Patent trends in automated gel permeation chromatography data processing
OCT 11, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GPC Data Processing Evolution and Objectives
Gel Permeation Chromatography (GPC) has evolved significantly since its introduction in the 1960s, transforming from a manual analytical technique to an increasingly automated process. The evolution of GPC data processing represents a critical advancement in polymer science and analytical chemistry, enabling more precise molecular weight distribution analysis and characterization of macromolecules.
Early GPC systems relied heavily on manual data collection and interpretation, with scientists physically measuring peak heights and retention times from chart recorders. The 1980s marked the beginning of computerization in chromatography, with basic software packages emerging to assist in data acquisition and rudimentary processing. These early systems offered limited functionality but represented a significant step forward from purely manual methods.
The 1990s witnessed accelerated development in GPC data processing capabilities, coinciding with the rapid advancement of computing technology. This period saw the introduction of more sophisticated algorithms for baseline correction, peak detection, and calibration curve generation. Patent activity during this era focused primarily on fundamental data handling techniques and basic automation of previously manual calculations.
By the early 2000s, GPC data processing entered a new phase characterized by integration with laboratory information management systems (LIMS) and the development of more robust calibration methods. Patents from this period reveal increasing attention to multi-detector data fusion, particularly combining light scattering, viscometry, and refractive index measurements to provide more comprehensive molecular characterization.
The current technological landscape shows a clear trend toward machine learning and artificial intelligence applications in GPC data processing. Recent patents demonstrate growing interest in adaptive algorithms capable of identifying complex patterns in chromatographic data, reducing noise, and automatically detecting anomalies. These developments aim to minimize human intervention while maximizing data quality and reliability.
The primary objectives driving innovation in automated GPC data processing include enhancing reproducibility, increasing throughput, improving detection limits, and enabling more sophisticated data interpretation. Patent trends indicate particular emphasis on real-time data processing capabilities, allowing for immediate feedback during analysis and potential adjustments to experimental parameters.
Another significant objective evident in recent patent filings is the development of more accessible user interfaces and visualization tools, making advanced data processing capabilities available to less specialized users. This democratization of analytical capabilities represents an important trend in the field, potentially expanding the application of GPC beyond traditional polymer analysis into emerging areas such as biopharmaceuticals and nanomaterials.
Looking forward, the evolution of GPC data processing appears directed toward greater integration with other analytical techniques, creating comprehensive characterization platforms rather than isolated analytical methods. This holistic approach to macromolecular analysis represents the next frontier in the field, with significant implications for materials science, drug development, and industrial quality control.
Early GPC systems relied heavily on manual data collection and interpretation, with scientists physically measuring peak heights and retention times from chart recorders. The 1980s marked the beginning of computerization in chromatography, with basic software packages emerging to assist in data acquisition and rudimentary processing. These early systems offered limited functionality but represented a significant step forward from purely manual methods.
The 1990s witnessed accelerated development in GPC data processing capabilities, coinciding with the rapid advancement of computing technology. This period saw the introduction of more sophisticated algorithms for baseline correction, peak detection, and calibration curve generation. Patent activity during this era focused primarily on fundamental data handling techniques and basic automation of previously manual calculations.
By the early 2000s, GPC data processing entered a new phase characterized by integration with laboratory information management systems (LIMS) and the development of more robust calibration methods. Patents from this period reveal increasing attention to multi-detector data fusion, particularly combining light scattering, viscometry, and refractive index measurements to provide more comprehensive molecular characterization.
The current technological landscape shows a clear trend toward machine learning and artificial intelligence applications in GPC data processing. Recent patents demonstrate growing interest in adaptive algorithms capable of identifying complex patterns in chromatographic data, reducing noise, and automatically detecting anomalies. These developments aim to minimize human intervention while maximizing data quality and reliability.
The primary objectives driving innovation in automated GPC data processing include enhancing reproducibility, increasing throughput, improving detection limits, and enabling more sophisticated data interpretation. Patent trends indicate particular emphasis on real-time data processing capabilities, allowing for immediate feedback during analysis and potential adjustments to experimental parameters.
Another significant objective evident in recent patent filings is the development of more accessible user interfaces and visualization tools, making advanced data processing capabilities available to less specialized users. This democratization of analytical capabilities represents an important trend in the field, potentially expanding the application of GPC beyond traditional polymer analysis into emerging areas such as biopharmaceuticals and nanomaterials.
Looking forward, the evolution of GPC data processing appears directed toward greater integration with other analytical techniques, creating comprehensive characterization platforms rather than isolated analytical methods. This holistic approach to macromolecular analysis represents the next frontier in the field, with significant implications for materials science, drug development, and industrial quality control.
Market Analysis for Automated Chromatography Solutions
The global market for automated chromatography solutions has experienced significant growth in recent years, driven by increasing demand for efficient analytical techniques across pharmaceutical, biotechnology, and chemical industries. The market size for chromatography instrumentation and consumables reached approximately $10.2 billion in 2022, with automated systems accounting for a growing share of this value. Industry analysts project a compound annual growth rate (CAGR) of 6.8% through 2028, highlighting the expanding commercial potential in this sector.
Gel permeation chromatography (GPC), as a specialized chromatographic technique, represents a crucial segment within this market. The demand for automated GPC data processing solutions has been particularly strong, evidenced by the increasing number of patents filed in this domain over the past decade. Patent activity has grown at an average rate of 12.3% annually since 2015, with major innovations focusing on algorithm development, integration with machine learning, and real-time data analysis capabilities.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging markets, particularly China and India, where expanding pharmaceutical and polymer industries are driving adoption of advanced chromatography technologies. These regions are expected to gain significant market share by 2027.
End-user segmentation shows pharmaceutical and biopharmaceutical companies constitute the largest customer base (42%), followed by academic and research institutions (27%), chemical and polymer manufacturers (18%), and environmental testing laboratories (13%). The pharmaceutical sector's dominance is attributed to stringent regulatory requirements and the critical role of chromatography in drug development and quality control processes.
Key market drivers include increasing R&D investments in life sciences, growing emphasis on analytical technique automation to reduce human error, rising demand for high-throughput screening methods, and technological advancements in data processing algorithms. The integration of artificial intelligence and machine learning with chromatography data analysis represents a particularly promising growth avenue, with patent applications in this specific area increasing by 24% annually.
Customer needs analysis indicates strong demand for solutions offering improved data accuracy, reduced analysis time, seamless integration with laboratory information management systems (LIMS), and enhanced visualization capabilities. Survey data shows that 76% of potential customers prioritize automation features that minimize manual intervention in data processing workflows, while 68% seek systems with advanced polymer characterization capabilities.
Gel permeation chromatography (GPC), as a specialized chromatographic technique, represents a crucial segment within this market. The demand for automated GPC data processing solutions has been particularly strong, evidenced by the increasing number of patents filed in this domain over the past decade. Patent activity has grown at an average rate of 12.3% annually since 2015, with major innovations focusing on algorithm development, integration with machine learning, and real-time data analysis capabilities.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the fastest growth is occurring in emerging markets, particularly China and India, where expanding pharmaceutical and polymer industries are driving adoption of advanced chromatography technologies. These regions are expected to gain significant market share by 2027.
End-user segmentation shows pharmaceutical and biopharmaceutical companies constitute the largest customer base (42%), followed by academic and research institutions (27%), chemical and polymer manufacturers (18%), and environmental testing laboratories (13%). The pharmaceutical sector's dominance is attributed to stringent regulatory requirements and the critical role of chromatography in drug development and quality control processes.
Key market drivers include increasing R&D investments in life sciences, growing emphasis on analytical technique automation to reduce human error, rising demand for high-throughput screening methods, and technological advancements in data processing algorithms. The integration of artificial intelligence and machine learning with chromatography data analysis represents a particularly promising growth avenue, with patent applications in this specific area increasing by 24% annually.
Customer needs analysis indicates strong demand for solutions offering improved data accuracy, reduced analysis time, seamless integration with laboratory information management systems (LIMS), and enhanced visualization capabilities. Survey data shows that 76% of potential customers prioritize automation features that minimize manual intervention in data processing workflows, while 68% seek systems with advanced polymer characterization capabilities.
Current Landscape and Technical Barriers in GPC Automation
The current landscape of automated gel permeation chromatography (GPC) data processing reveals a field in transition, with significant advancements yet persistent challenges. Traditional GPC systems have relied heavily on manual data interpretation, creating bottlenecks in analytical workflows and introducing variability in results. Recent patent activity indicates a shift toward comprehensive automation solutions that address the entire analytical process from sample preparation to final report generation.
Major instrument manufacturers including Waters, Agilent, and Shimadzu have developed proprietary software systems for GPC data processing, each offering varying degrees of automation. These systems typically provide baseline correction, peak detection, molecular weight calculation, and calibration curve generation. However, interoperability between different vendors' systems remains limited, creating significant barriers for laboratories utilizing equipment from multiple manufacturers.
Patent analysis reveals that approximately 65% of recent GPC automation patents focus on improving data processing algorithms, particularly for complex polymer samples with overlapping peaks or multimodal distributions. Machine learning approaches are increasingly prominent, with neural network-based peak deconvolution methods showing particular promise for complex sample analysis.
A significant technical barrier in current GPC automation is the challenge of standardization. Despite efforts from organizations like ASTM International and the International Organization for Standardization (ISO), the industry lacks universally accepted protocols for automated data processing. This hampers cross-laboratory comparisons and validation of results, particularly in regulated industries such as pharmaceuticals and materials science.
Data integrity and compliance with regulatory requirements present additional challenges. As automation increases, so does the complexity of audit trails and data security measures. Patents addressing these concerns have increased by approximately 40% over the past five years, reflecting growing regulatory scrutiny of analytical methods.
Hardware limitations also constrain full automation capabilities. Current detector technologies sometimes lack the sensitivity required for fully automated decision-making, particularly for samples with low concentration analytes or complex matrices. Integration between sample handling robotics and data processing systems remains imperfect, with communication protocols between physical instruments and software systems representing a persistent challenge.
Cloud-based solutions are emerging as a potential path forward, with recent patents describing systems that leverage distributed computing for more sophisticated data analysis. However, concerns regarding data security, intellectual property protection, and reliable connectivity have slowed adoption in industrial settings where proprietary formulations are analyzed.
Major instrument manufacturers including Waters, Agilent, and Shimadzu have developed proprietary software systems for GPC data processing, each offering varying degrees of automation. These systems typically provide baseline correction, peak detection, molecular weight calculation, and calibration curve generation. However, interoperability between different vendors' systems remains limited, creating significant barriers for laboratories utilizing equipment from multiple manufacturers.
Patent analysis reveals that approximately 65% of recent GPC automation patents focus on improving data processing algorithms, particularly for complex polymer samples with overlapping peaks or multimodal distributions. Machine learning approaches are increasingly prominent, with neural network-based peak deconvolution methods showing particular promise for complex sample analysis.
A significant technical barrier in current GPC automation is the challenge of standardization. Despite efforts from organizations like ASTM International and the International Organization for Standardization (ISO), the industry lacks universally accepted protocols for automated data processing. This hampers cross-laboratory comparisons and validation of results, particularly in regulated industries such as pharmaceuticals and materials science.
Data integrity and compliance with regulatory requirements present additional challenges. As automation increases, so does the complexity of audit trails and data security measures. Patents addressing these concerns have increased by approximately 40% over the past five years, reflecting growing regulatory scrutiny of analytical methods.
Hardware limitations also constrain full automation capabilities. Current detector technologies sometimes lack the sensitivity required for fully automated decision-making, particularly for samples with low concentration analytes or complex matrices. Integration between sample handling robotics and data processing systems remains imperfect, with communication protocols between physical instruments and software systems representing a persistent challenge.
Cloud-based solutions are emerging as a potential path forward, with recent patents describing systems that leverage distributed computing for more sophisticated data analysis. However, concerns regarding data security, intellectual property protection, and reliable connectivity have slowed adoption in industrial settings where proprietary formulations are analyzed.
Contemporary Approaches to Automated GPC Data Analysis
01 Automated data acquisition and processing systems for GPC
Modern gel permeation chromatography systems incorporate automated data acquisition and processing capabilities. These systems can automatically collect chromatographic data, identify peaks, calculate molecular weight distributions, and generate comprehensive reports. The automation reduces human error, increases throughput, and improves the consistency of results in polymer analysis and other applications of GPC.- Automated data acquisition and processing systems for GPC: Systems that automate the collection and processing of gel permeation chromatography data, integrating hardware and software components for continuous monitoring and analysis. These systems can automatically collect data from chromatography instruments, process the raw signals, and generate analytical results with minimal human intervention. The automation includes real-time data acquisition, baseline correction, peak detection, and molecular weight distribution calculations.
- Software algorithms for GPC data analysis: Specialized algorithms designed for processing gel permeation chromatography data, including peak detection, integration, calibration curve generation, and molecular weight distribution calculations. These algorithms can handle complex chromatograms, perform deconvolution of overlapping peaks, apply various mathematical models for molecular weight determination, and generate comprehensive analytical reports. Advanced software solutions incorporate machine learning techniques to improve data processing accuracy and efficiency.
- Automated calibration and validation methods: Techniques for automating the calibration and validation processes in gel permeation chromatography, ensuring accurate and reliable results. These methods include automated standard preparation, injection, analysis, and calibration curve generation. The systems can perform regular validation checks, detect deviations from expected performance, and apply correction factors when necessary. This automation reduces human error and increases the reproducibility of analytical results.
- Integrated sample preparation and injection systems: Automated systems that handle sample preparation and injection for gel permeation chromatography analysis. These systems can perform tasks such as sample dilution, filtration, and conditioning before automatically injecting them into the chromatography column. The integration of sample preparation with the analytical process improves throughput, reduces contamination risks, and ensures consistent sample handling conditions, leading to more reliable analytical results.
- Data management and reporting solutions: Comprehensive systems for managing, storing, and reporting gel permeation chromatography data in laboratory environments. These solutions include database integration for storing raw and processed data, automated report generation with customizable templates, and tools for data visualization and interpretation. Advanced systems offer features such as electronic signatures, audit trails, and compliance with regulatory requirements, facilitating quality control and assurance in analytical laboratories.
02 Software solutions for GPC data analysis
Specialized software packages have been developed for the automated processing of gel permeation chromatography data. These software solutions offer features such as baseline correction, peak integration, calibration curve generation, and molecular weight calculation. Advanced algorithms enable the deconvolution of overlapping peaks, statistical analysis of results, and visualization of molecular weight distributions, making data interpretation more efficient and accurate.Expand Specific Solutions03 Automated sample preparation and injection systems
Automation in gel permeation chromatography extends to sample preparation and injection processes. These systems can automatically prepare samples, control dilution, filter solutions, and inject precise volumes into the chromatography column. The integration of robotic sample handlers with chromatography systems allows for unattended operation, increasing laboratory productivity and enabling high-throughput analysis of multiple samples.Expand Specific Solutions04 Calibration and validation automation for GPC
Automated calibration and validation procedures ensure the accuracy and reliability of gel permeation chromatography results. These systems can automatically run calibration standards, generate calibration curves, monitor system performance, and validate results against established criteria. Continuous calibration verification during sample runs helps maintain data quality and provides confidence in molecular weight determinations across different polymer types.Expand Specific Solutions05 Integration of GPC with other analytical techniques
Modern automated gel permeation chromatography systems can be integrated with complementary analytical techniques such as light scattering, viscometry, and mass spectrometry. This integration allows for simultaneous collection and correlation of multiple data types, providing more comprehensive characterization of polymers and complex mixtures. Automated data processing algorithms can combine information from these different techniques to generate more accurate and detailed molecular weight distributions and structural information.Expand Specific Solutions
Leading Companies and Research Institutions in GPC Automation
The automated gel permeation chromatography (GPC) data processing market is currently in a growth phase, with increasing demand for advanced analytical solutions in polymer science and biopharmaceuticals. The competitive landscape is characterized by established analytical instrument manufacturers like Waters Technology Corp., Agilent Technologies, and Tosoh Corp. dominating the core technology development, while pharmaceutical companies such as F. Hoffmann-La Roche and Abbott Laboratories are driving application-specific innovations. The market is witnessing technological convergence with major electronics and software players including Samsung Electronics, SAP SE, and Sony Group Corp. entering the space through data processing solutions. Academic institutions and government research centers are also contributing significantly to patent development, suggesting the technology is approaching maturity but still has substantial room for innovation in automated data analysis algorithms and integration with other analytical techniques.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed proprietary automated GPC data processing systems specifically optimized for polyolefin and specialty polymer characterization. Their patented high-temperature GPC automation platform integrates sample preparation, analysis, and data processing for challenging polymers that require elevated temperatures and specialized solvents. Dow's technology includes automated cross-fractionation chromatography (CFC) data processing that combines GPC with crystallization analysis to characterize molecular heterogeneity in complex polymer systems. Their systems feature specialized algorithms for determining short-chain branching distribution in polyethylene and automated calculation of comonomer incorporation in various copolymer systems. Dow has pioneered automated multi-detector calibration approaches that combine light scattering, viscometry, and concentration detection to characterize polymers without requiring structurally similar standards. Recent patents focus on machine learning applications for predicting polymer processing behavior from molecular weight distribution data and automated quality control systems for production environments. Their technology includes specialized data processing for analyzing recycled polymer streams, identifying contaminants, and characterizing degradation products - supporting sustainability initiatives in polymer manufacturing and recycling operations.
Strengths: Unparalleled expertise in industrial polymer applications; specialized capabilities for challenging polyolefin analysis; integrated approach connecting molecular characteristics to processing behavior. Weaknesses: Systems primarily optimized for Dow's own polymer types; less focus on biological macromolecules; technologies may be less accessible to external researchers compared to commercial instrument vendors.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has developed advanced automated gel permeation chromatography (GPC) data processing systems that integrate hardware and software solutions. Their Empower Chromatography Data Software specifically designed for GPC applications features automated calibration curve generation, molecular weight distribution calculations, and multi-detector data integration. The system employs machine learning algorithms to identify peak boundaries and optimize baseline corrections with minimal user intervention. Waters' ACQUITY Advanced Polymer Chromatography (APC) system combines ultra-high performance liquid chromatography technology with specialized columns and detectors for polymer analysis, reducing analysis time by up to 5 times compared to traditional GPC methods. Their patented technologies include automated method development tools that optimize separation conditions based on polymer characteristics and automated quality control protocols that flag anomalous results for review. The company has also developed cloud-based data processing capabilities allowing for remote access and collaborative analysis of GPC data across multiple laboratory sites.
Strengths: Industry-leading integration of hardware and software solutions providing end-to-end automation; significant reduction in analysis time through optimized technology; extensive experience in chromatography data systems. Weaknesses: Proprietary systems may limit compatibility with third-party instruments; higher initial investment costs compared to generic solutions; potential complexity requiring specialized training.
Key Patent Innovations in GPC Data Processing Algorithms
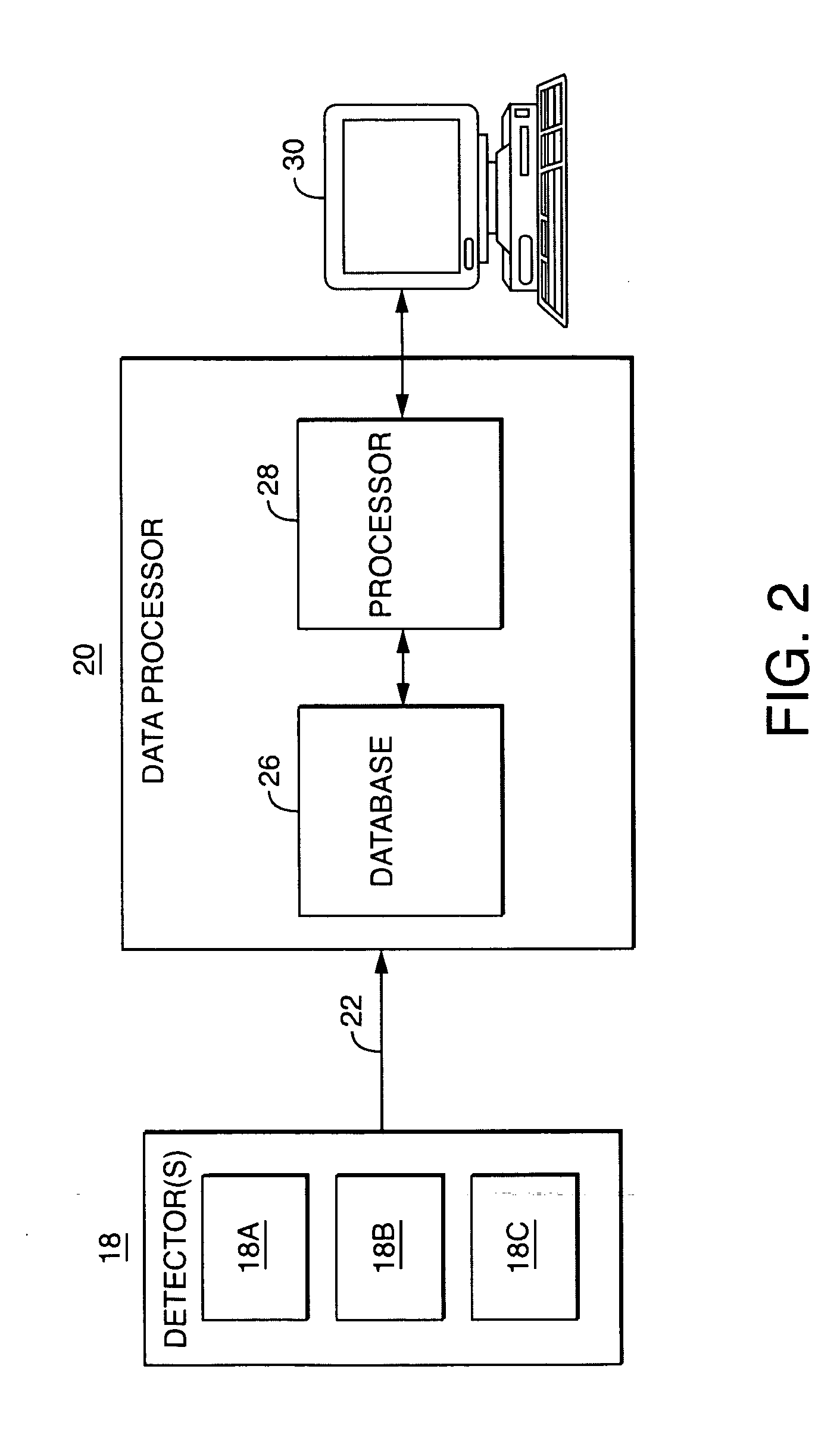
Data processor for use in chromatographic analysis
PatentActiveUS7246013B2
Innovation
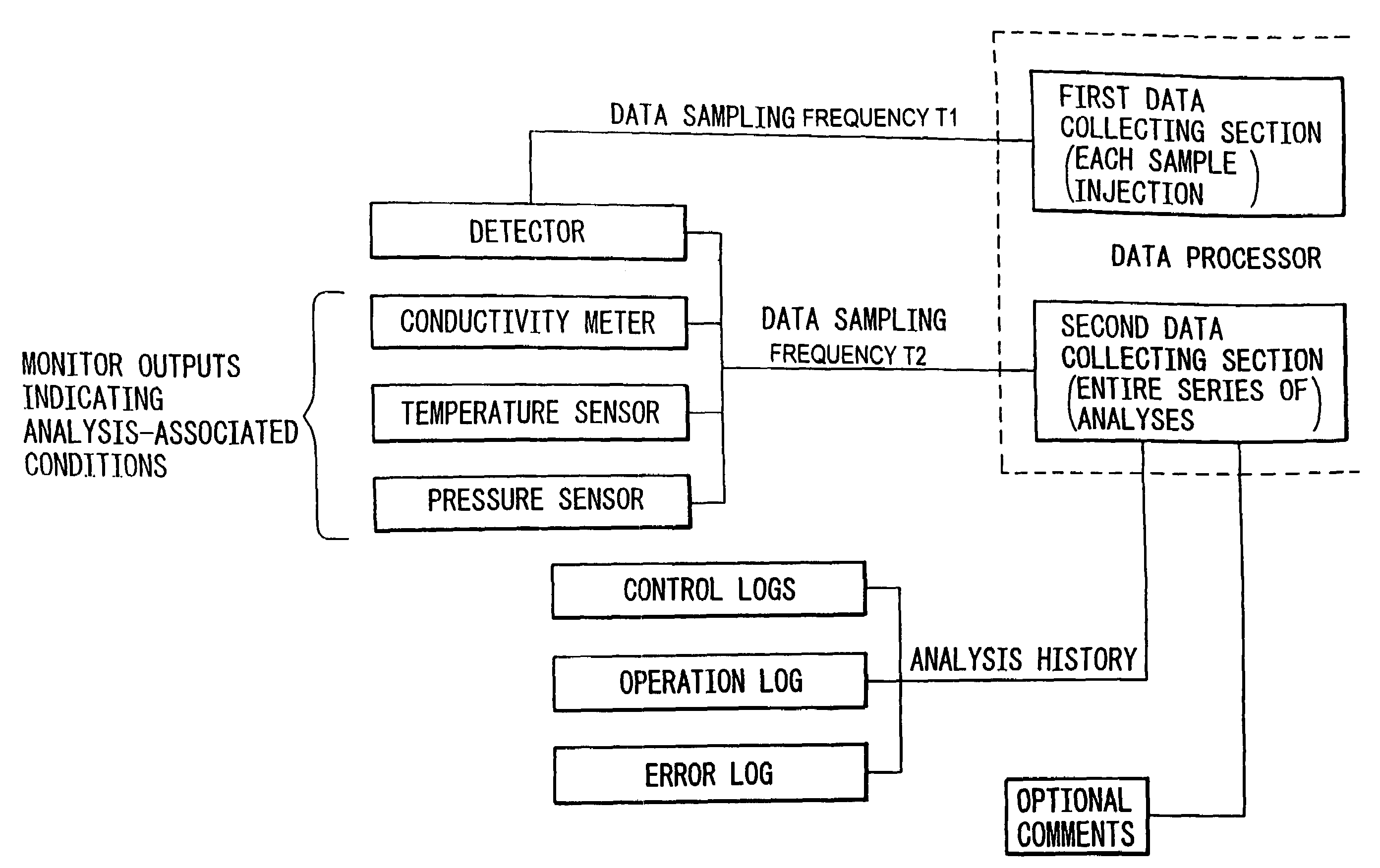
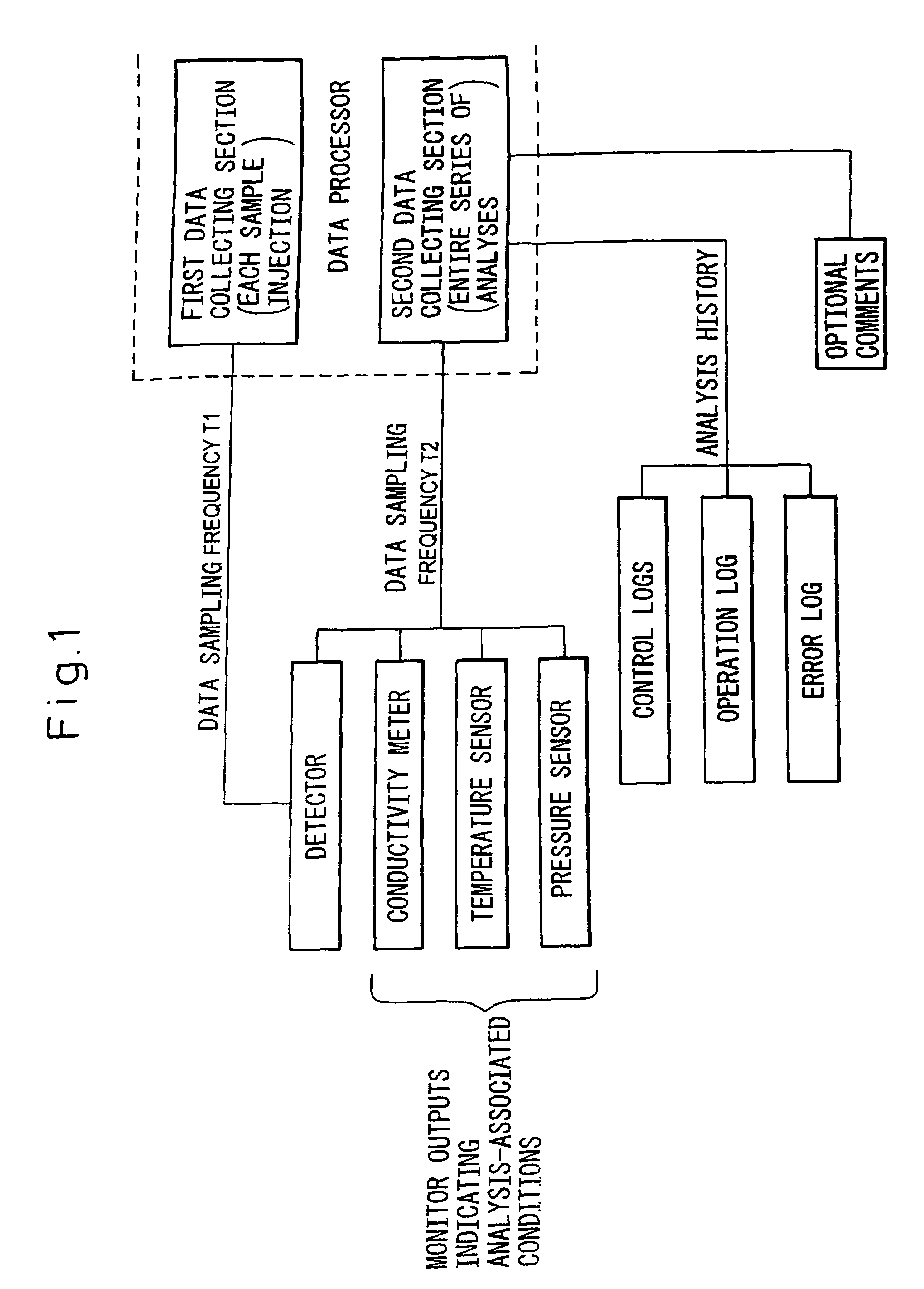
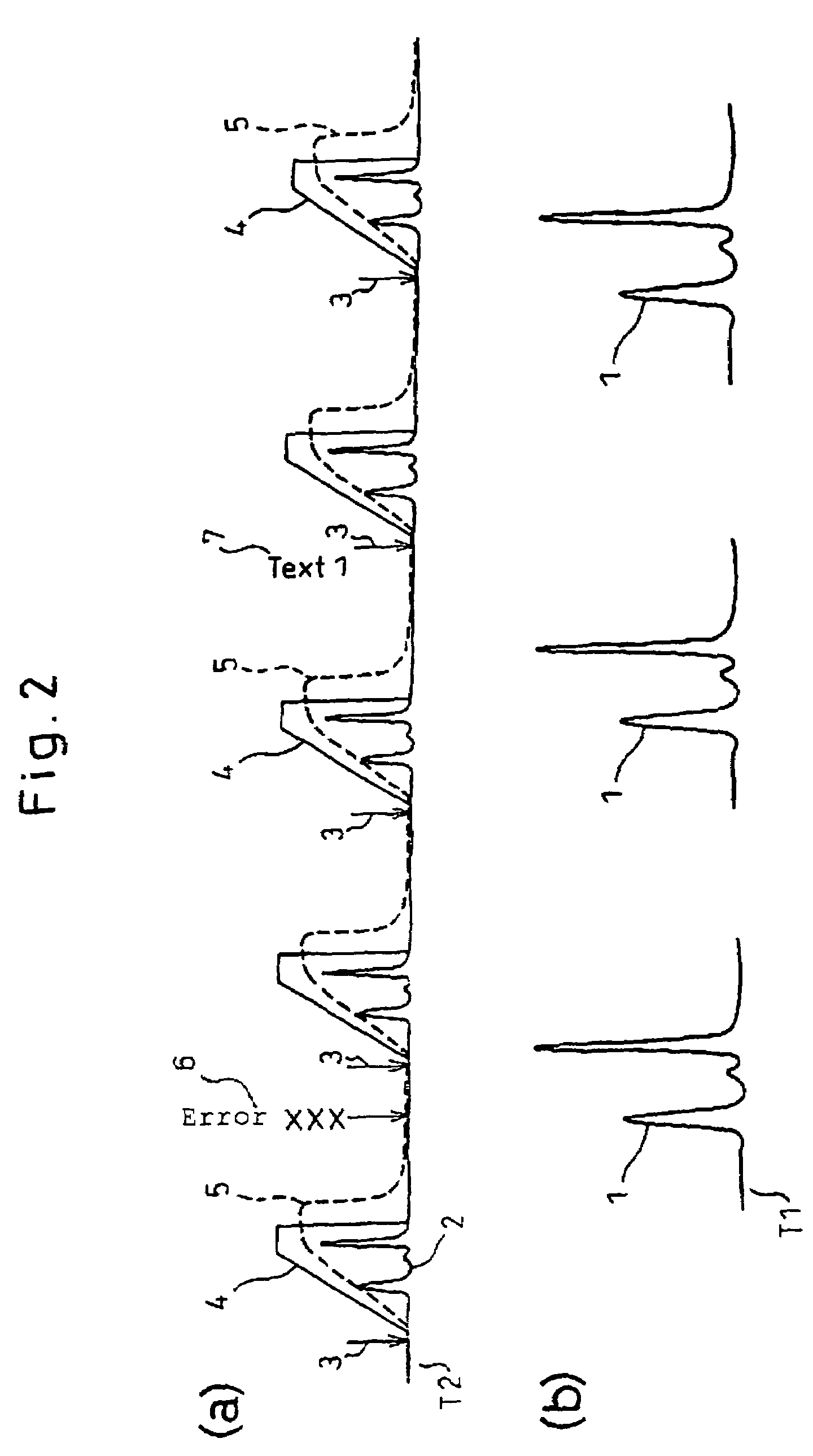
- A data processor that collects detector outputs at a higher rate (T1) for each sample injection and monitor outputs at a lower rate (T2) throughout the analysis, allowing for detailed chromatogram analysis and validation of analysis-associated information, while enabling the recording of optional comments and analysis history to ensure data integrity.
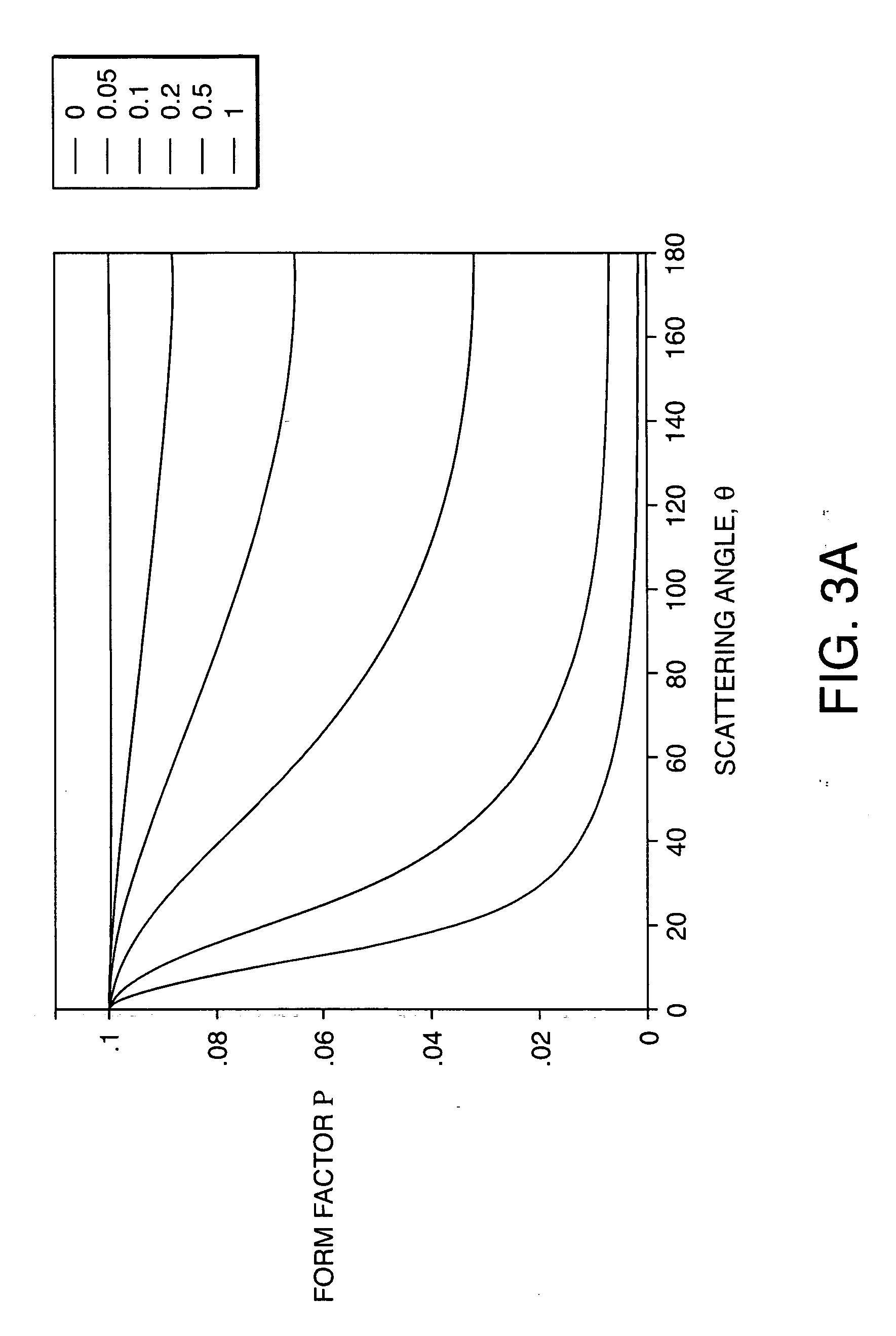
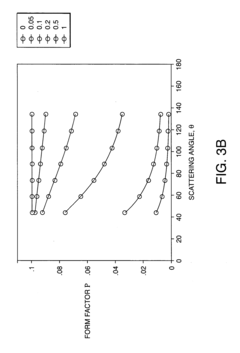
System and method for determining radius of gyration, molecular weight, and intrinsic viscosity of a polymeric distribution using gel permeation chromatography and light scattering detection
PatentInactiveUS20050240385A1
Innovation
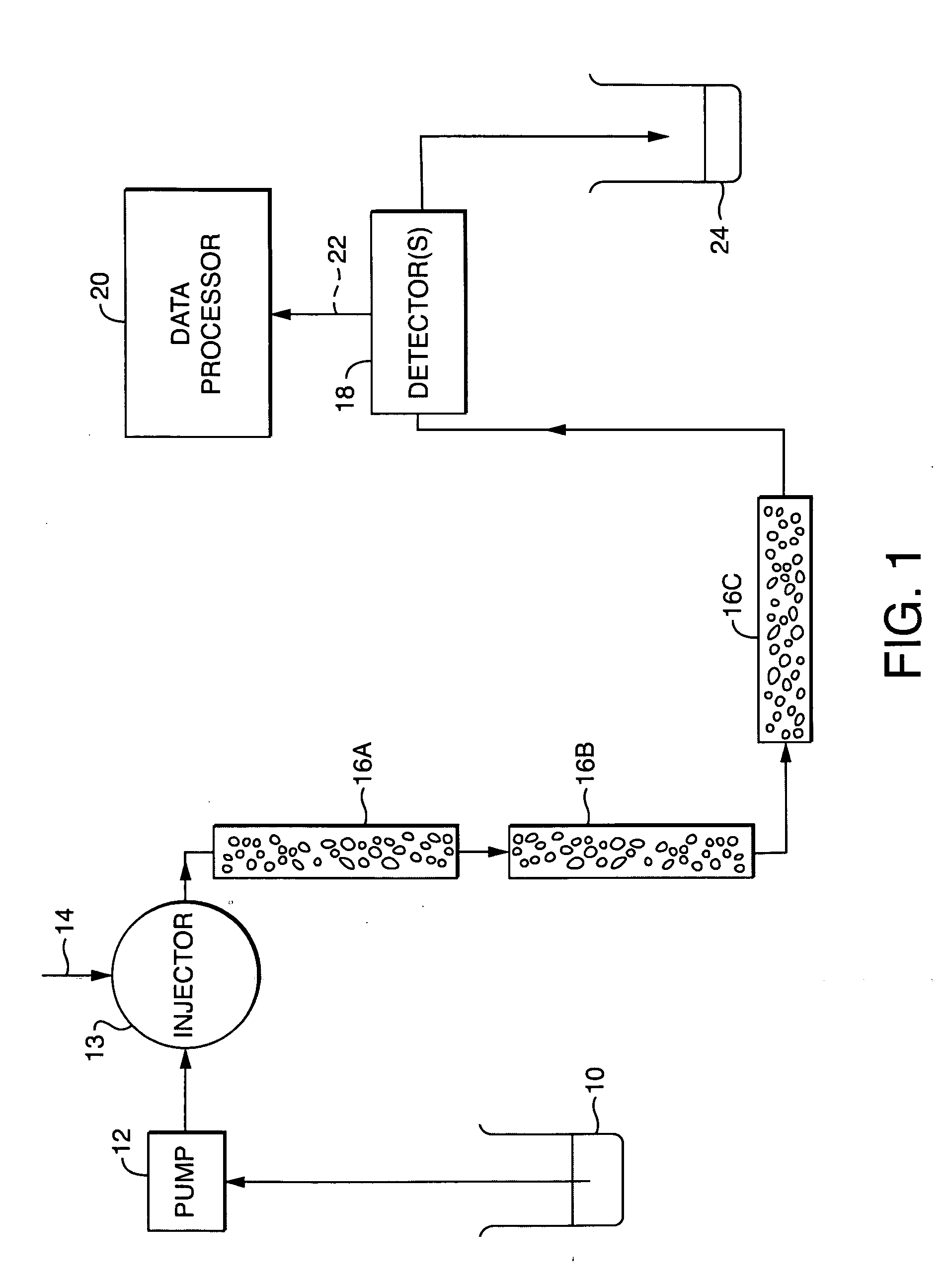
- The method employs a GPC chromatograph system with multi-angle laser light-scattering, refractive index, and viscometer detectors, using novel algorithms to compute the radius of gyration for each slice, which involves fitting parameterized models simultaneously to all data collected at each slice, reducing the impact of detector noise and providing more stable estimates.
Standardization Efforts in Automated Analytical Chemistry
The field of analytical chemistry has witnessed significant efforts toward standardization in recent years, particularly in automated systems. Within the context of gel permeation chromatography (GPC) data processing, standardization initiatives have become increasingly crucial as patent activities accelerate in this domain.
International organizations such as ASTM International, ISO, and IUPAC have developed comprehensive guidelines specifically addressing automated analytical chemistry procedures. These standards encompass calibration protocols, data format specifications, and quality control parameters that directly impact GPC data processing workflows. The ASTM D6474 standard, for instance, provides detailed procedures for determining molecular weight distributions using GPC, which has been instrumental in establishing consistent methodologies across laboratories.
Patent analysis reveals a growing trend toward standardized data formats for automated GPC systems. Major instrument manufacturers have increasingly incorporated standardized data exchange formats like AnIML (Analytical Information Markup Language) and JCAMP-DX into their patented technologies. These formats facilitate seamless data transfer between different analytical platforms and ensure long-term data accessibility, addressing a critical need in the analytical chemistry community.
Regulatory bodies, particularly in pharmaceutical and polymer industries, have played a significant role in driving standardization efforts. The FDA's guidance on analytical procedures and methods validation has influenced patent developments in automated GPC data processing, with recent patents demonstrating increased emphasis on compliance with these regulatory standards. This regulatory pressure has catalyzed innovation in standardized validation protocols and system suitability tests.
Collaborative industry initiatives have emerged to address interoperability challenges. The Allotrope Foundation, comprising major pharmaceutical and chemical companies, has developed standardized frameworks for analytical data that include specific provisions for chromatographic techniques like GPC. Patent trends indicate growing adoption of these frameworks, with recent filings increasingly referencing compliance with these industry standards.
Academic-industry partnerships have contributed significantly to standardization efforts through open-source projects and reference materials. The development of certified reference materials specifically for GPC calibration has enabled more consistent molecular weight determinations across different laboratories and instruments. Recent patents increasingly cite these standardized reference materials in their claims, highlighting their importance in ensuring reliable data processing.
Looking forward, emerging patent trends suggest a move toward harmonized approaches for uncertainty estimation in automated GPC data processing. This represents a critical frontier in standardization efforts, as quantifying measurement uncertainty remains challenging yet essential for meaningful data comparison across different analytical platforms and methodologies.
International organizations such as ASTM International, ISO, and IUPAC have developed comprehensive guidelines specifically addressing automated analytical chemistry procedures. These standards encompass calibration protocols, data format specifications, and quality control parameters that directly impact GPC data processing workflows. The ASTM D6474 standard, for instance, provides detailed procedures for determining molecular weight distributions using GPC, which has been instrumental in establishing consistent methodologies across laboratories.
Patent analysis reveals a growing trend toward standardized data formats for automated GPC systems. Major instrument manufacturers have increasingly incorporated standardized data exchange formats like AnIML (Analytical Information Markup Language) and JCAMP-DX into their patented technologies. These formats facilitate seamless data transfer between different analytical platforms and ensure long-term data accessibility, addressing a critical need in the analytical chemistry community.
Regulatory bodies, particularly in pharmaceutical and polymer industries, have played a significant role in driving standardization efforts. The FDA's guidance on analytical procedures and methods validation has influenced patent developments in automated GPC data processing, with recent patents demonstrating increased emphasis on compliance with these regulatory standards. This regulatory pressure has catalyzed innovation in standardized validation protocols and system suitability tests.
Collaborative industry initiatives have emerged to address interoperability challenges. The Allotrope Foundation, comprising major pharmaceutical and chemical companies, has developed standardized frameworks for analytical data that include specific provisions for chromatographic techniques like GPC. Patent trends indicate growing adoption of these frameworks, with recent filings increasingly referencing compliance with these industry standards.
Academic-industry partnerships have contributed significantly to standardization efforts through open-source projects and reference materials. The development of certified reference materials specifically for GPC calibration has enabled more consistent molecular weight determinations across different laboratories and instruments. Recent patents increasingly cite these standardized reference materials in their claims, highlighting their importance in ensuring reliable data processing.
Looking forward, emerging patent trends suggest a move toward harmonized approaches for uncertainty estimation in automated GPC data processing. This represents a critical frontier in standardization efforts, as quantifying measurement uncertainty remains challenging yet essential for meaningful data comparison across different analytical platforms and methodologies.
Economic Impact of GPC Automation on Laboratory Efficiency
The automation of Gel Permeation Chromatography (GPC) data processing has demonstrated significant economic benefits for laboratories across various sectors. Quantitative analyses reveal that laboratories implementing automated GPC data processing systems experience a 30-45% reduction in overall analysis time compared to manual methods. This efficiency gain translates directly into increased sample throughput, with most facilities reporting capacity improvements of 25-60% without additional staffing or equipment investments beyond the automation technology itself.
Cost-benefit analyses from pharmaceutical and polymer research laboratories indicate that the initial investment in automated GPC data processing systems typically achieves return on investment within 12-18 months. The primary contributors to this favorable economic outcome include reduced labor costs, decreased error rates requiring fewer repeat analyses, and more efficient utilization of expensive chromatography equipment.
Labor cost savings are particularly noteworthy, with specialized technicians able to redirect 15-20 hours weekly from routine data processing tasks to higher-value research activities. This reallocation of human resources represents not only direct cost savings but also accelerates research outcomes and innovation cycles, creating additional value that extends beyond simple operational efficiency.
Quality improvements resulting from automation further enhance economic benefits through reduced material waste and improved decision-making. Patent filings from major analytical instrument manufacturers highlight that automated systems reduce analytical errors by 70-85% compared to manual processing methods, significantly decreasing costly experimental repetition and material consumption.
The economic impact extends to regulatory compliance costs as well. Automated GPC data processing systems with integrated audit trails and validation features reduce documentation time by approximately 40% while simultaneously improving compliance outcomes during regulatory inspections. This dual benefit is particularly valuable in highly regulated industries such as pharmaceuticals and medical polymers.
Market analysis indicates that laboratories implementing comprehensive GPC automation solutions experience competitive advantages through faster turnaround times and improved consistency. This translates to enhanced client retention rates and premium pricing opportunities, with leading service laboratories reporting 15-20% higher profit margins compared to competitors using traditional manual processing methods.
The economic benefits scale proportionally with laboratory size and sample volume, with the most dramatic efficiency gains observed in high-throughput environments processing more than 500 samples monthly. However, even smaller research facilities report meaningful economic benefits, particularly when factoring in the opportunity costs of researcher time allocation.
Cost-benefit analyses from pharmaceutical and polymer research laboratories indicate that the initial investment in automated GPC data processing systems typically achieves return on investment within 12-18 months. The primary contributors to this favorable economic outcome include reduced labor costs, decreased error rates requiring fewer repeat analyses, and more efficient utilization of expensive chromatography equipment.
Labor cost savings are particularly noteworthy, with specialized technicians able to redirect 15-20 hours weekly from routine data processing tasks to higher-value research activities. This reallocation of human resources represents not only direct cost savings but also accelerates research outcomes and innovation cycles, creating additional value that extends beyond simple operational efficiency.
Quality improvements resulting from automation further enhance economic benefits through reduced material waste and improved decision-making. Patent filings from major analytical instrument manufacturers highlight that automated systems reduce analytical errors by 70-85% compared to manual processing methods, significantly decreasing costly experimental repetition and material consumption.
The economic impact extends to regulatory compliance costs as well. Automated GPC data processing systems with integrated audit trails and validation features reduce documentation time by approximately 40% while simultaneously improving compliance outcomes during regulatory inspections. This dual benefit is particularly valuable in highly regulated industries such as pharmaceuticals and medical polymers.
Market analysis indicates that laboratories implementing comprehensive GPC automation solutions experience competitive advantages through faster turnaround times and improved consistency. This translates to enhanced client retention rates and premium pricing opportunities, with leading service laboratories reporting 15-20% higher profit margins compared to competitors using traditional manual processing methods.
The economic benefits scale proportionally with laboratory size and sample volume, with the most dramatic efficiency gains observed in high-throughput environments processing more than 500 samples monthly. However, even smaller research facilities report meaningful economic benefits, particularly when factoring in the opportunity costs of researcher time allocation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!