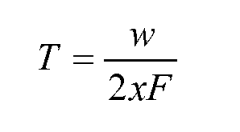Standards and ISO methods for gel permeation chromatography calibration
OCT 11, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GPC Calibration Standards Evolution and Objectives
Gel Permeation Chromatography (GPC) calibration standards have evolved significantly since the technique's inception in the 1960s. Initially, calibration methods relied on rudimentary polymer standards with broad molecular weight distributions, limiting the accuracy and reproducibility of results. The early development phase focused primarily on establishing basic separation principles rather than standardization.
The 1970s marked a turning point with the introduction of narrow molecular weight distribution polystyrene standards, which dramatically improved calibration precision. These standards enabled more reliable molecular weight determinations and became the foundation for subsequent calibration methodologies. During this period, researchers began recognizing the need for internationally accepted calibration protocols to ensure data comparability across laboratories.
By the 1980s, the International Organization for Standardization (ISO) initiated efforts to formalize GPC calibration methods, resulting in the first comprehensive guidelines. ISO 13885 emerged as a pivotal standard, addressing calibration procedures for various polymer types and establishing traceability requirements. This standardization effort coincided with technological advancements in detector systems and column technology, necessitating more sophisticated calibration approaches.
The 1990s witnessed the development of universal calibration concepts based on hydrodynamic volume relationships, allowing for more accurate molecular weight determinations across different polymer chemistries. Mark-Houwink parameters became essential components of calibration protocols, enabling the correlation between different polymer types and expanding the applicability of GPC analysis across diverse materials.
The early 2000s brought multi-detector GPC systems into prominence, incorporating light scattering, viscometry, and refractive index measurements. These technological advances required corresponding evolution in calibration standards and methodologies, leading to the development of ISO 16014 series standards specifically addressing multi-detector calibration approaches.
Current objectives in GPC calibration standardization focus on several key areas: enhancing the accuracy of absolute molecular weight determinations, expanding the range of available reference materials beyond traditional polystyrene standards, and developing robust calibration protocols for complex polymer architectures including branched, star-shaped, and comb polymers.
Additionally, there is growing emphasis on establishing calibration standards for specialized applications such as high-temperature GPC and aqueous systems. The industry is also working toward improved inter-laboratory reproducibility through more stringent validation protocols and reference material certification processes.
Future standardization goals include the development of calibration methodologies for emerging polymer classes, integration with computational modeling for enhanced data interpretation, and harmonization of standards across different chromatographic techniques to provide comprehensive polymer characterization frameworks.
The 1970s marked a turning point with the introduction of narrow molecular weight distribution polystyrene standards, which dramatically improved calibration precision. These standards enabled more reliable molecular weight determinations and became the foundation for subsequent calibration methodologies. During this period, researchers began recognizing the need for internationally accepted calibration protocols to ensure data comparability across laboratories.
By the 1980s, the International Organization for Standardization (ISO) initiated efforts to formalize GPC calibration methods, resulting in the first comprehensive guidelines. ISO 13885 emerged as a pivotal standard, addressing calibration procedures for various polymer types and establishing traceability requirements. This standardization effort coincided with technological advancements in detector systems and column technology, necessitating more sophisticated calibration approaches.
The 1990s witnessed the development of universal calibration concepts based on hydrodynamic volume relationships, allowing for more accurate molecular weight determinations across different polymer chemistries. Mark-Houwink parameters became essential components of calibration protocols, enabling the correlation between different polymer types and expanding the applicability of GPC analysis across diverse materials.
The early 2000s brought multi-detector GPC systems into prominence, incorporating light scattering, viscometry, and refractive index measurements. These technological advances required corresponding evolution in calibration standards and methodologies, leading to the development of ISO 16014 series standards specifically addressing multi-detector calibration approaches.
Current objectives in GPC calibration standardization focus on several key areas: enhancing the accuracy of absolute molecular weight determinations, expanding the range of available reference materials beyond traditional polystyrene standards, and developing robust calibration protocols for complex polymer architectures including branched, star-shaped, and comb polymers.
Additionally, there is growing emphasis on establishing calibration standards for specialized applications such as high-temperature GPC and aqueous systems. The industry is also working toward improved inter-laboratory reproducibility through more stringent validation protocols and reference material certification processes.
Future standardization goals include the development of calibration methodologies for emerging polymer classes, integration with computational modeling for enhanced data interpretation, and harmonization of standards across different chromatographic techniques to provide comprehensive polymer characterization frameworks.
Market Analysis for GPC Calibration Solutions
The global market for Gel Permeation Chromatography (GPC) calibration solutions is experiencing steady growth, driven by increasing applications in polymer science, pharmaceutical research, and materials development. Current market valuation stands at approximately 320 million USD, with projections indicating a compound annual growth rate of 5.7% through 2028, according to recent industry analyses.
North America dominates the GPC calibration standards market, accounting for roughly 38% of global revenue, followed by Europe at 31% and Asia-Pacific at 24%. This regional distribution reflects the concentration of polymer research facilities and pharmaceutical development centers in these regions. The remaining 7% is distributed across Latin America, Middle East, and Africa, where adoption is gradually increasing.
Demand patterns reveal distinct market segments based on calibration standard types. Polystyrene standards represent the largest segment with approximately 45% market share, followed by polyethylene glycol/oxide standards at 22%, and protein standards at 18%. Specialized standards for specific applications constitute the remaining 15%, showing the fastest growth rate as applications diversify.
End-user analysis indicates that academic and research institutions consume about 35% of GPC calibration solutions, while pharmaceutical and biotechnology companies account for 28%. The polymer and chemical manufacturing sector represents 25%, with other industries comprising the remaining 12%. This distribution highlights the critical role of GPC in both research and quality control applications across multiple sectors.
Market drivers include increasing regulatory requirements for polymer characterization, growing pharmaceutical research focusing on protein-based therapeutics, and rising demand for advanced materials with precise molecular weight distributions. The push toward standardization across laboratories has further stimulated demand for ISO-compliant calibration methods and certified reference materials.
Pricing trends show moderate increases of 2-3% annually, primarily due to the rising costs of producing highly characterized reference materials and the increasing complexity of calibration standards required for newer applications. Premium pricing is observed for standards with ISO certification and those offering traceability to international reference materials.
Future market expansion is anticipated in emerging economies, particularly in India, China, and Brazil, where investments in research infrastructure and analytical capabilities are growing. Additionally, the development of application-specific calibration kits tailored to particular industries represents a significant growth opportunity for market players seeking to differentiate their offerings in an increasingly competitive landscape.
North America dominates the GPC calibration standards market, accounting for roughly 38% of global revenue, followed by Europe at 31% and Asia-Pacific at 24%. This regional distribution reflects the concentration of polymer research facilities and pharmaceutical development centers in these regions. The remaining 7% is distributed across Latin America, Middle East, and Africa, where adoption is gradually increasing.
Demand patterns reveal distinct market segments based on calibration standard types. Polystyrene standards represent the largest segment with approximately 45% market share, followed by polyethylene glycol/oxide standards at 22%, and protein standards at 18%. Specialized standards for specific applications constitute the remaining 15%, showing the fastest growth rate as applications diversify.
End-user analysis indicates that academic and research institutions consume about 35% of GPC calibration solutions, while pharmaceutical and biotechnology companies account for 28%. The polymer and chemical manufacturing sector represents 25%, with other industries comprising the remaining 12%. This distribution highlights the critical role of GPC in both research and quality control applications across multiple sectors.
Market drivers include increasing regulatory requirements for polymer characterization, growing pharmaceutical research focusing on protein-based therapeutics, and rising demand for advanced materials with precise molecular weight distributions. The push toward standardization across laboratories has further stimulated demand for ISO-compliant calibration methods and certified reference materials.
Pricing trends show moderate increases of 2-3% annually, primarily due to the rising costs of producing highly characterized reference materials and the increasing complexity of calibration standards required for newer applications. Premium pricing is observed for standards with ISO certification and those offering traceability to international reference materials.
Future market expansion is anticipated in emerging economies, particularly in India, China, and Brazil, where investments in research infrastructure and analytical capabilities are growing. Additionally, the development of application-specific calibration kits tailored to particular industries represents a significant growth opportunity for market players seeking to differentiate their offerings in an increasingly competitive landscape.
Current ISO Methods and Technical Challenges
Gel Permeation Chromatography (GPC) calibration is currently governed by several ISO standards that provide methodological frameworks for ensuring accuracy and reproducibility in polymer analysis. ISO 13885 series represents the cornerstone of standardization in this field, with ISO 13885-1 specifically addressing the calibration of GPC columns using polystyrene standards. This standard details procedures for preparing calibration curves and validating system performance.
The ISO 16014 series focuses on the determination of molecular mass averages and distributions by GPC, with specific parts dedicated to calibration procedures for different polymer types. These standards establish protocols for narrow molecular weight distribution calibrants and broad standard reference materials.
Despite these established standards, significant technical challenges persist in GPC calibration. The primary challenge stems from the relative nature of GPC measurements, where molecular weights are determined by comparison with standards of known molecular weight. This approach introduces inherent inaccuracies when analyzing polymers with different chemical compositions or architectures from the calibration standards.
Column resolution limitations represent another substantial challenge, particularly for high molecular weight polymers or complex mixtures. Current ISO methods struggle to provide adequate guidance for resolving closely spaced molecular weight distributions, leading to potential misinterpretation of results in complex polymer systems.
Inter-laboratory variability remains problematic despite standardization efforts. Studies have shown that even when following identical ISO protocols, different laboratories may produce varying results due to subtle differences in equipment, operator techniques, or environmental conditions. This variability undermines the reliability of GPC data across research institutions and industrial settings.
The calibration of advanced GPC systems, particularly those coupled with multiple detectors (light scattering, viscometry, etc.), presents additional challenges not fully addressed by current ISO standards. These multi-detector systems require more sophisticated calibration approaches that extend beyond traditional single-detector methodologies.
Temperature and solvent effects on calibration stability represent ongoing challenges, with current standards providing insufficient guidance on compensating for these variables. Polymer-solvent interactions can significantly alter hydrodynamic volumes, affecting retention times and calibration accuracy.
Emerging polymer technologies, including branched polymers, block copolymers, and biopolymers, pose unique calibration challenges that exceed the scope of existing ISO methods. These complex architectures often exhibit non-standard elution behaviors that cannot be accurately characterized using conventional calibration approaches.
The lack of universally applicable calibration standards across different polymer classes remains a fundamental limitation. While polystyrene standards are widely used, their application to dissimilar polymers introduces systematic errors that current ISO methods struggle to address comprehensively.
The ISO 16014 series focuses on the determination of molecular mass averages and distributions by GPC, with specific parts dedicated to calibration procedures for different polymer types. These standards establish protocols for narrow molecular weight distribution calibrants and broad standard reference materials.
Despite these established standards, significant technical challenges persist in GPC calibration. The primary challenge stems from the relative nature of GPC measurements, where molecular weights are determined by comparison with standards of known molecular weight. This approach introduces inherent inaccuracies when analyzing polymers with different chemical compositions or architectures from the calibration standards.
Column resolution limitations represent another substantial challenge, particularly for high molecular weight polymers or complex mixtures. Current ISO methods struggle to provide adequate guidance for resolving closely spaced molecular weight distributions, leading to potential misinterpretation of results in complex polymer systems.
Inter-laboratory variability remains problematic despite standardization efforts. Studies have shown that even when following identical ISO protocols, different laboratories may produce varying results due to subtle differences in equipment, operator techniques, or environmental conditions. This variability undermines the reliability of GPC data across research institutions and industrial settings.
The calibration of advanced GPC systems, particularly those coupled with multiple detectors (light scattering, viscometry, etc.), presents additional challenges not fully addressed by current ISO standards. These multi-detector systems require more sophisticated calibration approaches that extend beyond traditional single-detector methodologies.
Temperature and solvent effects on calibration stability represent ongoing challenges, with current standards providing insufficient guidance on compensating for these variables. Polymer-solvent interactions can significantly alter hydrodynamic volumes, affecting retention times and calibration accuracy.
Emerging polymer technologies, including branched polymers, block copolymers, and biopolymers, pose unique calibration challenges that exceed the scope of existing ISO methods. These complex architectures often exhibit non-standard elution behaviors that cannot be accurately characterized using conventional calibration approaches.
The lack of universally applicable calibration standards across different polymer classes remains a fundamental limitation. While polystyrene standards are widely used, their application to dissimilar polymers introduces systematic errors that current ISO methods struggle to address comprehensively.
Established ISO Protocols for GPC Calibration
01 Calibration standards and reference materials
Various calibration standards and reference materials are used in GPC calibration to establish accurate molecular weight determinations. These standards typically include well-characterized polymers with narrow molecular weight distributions. The choice of calibration standards should match or closely resemble the chemical nature of the samples being analyzed to minimize errors due to differences in hydrodynamic volume. Common standards include polystyrene, poly(methyl methacrylate), polyethylene glycol, and pullulan for different applications.- Standard calibration methods for GPC: Gel permeation chromatography requires proper calibration to ensure accurate molecular weight determination. Standard calibration methods involve using reference materials with known molecular weights to create calibration curves. These standards typically include polystyrene, polyethylene glycol, or other well-characterized polymers. The calibration process establishes the relationship between elution volume and molecular weight, which is essential for accurate analysis of unknown samples.
- Universal calibration techniques: Universal calibration approaches in GPC overcome limitations of conventional calibration by accounting for differences in polymer structure and hydrodynamic volume. This technique uses the Mark-Houwink equation to relate intrinsic viscosity to molecular weight, allowing calibration curves to be applied across different polymer types. Universal calibration often employs viscometry detectors alongside concentration detectors to provide more accurate molecular weight distributions for structurally diverse polymers.
- Multi-detector calibration systems: Advanced GPC calibration incorporates multiple detection methods to enhance accuracy and provide additional polymer characterization data. These systems typically combine refractive index, light scattering, viscometry, and UV detectors. Multi-detector approaches allow for absolute molecular weight determination without relying on calibration standards with similar chemical structures to the analyte. This method provides comprehensive information about molecular weight distribution, branching, and conformation of polymers.
- Automated calibration software and algorithms: Modern GPC systems utilize specialized software and algorithms to streamline the calibration process and improve data analysis. These automated systems can perform calibration verification, data processing, and error detection with minimal user intervention. Advanced algorithms can correct for peak broadening, system dispersion, and other instrumental factors that affect calibration accuracy. Machine learning approaches are increasingly being applied to improve calibration model robustness across different sample types.
- Calibration standards and reference materials: The development and use of specialized calibration standards is critical for accurate GPC analysis. These reference materials include narrow molecular weight distribution polymers, oligomeric standards, and certified reference materials with precisely characterized molecular weights. For specific applications, custom calibration standards may be developed to match the chemical composition of analytes. Proper handling, storage, and preparation of calibration standards is essential to maintain their integrity and ensure reliable calibration results.
02 Calibration methods and procedures
Different calibration methods are employed in GPC analysis, including conventional calibration with standards of known molecular weight, universal calibration based on hydrodynamic volume, and multi-detector calibration approaches. The procedures involve preparing a series of standard solutions, analyzing them under identical conditions as the samples, and constructing calibration curves by plotting the logarithm of molecular weight against retention time or elution volume. These methods ensure accurate and reproducible molecular weight determinations across different polymer types.Expand Specific Solutions03 Advanced instrumentation and detector calibration
Modern GPC systems incorporate multiple detectors that require specific calibration procedures. These may include refractive index detectors, UV-visible detectors, light scattering detectors, and viscometers. Each detector type requires specific calibration protocols to ensure accurate response factors and baseline corrections. Multi-detector approaches allow for absolute molecular weight determination without relying solely on relative calibration standards, improving the accuracy of molecular weight distribution analysis for complex polymer systems.Expand Specific Solutions04 Software and data processing for calibration
Specialized software tools are essential for GPC calibration data processing and analysis. These software packages facilitate the construction of calibration curves, apply various calibration models, and perform molecular weight calculations. Advanced algorithms can correct for band broadening, inter-detector delays, and other systematic errors. The software may also include validation tools to assess the quality of calibration and provide statistical analysis of calibration parameters, ensuring reliable and reproducible molecular weight determinations.Expand Specific Solutions05 Calibration for specific polymer types and applications
Different polymer types and specific applications require tailored GPC calibration approaches. For example, water-soluble polymers, high-temperature GPC for polyolefins, and branched polymers each present unique calibration challenges. Industry-specific applications in pharmaceuticals, biopolymers, and synthetic materials may require specialized calibration protocols to meet regulatory requirements or specific analytical needs. These specialized calibration approaches often involve selecting appropriate standards, solvents, and column systems that match the physicochemical properties of the target polymers.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Gel permeation chromatography (GPC) calibration standards and ISO methods represent a mature yet evolving technical field, currently in the growth phase with an estimated market size of $300-400 million annually. The competitive landscape is dominated by established analytical instrumentation companies like Waters Technology Corp. and Dionex Corp., which provide comprehensive GPC solutions with standardized calibration methods. Chemical industry players including ExxonMobil Chemical Patents, Dow Global Technologies, and Covestro Deutschland AG have developed proprietary calibration techniques for polymer characterization. Academic institutions collaborate with industry to advance ISO standardization efforts, while specialized companies like SPEX CertiPrep focus exclusively on reference materials. The technology continues to mature with increasing emphasis on cross-validation between different calibration methods and international standardization.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil Chemical has developed proprietary GPC calibration methodologies specifically optimized for petroleum-derived polymers and complex hydrocarbon mixtures. Their approach integrates multi-angle light scattering (MALS) with differential refractive index detection to establish absolute molecular weight calibrations that overcome the limitations of conventional relative calibration methods. ExxonMobil's calibration protocol incorporates specialized standards with precisely controlled branching characteristics that enable accurate characterization of polyolefins and other non-linear polymers in accordance with ISO 16014-2 guidelines. Their methodology includes temperature-dependent calibration parameters that account for the conformational changes of polymers in solution at different temperatures, critical for accurate analysis of temperature-sensitive materials. The company has pioneered the use of broad standard calibration techniques that better represent the actual molecular weight distribution of industrial polymers, moving beyond the traditional narrow standard approach outlined in ISO 13885-3. ExxonMobil's calibration software implements advanced statistical methods for uncertainty estimation, providing confidence intervals for molecular weight determinations as recommended by ISO/IEC 17025 for analytical testing laboratories.
Strengths: Specialized expertise in petroleum-derived and complex polymers; temperature-dependent calibration parameters improve accuracy for diverse operating conditions; absolute molecular weight determination reduces polymer chemistry dependence. Weaknesses: Calibration methods optimized primarily for petroleum industry applications; proprietary nature limits broader standardization efforts; requires sophisticated equipment beyond basic GPC setups.
Dionex Corp.
Technical Solution: Dionex Corporation has pioneered standardized approaches to GPC calibration through their UltiMate 3000 chromatography systems specifically optimized for polymer analysis. Their technical solution incorporates universal calibration methodologies based on the Mark-Houwink relationship, allowing for accurate molecular weight determination across different polymer types using a single calibration curve. Dionex's approach includes specialized software algorithms that implement the ISO 13885-1 requirements for calibration curve generation with automatic detection of outliers and statistical validation of calibration quality. Their calibration standards feature narrow polydispersity index values (<1.1) and are characterized using multiple independent techniques to ensure accuracy. The company has developed a proprietary "Smart Calibration" protocol that monitors system performance over time and automatically adjusts for instrumental drift, maintaining calibration integrity in accordance with ISO 16014-4 guidelines for quality control procedures. Dionex also provides industry-specific calibration kits tailored for particular polymer classes with documented traceability to international standards.
Strengths: Universal calibration approach enables analysis of diverse polymer types with single calibration; automated drift correction ensures long-term stability; specialized industry-specific calibration kits improve accuracy for targeted applications. Weaknesses: Proprietary calibration algorithms create potential vendor lock-in; higher initial investment compared to basic calibration methods; requires regular purchase of certified calibration standards.
Key Patents and Innovations in Calibration Standards
POLYSTYRENE STANDARDS kit FOR GEL PERMEATION CHROMATOGRAPHY (GPC) CALIBRATION, PROCESS OF OBTAINING AND USING THE BENEFIT KIT
PatentActiveBRPI1103568A2
Innovation
- A kit of polystyrene standards with varying molar masses and controlled polydispersity indices, produced through nitroxide-mediated radical polymerization (NMRP), using mixtures of initiators with different decomposition constants to achieve narrow molar mass distributions and polydispersity indices close to 1.0, packaged in vials for easy use in GPC calibration.
GEL filtration standard
PatentWO2009058796A1
Innovation
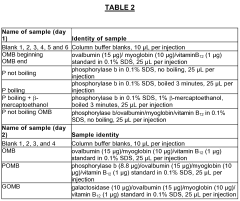
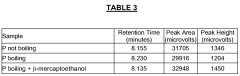
- A gel filtration standard comprising specific protein combinations such as ovalbumin, myoglobin, and vitamin B12, or phosphorylase b, ovalbumin, and myoglobin, with optional reducing agents like dithiothreitol, is developed to provide sharp, symmetrical peaks suitable for denatured conditions, covering molecular weight ranges from 1.35 kDa to 97 kDa, enabling effective system suitability testing.
Regulatory Compliance and Quality Assurance
Regulatory compliance in gel permeation chromatography (GPC) calibration is governed by stringent international standards that ensure consistency, reliability, and comparability of analytical results across laboratories worldwide. The International Organization for Standardization (ISO) has established several key standards specifically addressing GPC calibration methodologies, including ISO 13885 and ISO 16014 series, which provide comprehensive frameworks for polymer characterization using GPC techniques.
These standards define specific protocols for calibration procedures, including the selection of appropriate calibration standards, preparation methods, and data analysis techniques. Compliance with these standards is essential for laboratories seeking accreditation and for manufacturers ensuring product quality meets international requirements.
Quality assurance in GPC calibration encompasses systematic monitoring and documentation of calibration processes. This includes regular verification of instrument performance, validation of calibration curves, and implementation of quality control samples. The United States Pharmacopeia (USP) and European Pharmacopoeia (EP) provide additional guidance specific to pharmaceutical applications, detailing requirements for system suitability tests and acceptance criteria.
For regulated industries such as pharmaceuticals and medical devices, adherence to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory when performing GPC analyses. These regulations require comprehensive documentation of calibration procedures, including certificates of analysis for reference standards, maintenance records, and training documentation for personnel.
Traceability to internationally recognized reference materials is a fundamental aspect of regulatory compliance in GPC calibration. The National Institute of Standards and Technology (NIST) and other national metrology institutes provide certified reference materials that serve as primary standards for calibration. Laboratories must maintain clear documentation demonstrating the traceability chain from their working standards to these primary references.
Audit readiness represents another critical component of regulatory compliance. Laboratories must maintain detailed records of calibration activities, including raw data, calculations, and final reports. These records must be readily accessible during regulatory inspections and customer audits, with electronic data requiring compliance with data integrity principles outlined in 21 CFR Part 11 or equivalent regulations.
Risk assessment methodologies are increasingly incorporated into regulatory frameworks for analytical techniques including GPC. This involves identifying potential sources of error in calibration procedures, implementing appropriate control measures, and establishing acceptance criteria that ensure the reliability of molecular weight determinations for critical applications.
These standards define specific protocols for calibration procedures, including the selection of appropriate calibration standards, preparation methods, and data analysis techniques. Compliance with these standards is essential for laboratories seeking accreditation and for manufacturers ensuring product quality meets international requirements.
Quality assurance in GPC calibration encompasses systematic monitoring and documentation of calibration processes. This includes regular verification of instrument performance, validation of calibration curves, and implementation of quality control samples. The United States Pharmacopeia (USP) and European Pharmacopoeia (EP) provide additional guidance specific to pharmaceutical applications, detailing requirements for system suitability tests and acceptance criteria.
For regulated industries such as pharmaceuticals and medical devices, adherence to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory when performing GPC analyses. These regulations require comprehensive documentation of calibration procedures, including certificates of analysis for reference standards, maintenance records, and training documentation for personnel.
Traceability to internationally recognized reference materials is a fundamental aspect of regulatory compliance in GPC calibration. The National Institute of Standards and Technology (NIST) and other national metrology institutes provide certified reference materials that serve as primary standards for calibration. Laboratories must maintain clear documentation demonstrating the traceability chain from their working standards to these primary references.
Audit readiness represents another critical component of regulatory compliance. Laboratories must maintain detailed records of calibration activities, including raw data, calculations, and final reports. These records must be readily accessible during regulatory inspections and customer audits, with electronic data requiring compliance with data integrity principles outlined in 21 CFR Part 11 or equivalent regulations.
Risk assessment methodologies are increasingly incorporated into regulatory frameworks for analytical techniques including GPC. This involves identifying potential sources of error in calibration procedures, implementing appropriate control measures, and establishing acceptance criteria that ensure the reliability of molecular weight determinations for critical applications.
Cross-Laboratory Validation Methods
Cross-laboratory validation represents a critical component in establishing reliable and reproducible gel permeation chromatography (GPC) calibration methods. The variability between different laboratory environments, equipment configurations, and operator techniques necessitates robust validation protocols to ensure consistent results across multiple facilities.
International standards organizations, particularly ISO, have developed comprehensive frameworks for cross-laboratory validation of GPC calibration. ISO 13885 series specifically addresses polymer characterization by GPC, with ISO 13885-1 focusing on calibration reference materials. These standards outline interlaboratory comparison procedures, statistical analysis methods, and acceptance criteria for validating calibration curves across different laboratories.
The round-robin testing approach forms the backbone of cross-laboratory validation for GPC calibration. This methodology involves multiple laboratories analyzing identical samples using standardized protocols, followed by statistical evaluation of the results. ASTM D6474 provides guidelines for conducting such collaborative studies, emphasizing the importance of sample homogeneity and stability during distribution to participating laboratories.
Statistical methods for evaluating cross-laboratory data include calculation of reproducibility limits (R), repeatability limits (r), and Horwitz ratios (HorRat). These metrics help quantify the expected variability between different laboratories and determine whether observed differences fall within acceptable ranges. ISO 5725 series details the statistical approaches for assessing accuracy and precision in measurement methods across different testing facilities.
Proficiency testing programs, such as those operated by organizations like NIST and LGC Standards, provide external quality assessment for laboratories performing GPC calibration. These programs distribute calibration standards with undisclosed molecular weight values, allowing laboratories to demonstrate their calibration accuracy against reference values. Performance is typically evaluated using z-scores, with values between -2 and +2 considered satisfactory.
Digital data exchange formats have emerged as important tools for cross-laboratory validation. The JCAMP-DX and AnIML formats facilitate the sharing of raw chromatographic data between laboratories, enabling more detailed comparisons beyond just the final calibration parameters. This approach allows identification of potential sources of variability in data processing algorithms and integration methods.
Continuous monitoring programs represent the most comprehensive approach to cross-laboratory validation. These programs involve regular exchange of calibration standards between participating laboratories, with statistical control charts tracking performance over time. Such longitudinal monitoring helps identify systematic shifts in calibration accuracy and provides early warning of potential issues with laboratory procedures or equipment performance.
International standards organizations, particularly ISO, have developed comprehensive frameworks for cross-laboratory validation of GPC calibration. ISO 13885 series specifically addresses polymer characterization by GPC, with ISO 13885-1 focusing on calibration reference materials. These standards outline interlaboratory comparison procedures, statistical analysis methods, and acceptance criteria for validating calibration curves across different laboratories.
The round-robin testing approach forms the backbone of cross-laboratory validation for GPC calibration. This methodology involves multiple laboratories analyzing identical samples using standardized protocols, followed by statistical evaluation of the results. ASTM D6474 provides guidelines for conducting such collaborative studies, emphasizing the importance of sample homogeneity and stability during distribution to participating laboratories.
Statistical methods for evaluating cross-laboratory data include calculation of reproducibility limits (R), repeatability limits (r), and Horwitz ratios (HorRat). These metrics help quantify the expected variability between different laboratories and determine whether observed differences fall within acceptable ranges. ISO 5725 series details the statistical approaches for assessing accuracy and precision in measurement methods across different testing facilities.
Proficiency testing programs, such as those operated by organizations like NIST and LGC Standards, provide external quality assessment for laboratories performing GPC calibration. These programs distribute calibration standards with undisclosed molecular weight values, allowing laboratories to demonstrate their calibration accuracy against reference values. Performance is typically evaluated using z-scores, with values between -2 and +2 considered satisfactory.
Digital data exchange formats have emerged as important tools for cross-laboratory validation. The JCAMP-DX and AnIML formats facilitate the sharing of raw chromatographic data between laboratories, enabling more detailed comparisons beyond just the final calibration parameters. This approach allows identification of potential sources of variability in data processing algorithms and integration methods.
Continuous monitoring programs represent the most comprehensive approach to cross-laboratory validation. These programs involve regular exchange of calibration standards between participating laboratories, with statistical control charts tracking performance over time. Such longitudinal monitoring helps identify systematic shifts in calibration accuracy and provides early warning of potential issues with laboratory procedures or equipment performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!