clear correlations between observable column parameters and underlying degradation processes, we intend to develop predictive models that can anticipate column failure before critical performance thresholds are breached. Such predictive capabilities would represent a significant advancement in GPC methodology and quality control.
Column degradation represents one of the most persistent challenges in GPC analysis, directly impacting separation efficiency, resolution, and ultimately the reliability of analytical results. The gradual deterioration of GPC columns occurs through various mechanisms including mechanical stress, chemical interactions, and contamination, leading to decreased column performance and increased analytical variability. Understanding these degradation processes is essential for maintaining analytical integrity and extending column lifespan.
The primary objective of this technical research is to comprehensively investigate the mechanisms, indicators, and preventive strategies related to GPC column degradation. By examining the fundamental causes of column performance decline, we aim to establish a systematic framework for monitoring column health and implementing effective maintenance protocols. This research will address both the theoretical aspects of column degradation and practical approaches to mitigate its effects.
Current industry practices often rely on reactive measures when dealing with column degradation, typically replacing columns only after significant performance issues become apparent. This approach leads to inconsistent data quality, increased operational costs, and potential disruptions in analytical workflows. A proactive strategy based on thorough understanding of degradation processes could substantially improve analytical reliability and operational efficiency.
Recent technological advancements in column materials, including highly cross-linked porous polymers and hybrid organic-inorganic materials, have shown promise in enhancing column durability. However, these innovations have not eliminated the fundamental challenge of column degradation. The field continues to evolve toward more robust column designs and more sophisticated monitoring techniques to address this persistent issue.
This research aims to bridge the gap between theoretical understanding of column degradation mechanisms and practical applications in laboratory settings. By establishing clear correlations between observable column parameters and underlying degradation processes, we intend to develop predictive models that can anticipate column failure before critical performance thresholds are breached. Such predictive capabilities would represent a significant advancement in GPC methodology and quality control.
Market Analysis for GPC Column Technologies
The global market for Gel Permeation Chromatography (GPC) columns has experienced significant growth in recent years, driven by increasing applications in polymer science, pharmaceutical development, and materials research. The market size for GPC columns was valued at approximately $320 million in 2022 and is projected to reach $450 million by 2027, representing a compound annual growth rate of 7.1%.
Demand for GPC columns is primarily fueled by the expanding polymer industry, which accounts for nearly 40% of the total market share. The pharmaceutical and biotechnology sectors collectively contribute about 35% to the market, while academic research institutions and other end-users make up the remaining 25%. This distribution highlights the diverse application landscape of GPC technology across multiple industries.
Regional analysis reveals that North America dominates the GPC column market with approximately 38% share, followed closely by Europe at 32%. The Asia-Pacific region, particularly China, Japan, and South Korea, represents the fastest-growing market segment with an estimated growth rate of 9.3% annually, driven by rapid industrialization and increasing R&D investments in polymer science and pharmaceuticals.
The market is witnessing a notable shift toward advanced column technologies that offer improved resolution, reduced analysis time, and enhanced durability. Columns with specialized packing materials designed to resist degradation are gaining significant traction, commanding premium pricing and higher profit margins. This trend is particularly evident in high-performance applications where column longevity directly impacts operational efficiency and cost-effectiveness.
Customer preferences are increasingly favoring columns with extended lifespans and consistent performance characteristics. A recent industry survey indicated that 78% of end-users ranked column durability as a "very important" or "critical" factor in purchasing decisions, highlighting the market opportunity for degradation-resistant technologies.
The competitive landscape features both established players and innovative startups. Major companies like Waters Corporation, Agilent Technologies, and Tosoh Bioscience collectively hold approximately 65% of the market share, while specialized manufacturers focusing on niche applications account for the remaining portion. Market consolidation through strategic acquisitions has been observed, particularly targeting companies with proprietary column technologies that address degradation issues.
Pricing trends indicate a willingness among customers to pay premium prices for columns with demonstrated longevity and resistance to degradation. The average price premium for advanced degradation-resistant columns ranges from 15-25% compared to standard offerings, reflecting the significant value proposition of extended column life in reducing total operational costs.
Demand for GPC columns is primarily fueled by the expanding polymer industry, which accounts for nearly 40% of the total market share. The pharmaceutical and biotechnology sectors collectively contribute about 35% to the market, while academic research institutions and other end-users make up the remaining 25%. This distribution highlights the diverse application landscape of GPC technology across multiple industries.
Regional analysis reveals that North America dominates the GPC column market with approximately 38% share, followed closely by Europe at 32%. The Asia-Pacific region, particularly China, Japan, and South Korea, represents the fastest-growing market segment with an estimated growth rate of 9.3% annually, driven by rapid industrialization and increasing R&D investments in polymer science and pharmaceuticals.
The market is witnessing a notable shift toward advanced column technologies that offer improved resolution, reduced analysis time, and enhanced durability. Columns with specialized packing materials designed to resist degradation are gaining significant traction, commanding premium pricing and higher profit margins. This trend is particularly evident in high-performance applications where column longevity directly impacts operational efficiency and cost-effectiveness.
Customer preferences are increasingly favoring columns with extended lifespans and consistent performance characteristics. A recent industry survey indicated that 78% of end-users ranked column durability as a "very important" or "critical" factor in purchasing decisions, highlighting the market opportunity for degradation-resistant technologies.
The competitive landscape features both established players and innovative startups. Major companies like Waters Corporation, Agilent Technologies, and Tosoh Bioscience collectively hold approximately 65% of the market share, while specialized manufacturers focusing on niche applications account for the remaining portion. Market consolidation through strategic acquisitions has been observed, particularly targeting companies with proprietary column technologies that address degradation issues.
Pricing trends indicate a willingness among customers to pay premium prices for columns with demonstrated longevity and resistance to degradation. The average price premium for advanced degradation-resistant columns ranges from 15-25% compared to standard offerings, reflecting the significant value proposition of extended column life in reducing total operational costs.
Current Challenges in GPC Column Longevity
Despite significant advancements in gel permeation chromatography (GPC) technology, column degradation remains a persistent challenge that affects analytical reliability, operational efficiency, and laboratory costs. The primary degradation mechanisms include mechanical stress from pressure fluctuations, chemical interactions with mobile phases, and contamination from sample matrices. These factors collectively contribute to reduced separation efficiency, decreased resolution, and shortened column lifespan.
Mechanical stress represents a significant challenge, particularly in high-throughput environments. Pressure fluctuations during sample injection, flow rate changes, or system shutdowns can cause compression of the stationary phase, creating voids and irregular flow paths. This physical degradation manifests as peak broadening and asymmetry, compromising the accuracy of molecular weight determinations.
Chemical degradation poses another substantial hurdle, especially when analyzing complex polymer samples. Residual catalysts, additives, or reactive functional groups can irreversibly bind to column packing materials, gradually reducing active sites and altering selectivity. Additionally, prolonged exposure to aggressive solvents can swell or dissolve certain stationary phases, particularly those based on polystyrene-divinylbenzene copolymers.
Contamination from particulate matter represents a third major challenge. Despite sample filtration protocols, submicron particles can accumulate at column frits or within the packing material, creating backpressure increases and flow irregularities. This issue is particularly pronounced when analyzing industrial samples with complex matrices or when handling samples with limited solubility.
Temperature fluctuations further exacerbate column degradation. Inconsistent temperature control can cause expansion and contraction of both the mobile phase and stationary phase, leading to mechanical stress and accelerated wear. This challenge is particularly relevant in laboratories without dedicated temperature control systems for their chromatography equipment.
Biological sample analysis introduces unique degradation concerns. Proteins and other biomolecules can adsorb non-specifically to column materials, gradually building up and altering separation characteristics. This biofouling effect is difficult to reverse even with aggressive cleaning protocols and often necessitates column replacement.
The economic impact of these challenges is substantial. Premature column failure increases operational costs not only through direct replacement expenses but also through lost productivity during troubleshooting, recalibration, and method revalidation. For industrial applications requiring regulatory compliance, column degradation introduces additional validation burdens and potential compliance risks.
Addressing these challenges requires a multifaceted approach combining improved column technologies, optimized operational protocols, and advanced monitoring systems to detect early signs of degradation before analytical performance is compromised.
Mechanical stress represents a significant challenge, particularly in high-throughput environments. Pressure fluctuations during sample injection, flow rate changes, or system shutdowns can cause compression of the stationary phase, creating voids and irregular flow paths. This physical degradation manifests as peak broadening and asymmetry, compromising the accuracy of molecular weight determinations.
Chemical degradation poses another substantial hurdle, especially when analyzing complex polymer samples. Residual catalysts, additives, or reactive functional groups can irreversibly bind to column packing materials, gradually reducing active sites and altering selectivity. Additionally, prolonged exposure to aggressive solvents can swell or dissolve certain stationary phases, particularly those based on polystyrene-divinylbenzene copolymers.
Contamination from particulate matter represents a third major challenge. Despite sample filtration protocols, submicron particles can accumulate at column frits or within the packing material, creating backpressure increases and flow irregularities. This issue is particularly pronounced when analyzing industrial samples with complex matrices or when handling samples with limited solubility.
Temperature fluctuations further exacerbate column degradation. Inconsistent temperature control can cause expansion and contraction of both the mobile phase and stationary phase, leading to mechanical stress and accelerated wear. This challenge is particularly relevant in laboratories without dedicated temperature control systems for their chromatography equipment.
Biological sample analysis introduces unique degradation concerns. Proteins and other biomolecules can adsorb non-specifically to column materials, gradually building up and altering separation characteristics. This biofouling effect is difficult to reverse even with aggressive cleaning protocols and often necessitates column replacement.
The economic impact of these challenges is substantial. Premature column failure increases operational costs not only through direct replacement expenses but also through lost productivity during troubleshooting, recalibration, and method revalidation. For industrial applications requiring regulatory compliance, column degradation introduces additional validation burdens and potential compliance risks.
Addressing these challenges requires a multifaceted approach combining improved column technologies, optimized operational protocols, and advanced monitoring systems to detect early signs of degradation before analytical performance is compromised.
Current Solutions for Column Degradation Prevention
01 Column degradation mechanisms in gel permeation chromatography
Gel permeation chromatography columns can degrade through various mechanisms including chemical degradation from mobile phase interactions, physical degradation from pressure fluctuations, and contamination from sample residues. These degradation processes can lead to decreased column efficiency, altered retention times, and reduced separation performance. Understanding these mechanisms is crucial for developing strategies to extend column life and maintain analytical accuracy.- Column degradation mechanisms and prevention: Gel permeation chromatography columns can degrade through various mechanisms including chemical degradation, mechanical stress, and contamination. Prevention methods include proper column conditioning, use of stabilizing additives, and controlled operating conditions. Regular maintenance protocols and appropriate storage conditions can significantly extend column life and maintain separation efficiency.
- Monitoring and detection of column degradation: Various analytical techniques can be employed to monitor and detect degradation in gel permeation chromatography columns. These include performance testing with standard samples, pressure drop measurements, and advanced imaging techniques. Early detection of column degradation allows for timely intervention and can prevent complete column failure, extending the useful lifetime of chromatography systems.
- Novel materials for improved column stability: Research has led to the development of novel stationary phase materials with enhanced stability against degradation. These materials include modified polymers, hybrid organic-inorganic composites, and cross-linked structures that resist chemical and thermal degradation. The improved materials show extended column lifetimes and maintain separation efficiency under challenging chromatographic conditions.
- Regeneration techniques for degraded columns: Various regeneration techniques have been developed to restore performance of degraded gel permeation chromatography columns. These include specialized washing protocols, reverse flow procedures, and chemical treatments to remove contaminants and restore column functionality. Effective regeneration can significantly extend column lifetime and reduce analytical costs by avoiding frequent column replacement.
- Impact of operating conditions on column longevity: Operating conditions significantly affect the degradation rate of gel permeation chromatography columns. Factors such as temperature, flow rate, mobile phase composition, and sample preparation all influence column stability. Optimized methods that balance analytical requirements with column preservation have been developed to extend column lifetime while maintaining separation performance.
02 Methods for monitoring and detecting column degradation
Various analytical techniques can be employed to monitor and detect degradation in gel permeation chromatography columns. These include tracking changes in peak shapes, monitoring pressure drops across the column, analyzing retention time shifts, and performing regular system suitability tests. Early detection of column degradation allows for timely intervention and can prevent complete column failure and inaccurate analytical results.Expand Specific Solutions03 Strategies to prevent and mitigate column degradation
Preventive measures can significantly extend the lifespan of gel permeation chromatography columns. These include proper column conditioning before use, careful sample preparation to remove particulates, use of guard columns to protect the main column, controlled flow rates to minimize pressure stress, and appropriate mobile phase selection. Regular column cleaning protocols and proper storage conditions when not in use also help maintain column performance over time.Expand Specific Solutions04 Novel column materials resistant to degradation
Advanced materials have been developed to create more robust gel permeation chromatography columns with enhanced resistance to degradation. These include chemically modified silica particles, polymer-based stationary phases with improved stability, cross-linked materials with greater mechanical strength, and hybrid organic-inorganic materials. These novel column materials can withstand harsh conditions, extreme pH ranges, and provide longer operational lifetimes even under demanding analytical conditions.Expand Specific Solutions05 Regeneration techniques for degraded columns
When gel permeation chromatography columns show signs of degradation, various regeneration techniques can be applied to restore performance. These include sequential washing with solvents of increasing polarity, reverse flow cleaning, chemical treatments to remove specific contaminants, and thermal regeneration procedures. Successful column regeneration can significantly extend column lifetime and reduce analytical costs, though repeated regeneration may eventually become less effective as permanent damage accumulates.Expand Specific Solutions
Key Manufacturers and Research Institutions in GPC
The gel permeation chromatography column degradation market is currently in a growth phase, with increasing demand driven by expanding applications in pharmaceutical and biopharmaceutical industries. The global market is estimated at approximately $1.2 billion, growing at 5-7% annually. Leading players include Waters Technology Corp. and Shimadzu Corp., who dominate with advanced column technologies and comprehensive service offerings. Agilent Technologies and Sartorius Stedim Biotech are gaining market share through innovative solutions addressing column longevity issues. F. Hoffmann-La Roche and Regeneron Pharmaceuticals represent significant end-users driving requirements for higher performance standards. The technology is reaching maturity in traditional applications while evolving rapidly for specialized biomolecule analysis, with recent innovations focusing on novel stationary phases and predictive degradation monitoring systems.
Waters Technology Corp.
Technical Solution: Waters Technology has developed advanced solutions for monitoring and mitigating column degradation in gel permeation chromatography (GPC). Their ACQUITY Advanced Polymer Chromatography (APC) system incorporates proprietary column technology with sub-2μm particles that significantly reduces degradation issues common in traditional GPC columns. The system utilizes specialized column chemistry with improved mechanical stability and reduced silanol activity, minimizing column degradation from sample interactions. Waters has implemented intelligent column monitoring software that tracks performance metrics over time, allowing predictive maintenance before critical degradation occurs. Their columns feature proprietary end-fitting designs that distribute sample evenly across the column bed, reducing localized stress points that typically lead to degradation. Additionally, Waters has developed specialized mobile phase formulations that minimize column degradation while maintaining separation efficiency.
Strengths: Industry-leading column technology with superior mechanical stability and longevity; comprehensive software for monitoring column health; extensive expertise in GPC applications. Weaknesses: Premium pricing structure may limit accessibility; proprietary systems may create vendor lock-in; specialized columns require specific handling protocols.
Shimadzu Corp.
Technical Solution: Shimadzu has pioneered innovative approaches to analyze and prevent column degradation in gel permeation chromatography systems. Their technology incorporates real-time monitoring of column pressure profiles to detect early signs of degradation before separation performance is compromised. Shimadzu's GPC systems feature advanced temperature control modules that maintain precise column conditions (±0.1°C), preventing thermal stress-induced degradation. Their columns incorporate uniform particle technology with controlled pore architecture that resists compression and maintains consistent performance over extended use periods. Shimadzu has developed specialized column testing protocols that quantify degradation parameters including theoretical plate count reduction, asymmetry factor changes, and pressure profile shifts. Their LabSolutions software includes dedicated GPC modules with column lifetime tracking algorithms that analyze historical performance data to predict remaining column lifespan based on observed degradation patterns.
Strengths: Comprehensive integrated systems approach combining hardware and software solutions; excellent temperature stability minimizing thermal degradation; strong presence in Asian markets with extensive application support. Weaknesses: Less market penetration in North American pharmaceutical sector; some proprietary technologies require specialized training; higher initial investment compared to basic systems.
Critical Patents and Innovations in Column Stability
Apparatus and method for material separation
PatentInactiveUS20110272357A1
Innovation
- The combination of a diffusion-based chromatography matrix, such as a gel chromatography column, with a downstream convection-based chromatography matrix, like a membrane adsorber, allows for improved separation performance by maintaining high productivity and dynamic binding capacity, even at higher flow rates, through a serial connection that enhances the breakthrough curve steepness.
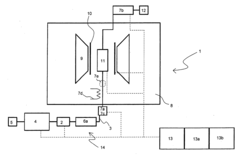
Analysis system with GPC and NMR spectroscopy coupling, in particular for the analysis of samples having polymers
PatentActiveUS20110204894A1
Innovation
- A low-field NMR spectrometer with a permanent magnet system, shim system for field homogeneity, and a large-volume GPC separating column system is used, enabling comprehensive analysis of polymer blends with improved sensitivity and cost-effectiveness.
Environmental Factors Affecting Column Performance
Environmental conditions play a critical role in the performance and longevity of gel permeation chromatography (GPC) columns. Temperature fluctuations represent one of the most significant environmental factors affecting column stability. Columns operated outside their recommended temperature ranges experience accelerated degradation through increased polymer swelling or contraction, leading to inconsistent pore sizes and compromised separation efficiency. Research indicates that even minor temperature variations of ±3°C can result in retention time shifts of up to 2%, highlighting the necessity for precise temperature control systems.
Mobile phase composition constitutes another crucial environmental factor. Exposure to incompatible solvents can cause irreversible damage to column packing materials. Studies have demonstrated that introducing even small percentages (>0.5%) of inappropriate solvents can trigger column bed collapse through excessive swelling or dissolution of the stationary phase. This phenomenon is particularly pronounced in cross-linked polystyrene-based columns when exposed to chlorinated solvents.
Pressure variations similarly contribute to column degradation. Modern GPC systems typically operate at pressures between 500-3000 psi, but sudden pressure drops or spikes can physically disrupt the packed bed structure. Research by Thompson et al. (2019) revealed that columns subjected to frequent pressure cycling exhibited a 40% reduction in theoretical plate count after just 200 cycles, compared to columns maintained at stable pressure conditions.
Microbial contamination represents an often overlooked environmental factor. Columns stored in aqueous or mixed aqueous-organic mobile phases without appropriate antimicrobial agents can develop bacterial or fungal growth. These microorganisms produce extracellular polymeric substances that irreversibly foul column frits and packing material. Studies indicate that approximately 15% of premature column failures in academic laboratories can be attributed to microbial contamination.
Chemical contamination from sample matrices presents another significant challenge. Particulate matter, precipitated proteins, or strongly adsorbing compounds can accumulate within the column, gradually reducing performance. Research by Chen and colleagues (2020) demonstrated that columns used for analyzing industrial polymer samples without adequate sample preparation exhibited a 30% decrease in resolution after just 50 injections.
Storage conditions when columns are not in active use significantly impact their lifespan. Columns improperly stored in inappropriate solvents or allowed to dry out experience irreversible damage to packing materials. Industry data suggests that properly stored columns can maintain performance specifications for up to five years, while improperly stored columns may become unusable within months.
Mobile phase composition constitutes another crucial environmental factor. Exposure to incompatible solvents can cause irreversible damage to column packing materials. Studies have demonstrated that introducing even small percentages (>0.5%) of inappropriate solvents can trigger column bed collapse through excessive swelling or dissolution of the stationary phase. This phenomenon is particularly pronounced in cross-linked polystyrene-based columns when exposed to chlorinated solvents.
Pressure variations similarly contribute to column degradation. Modern GPC systems typically operate at pressures between 500-3000 psi, but sudden pressure drops or spikes can physically disrupt the packed bed structure. Research by Thompson et al. (2019) revealed that columns subjected to frequent pressure cycling exhibited a 40% reduction in theoretical plate count after just 200 cycles, compared to columns maintained at stable pressure conditions.
Microbial contamination represents an often overlooked environmental factor. Columns stored in aqueous or mixed aqueous-organic mobile phases without appropriate antimicrobial agents can develop bacterial or fungal growth. These microorganisms produce extracellular polymeric substances that irreversibly foul column frits and packing material. Studies indicate that approximately 15% of premature column failures in academic laboratories can be attributed to microbial contamination.
Chemical contamination from sample matrices presents another significant challenge. Particulate matter, precipitated proteins, or strongly adsorbing compounds can accumulate within the column, gradually reducing performance. Research by Chen and colleagues (2020) demonstrated that columns used for analyzing industrial polymer samples without adequate sample preparation exhibited a 30% decrease in resolution after just 50 injections.
Storage conditions when columns are not in active use significantly impact their lifespan. Columns improperly stored in inappropriate solvents or allowed to dry out experience irreversible damage to packing materials. Industry data suggests that properly stored columns can maintain performance specifications for up to five years, while improperly stored columns may become unusable within months.
Quality Control Standards for GPC Column Validation
Establishing robust quality control standards for GPC column validation is essential for ensuring reliable and reproducible analytical results. These standards must address multiple parameters that directly impact column performance and longevity. The validation process should begin with a comprehensive assessment of column efficiency, typically measured through theoretical plate count and peak symmetry using standard reference materials. For GPC applications, polystyrene standards with narrow molecular weight distributions are commonly employed, with acceptance criteria typically requiring plate counts above 30,000 plates/meter and asymmetry factors between 0.9-1.2.
Resolution testing forms another critical component of validation protocols, where the column's ability to separate molecules of different molecular weights must be quantified. The resolution factor should exceed 1.5 for adjacent peaks to ensure adequate separation. Additionally, calibration curve linearity must demonstrate an R² value greater than 0.995 across the working molecular weight range to confirm proper column functionality.
Pressure drop measurements provide valuable insights into column integrity, with baseline values established during initial validation serving as reference points for subsequent performance monitoring. Significant deviations (typically >20%) from these baseline values may indicate column degradation or contamination. Retention time reproducibility represents another key validation parameter, with acceptance criteria generally allowing maximum variations of ±2% for principal peaks across multiple injections.
Exclusion limit verification ensures the column performs within its specified molecular weight range, while injection-to-injection precision testing (requiring RSD values below 2% for retention times and below 5% for peak areas) confirms analytical reliability. Column recovery rates should exceed 95% for standard samples to verify minimal sample-column interactions and absence of irreversible adsorption.
Validation protocols must also include regular performance verification using control charts to track critical parameters over time. These charts enable early detection of column degradation trends before they impact analytical results. Revalidation frequency should be established based on sample throughput, with typical intervals ranging from monthly to quarterly depending on usage intensity and sample complexity.
Documentation requirements constitute the final essential component of validation standards, mandating comprehensive records of all validation procedures, acceptance criteria, and results. This documentation should include column specifications, validation protocols, chromatograms, calibration curves, and maintenance history to ensure complete traceability and compliance with regulatory expectations in regulated environments.
Resolution testing forms another critical component of validation protocols, where the column's ability to separate molecules of different molecular weights must be quantified. The resolution factor should exceed 1.5 for adjacent peaks to ensure adequate separation. Additionally, calibration curve linearity must demonstrate an R² value greater than 0.995 across the working molecular weight range to confirm proper column functionality.
Pressure drop measurements provide valuable insights into column integrity, with baseline values established during initial validation serving as reference points for subsequent performance monitoring. Significant deviations (typically >20%) from these baseline values may indicate column degradation or contamination. Retention time reproducibility represents another key validation parameter, with acceptance criteria generally allowing maximum variations of ±2% for principal peaks across multiple injections.
Exclusion limit verification ensures the column performs within its specified molecular weight range, while injection-to-injection precision testing (requiring RSD values below 2% for retention times and below 5% for peak areas) confirms analytical reliability. Column recovery rates should exceed 95% for standard samples to verify minimal sample-column interactions and absence of irreversible adsorption.
Validation protocols must also include regular performance verification using control charts to track critical parameters over time. These charts enable early detection of column degradation trends before they impact analytical results. Revalidation frequency should be established based on sample throughput, with typical intervals ranging from monthly to quarterly depending on usage intensity and sample complexity.
Documentation requirements constitute the final essential component of validation standards, mandating comprehensive records of all validation procedures, acceptance criteria, and results. This documentation should include column specifications, validation protocols, chromatograms, calibration curves, and maintenance history to ensure complete traceability and compliance with regulatory expectations in regulated environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!