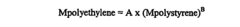Patent landscape of gel permeation chromatography systems and columns
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
GPC Technology Evolution and Research Objectives
Gel Permeation Chromatography (GPC) has evolved significantly since its inception in the 1960s, transforming from a rudimentary analytical technique to a sophisticated method for polymer characterization. The historical trajectory of GPC technology reveals a continuous refinement in separation mechanisms, detection capabilities, and data analysis methodologies. Initially developed as a size-exclusion technique for polymer analysis, GPC has expanded its applications across pharmaceutical, environmental, and materials science domains.
The evolution of GPC systems has been characterized by several key technological advancements. Early systems utilized simple glass columns with limited resolution and reproducibility. The 1980s witnessed the introduction of high-performance liquid chromatography (HPLC) principles to GPC, resulting in higher pressure capabilities and improved column efficiency. By the 1990s, multi-detector arrays began to emerge, combining light scattering, viscometry, and refractive index detection to provide comprehensive molecular weight distribution analysis.
Column technology has undergone parallel advancement, transitioning from irregular silica particles to precisely engineered porous media with controlled pore size distributions. Modern columns feature sub-2μm particles, monolithic structures, and hybrid organic-inorganic materials that offer superior resolution and stability across diverse mobile phase conditions. The development of specialized stationary phases has enabled the analysis of increasingly complex polymer architectures, including branched, star-shaped, and block copolymers.
Current research objectives in GPC technology focus on several critical areas. First, enhancing separation efficiency through novel stationary phase designs and column geometries to achieve higher resolution with shorter analysis times. Second, improving detector sensitivity and selectivity to characterize polymers at increasingly lower concentrations, addressing challenges in trace analysis. Third, developing robust calibration methodologies and universal calibration approaches to provide accurate molecular weight determinations across diverse polymer types.
The integration of GPC with complementary analytical techniques represents another significant research direction. Hyphenated systems combining GPC with mass spectrometry, infrared spectroscopy, or nuclear magnetic resonance offer unprecedented insights into polymer composition and structure. Additionally, miniaturization efforts aim to reduce sample volume requirements and increase throughput through microfluidic GPC platforms.
Patent landscape analysis reveals concentrated innovation efforts in automated sample preparation, column lifetime extension, and data processing algorithms. The technical objectives driving current patent activity include achieving greater reproducibility, minimizing inter-laboratory variations, and developing predictive models for polymer behavior based on GPC-derived parameters. These advancements collectively aim to transform GPC from a primarily analytical tool to a predictive technology capable of informing polymer design and processing decisions.
The evolution of GPC systems has been characterized by several key technological advancements. Early systems utilized simple glass columns with limited resolution and reproducibility. The 1980s witnessed the introduction of high-performance liquid chromatography (HPLC) principles to GPC, resulting in higher pressure capabilities and improved column efficiency. By the 1990s, multi-detector arrays began to emerge, combining light scattering, viscometry, and refractive index detection to provide comprehensive molecular weight distribution analysis.
Column technology has undergone parallel advancement, transitioning from irregular silica particles to precisely engineered porous media with controlled pore size distributions. Modern columns feature sub-2μm particles, monolithic structures, and hybrid organic-inorganic materials that offer superior resolution and stability across diverse mobile phase conditions. The development of specialized stationary phases has enabled the analysis of increasingly complex polymer architectures, including branched, star-shaped, and block copolymers.
Current research objectives in GPC technology focus on several critical areas. First, enhancing separation efficiency through novel stationary phase designs and column geometries to achieve higher resolution with shorter analysis times. Second, improving detector sensitivity and selectivity to characterize polymers at increasingly lower concentrations, addressing challenges in trace analysis. Third, developing robust calibration methodologies and universal calibration approaches to provide accurate molecular weight determinations across diverse polymer types.
The integration of GPC with complementary analytical techniques represents another significant research direction. Hyphenated systems combining GPC with mass spectrometry, infrared spectroscopy, or nuclear magnetic resonance offer unprecedented insights into polymer composition and structure. Additionally, miniaturization efforts aim to reduce sample volume requirements and increase throughput through microfluidic GPC platforms.
Patent landscape analysis reveals concentrated innovation efforts in automated sample preparation, column lifetime extension, and data processing algorithms. The technical objectives driving current patent activity include achieving greater reproducibility, minimizing inter-laboratory variations, and developing predictive models for polymer behavior based on GPC-derived parameters. These advancements collectively aim to transform GPC from a primarily analytical tool to a predictive technology capable of informing polymer design and processing decisions.
Market Analysis for GPC Systems and Columns
The global market for Gel Permeation Chromatography (GPC) systems and columns has experienced steady growth over the past decade, driven primarily by increasing applications in pharmaceutical research, polymer science, and academic research. The current market size is estimated at approximately $1.2 billion, with a compound annual growth rate (CAGR) of 5.8% projected through 2028.
North America dominates the GPC market with roughly 40% market share, followed by Europe (30%) and Asia-Pacific (25%). The remaining 5% is distributed across other regions. This regional distribution reflects the concentration of pharmaceutical and chemical industries, as well as research institutions in these areas. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth due to expanding research infrastructure and increasing investments in life sciences.
By application segment, pharmaceutical and biotechnology sectors account for the largest market share (45%), followed by academic and research institutions (30%), and industrial applications including polymer manufacturing (25%). The pharmaceutical sector's dominance stems from stringent regulatory requirements for drug development and quality control processes where GPC plays a crucial role in molecular weight determination and protein characterization.
Key market drivers include technological advancements in GPC systems, increasing R&D investments in pharmaceutical and biotechnology sectors, and growing demand for high-performance analytical techniques. The integration of GPC with other analytical methods, such as mass spectrometry and multi-angle light scattering (MALS), has expanded its application scope and market potential.
Market restraints include the high cost of advanced GPC systems, technical complexity requiring specialized training, and competition from alternative technologies like field-flow fractionation. Additionally, budget constraints in academic institutions and small research facilities limit market penetration in these segments.
The consumables segment, particularly columns, represents a significant revenue stream with higher replacement frequency compared to instruments. Specialty columns designed for specific applications command premium pricing and contribute substantially to market revenues. The service segment, including maintenance contracts and technical support, is growing at a faster rate than hardware sales, indicating a shift toward service-oriented business models among major vendors.
Future market trends point toward miniaturization of GPC systems, development of hybrid technologies combining multiple separation techniques, and increasing automation to improve throughput and reproducibility. The emergence of bio-based polymers and nanomaterials is expected to create new application niches for GPC technology, potentially expanding the market further in the coming years.
North America dominates the GPC market with roughly 40% market share, followed by Europe (30%) and Asia-Pacific (25%). The remaining 5% is distributed across other regions. This regional distribution reflects the concentration of pharmaceutical and chemical industries, as well as research institutions in these areas. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth due to expanding research infrastructure and increasing investments in life sciences.
By application segment, pharmaceutical and biotechnology sectors account for the largest market share (45%), followed by academic and research institutions (30%), and industrial applications including polymer manufacturing (25%). The pharmaceutical sector's dominance stems from stringent regulatory requirements for drug development and quality control processes where GPC plays a crucial role in molecular weight determination and protein characterization.
Key market drivers include technological advancements in GPC systems, increasing R&D investments in pharmaceutical and biotechnology sectors, and growing demand for high-performance analytical techniques. The integration of GPC with other analytical methods, such as mass spectrometry and multi-angle light scattering (MALS), has expanded its application scope and market potential.
Market restraints include the high cost of advanced GPC systems, technical complexity requiring specialized training, and competition from alternative technologies like field-flow fractionation. Additionally, budget constraints in academic institutions and small research facilities limit market penetration in these segments.
The consumables segment, particularly columns, represents a significant revenue stream with higher replacement frequency compared to instruments. Specialty columns designed for specific applications command premium pricing and contribute substantially to market revenues. The service segment, including maintenance contracts and technical support, is growing at a faster rate than hardware sales, indicating a shift toward service-oriented business models among major vendors.
Future market trends point toward miniaturization of GPC systems, development of hybrid technologies combining multiple separation techniques, and increasing automation to improve throughput and reproducibility. The emergence of bio-based polymers and nanomaterials is expected to create new application niches for GPC technology, potentially expanding the market further in the coming years.
Current Technical Challenges in GPC Technology
Despite significant advancements in gel permeation chromatography (GPC) technology over the past decades, several technical challenges continue to impede optimal performance and broader application. One of the most persistent issues is resolution limitations, particularly when analyzing complex polymer mixtures with similar molecular weights or when attempting to separate macromolecules with broad molecular weight distributions. Current column technologies struggle to provide sufficient resolution for these challenging separations, often resulting in peak overlapping and inaccurate molecular weight determinations.
Column efficiency degradation represents another significant challenge. GPC columns typically experience performance deterioration over time due to mechanical stress, chemical degradation, and particulate contamination. This leads to increased back pressure, peak broadening, and reduced resolution, ultimately shortening column lifespan and increasing operational costs. The development of more robust stationary phases that maintain separation efficiency over extended periods remains an active area of research.
Calibration accuracy presents a fundamental challenge in GPC analysis. The reliance on relative calibration using polymer standards introduces inherent errors when analyzing polymers with different chemical compositions or architectures from the calibration standards. While universal calibration approaches using viscometry or light scattering detection have improved accuracy, they add complexity and cost to GPC systems, limiting widespread adoption in routine analysis settings.
Sample preparation challenges also persist in GPC technology. Complete dissolution of polymer samples without degradation or aggregation is critical for accurate analysis but remains difficult for many complex materials. Incomplete dissolution leads to filtration issues, column clogging, and erroneous molecular weight determinations. Additionally, the presence of charged groups or specific interactions between analytes and the stationary phase can cause non-size-exclusion separation mechanisms, complicating data interpretation.
Detection sensitivity limitations represent a significant barrier, particularly for analyzing low concentration samples or polymers with weak UV absorption. While multi-detector systems combining UV, refractive index, light scattering, and viscometry have enhanced detection capabilities, they introduce additional complexity in instrument operation and data analysis. The integration and synchronization of multiple detectors remain technically challenging and often require specialized expertise.
Throughput constraints continue to limit GPC applications in high-volume testing environments. Traditional GPC analyses typically require 30-60 minutes per sample, making the technique less suitable for rapid screening applications. Recent developments in ultra-high-performance liquid chromatography (UHPLC) applied to GPC have improved speed, but often at the expense of resolution or sample capacity, creating a persistent trade-off between analysis time and separation quality.
Column efficiency degradation represents another significant challenge. GPC columns typically experience performance deterioration over time due to mechanical stress, chemical degradation, and particulate contamination. This leads to increased back pressure, peak broadening, and reduced resolution, ultimately shortening column lifespan and increasing operational costs. The development of more robust stationary phases that maintain separation efficiency over extended periods remains an active area of research.
Calibration accuracy presents a fundamental challenge in GPC analysis. The reliance on relative calibration using polymer standards introduces inherent errors when analyzing polymers with different chemical compositions or architectures from the calibration standards. While universal calibration approaches using viscometry or light scattering detection have improved accuracy, they add complexity and cost to GPC systems, limiting widespread adoption in routine analysis settings.
Sample preparation challenges also persist in GPC technology. Complete dissolution of polymer samples without degradation or aggregation is critical for accurate analysis but remains difficult for many complex materials. Incomplete dissolution leads to filtration issues, column clogging, and erroneous molecular weight determinations. Additionally, the presence of charged groups or specific interactions between analytes and the stationary phase can cause non-size-exclusion separation mechanisms, complicating data interpretation.
Detection sensitivity limitations represent a significant barrier, particularly for analyzing low concentration samples or polymers with weak UV absorption. While multi-detector systems combining UV, refractive index, light scattering, and viscometry have enhanced detection capabilities, they introduce additional complexity in instrument operation and data analysis. The integration and synchronization of multiple detectors remain technically challenging and often require specialized expertise.
Throughput constraints continue to limit GPC applications in high-volume testing environments. Traditional GPC analyses typically require 30-60 minutes per sample, making the technique less suitable for rapid screening applications. Recent developments in ultra-high-performance liquid chromatography (UHPLC) applied to GPC have improved speed, but often at the expense of resolution or sample capacity, creating a persistent trade-off between analysis time and separation quality.
Current Patent-Protected GPC Solutions
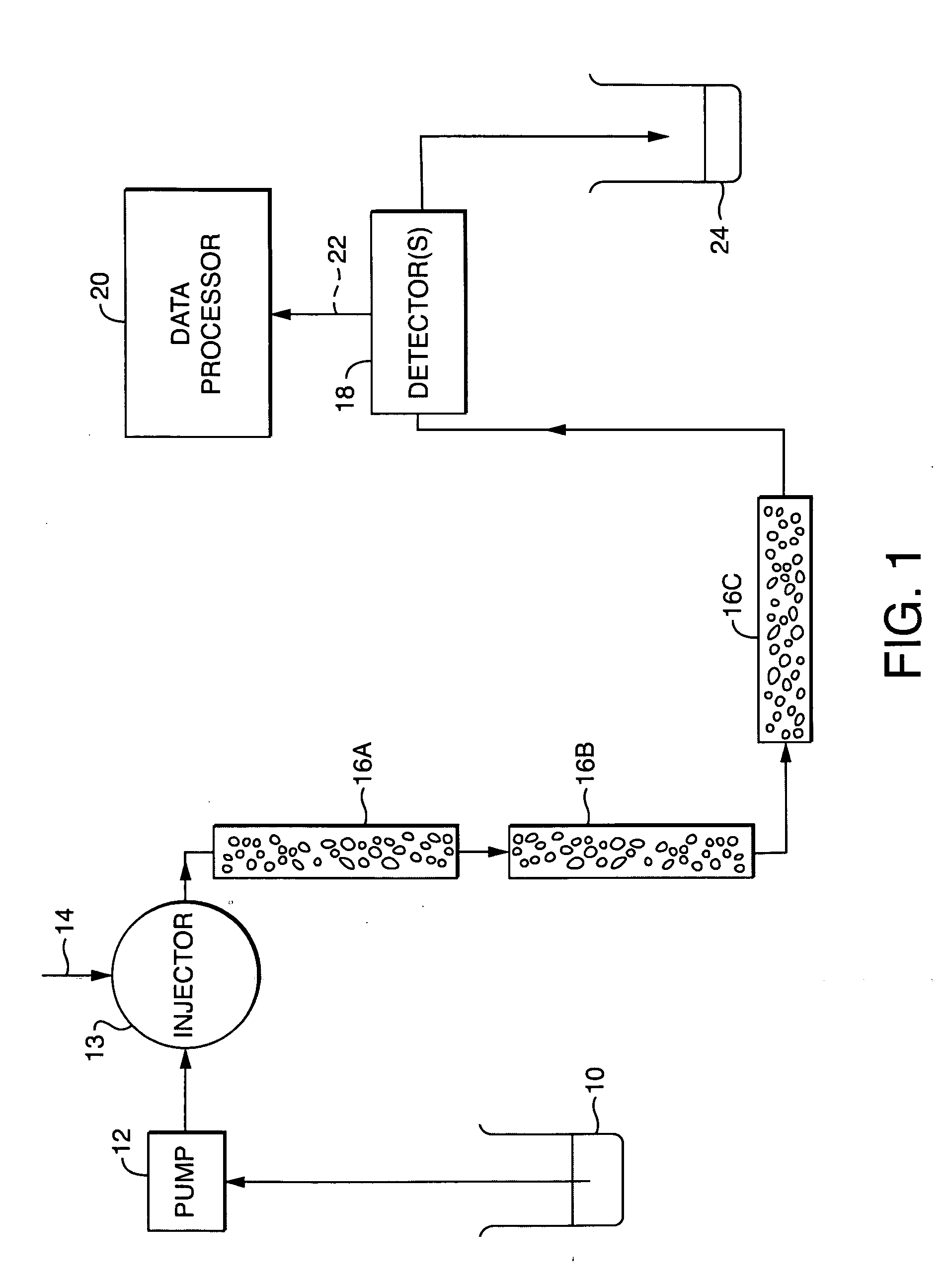
01 GPC system design and components
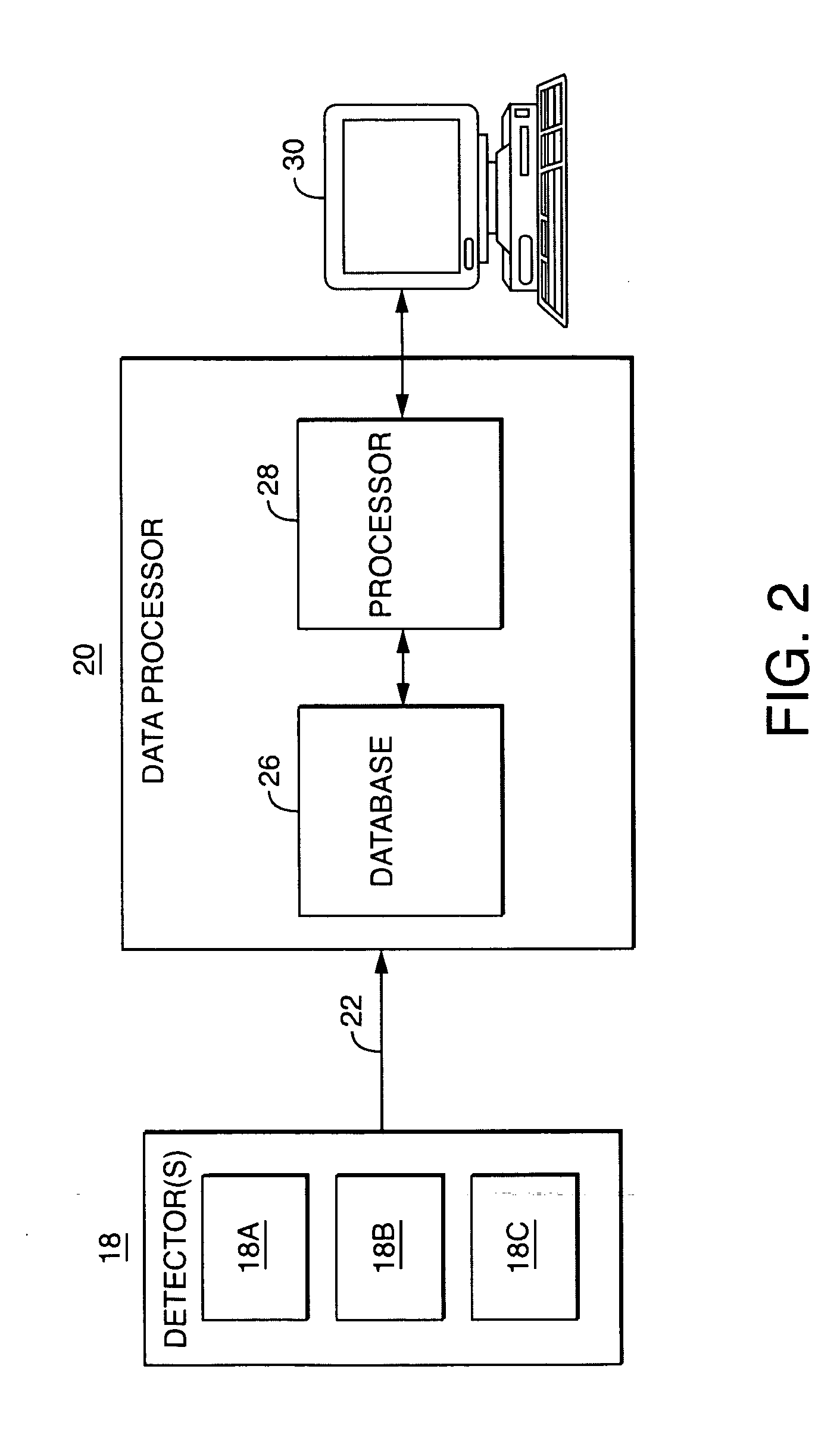
Gel permeation chromatography systems consist of various components including pumps, detectors, columns, and data processing units. These systems are designed for efficient separation and analysis of polymers based on their molecular size. Advanced designs incorporate features for improved flow control, temperature regulation, and automated sample handling to enhance separation efficiency and reproducibility of results.- GPC system design and components: Gel permeation chromatography systems consist of various components including pumps, detectors, columns, and data processing units. These systems are designed to separate molecules based on their size as they pass through a porous gel matrix. Advanced designs incorporate features for improved flow control, temperature regulation, and automated sample handling to enhance separation efficiency and reproducibility.
- Column technology and packing materials: GPC columns utilize specialized packing materials with controlled pore sizes to achieve effective molecular separation. Various materials such as cross-linked polystyrene, silica-based gels, and organic polymers are used as stationary phases. Column design factors including length, diameter, particle size, and pore distribution significantly impact separation performance, resolution, and analysis time.
- Detection and analysis methods: Modern GPC systems employ various detection technologies including refractive index detectors, UV-visible spectrophotometers, light scattering detectors, and viscometers. Multi-detector arrangements allow for comprehensive characterization of molecular weight distribution and polymer properties. Advanced data analysis algorithms and software enable accurate interpretation of chromatograms and calculation of molecular weight parameters.
- Calibration and standardization techniques: Accurate GPC analysis requires proper calibration using reference standards with known molecular weights. Calibration methods include conventional calibration with narrow molecular weight standards, universal calibration based on hydrodynamic volume, and absolute molecular weight determination using light scattering. Standardization procedures ensure reproducibility and comparability of results across different laboratories and instruments.
- Specialized applications and modifications: GPC systems have been adapted for specialized applications including high-temperature GPC for analyzing high molecular weight polymers, aqueous GPC for water-soluble polymers, and micro-GPC for small sample volumes. Modifications to conventional systems include coupling with mass spectrometry, incorporating online viscometers, and developing high-throughput systems for industrial quality control and research applications.
02 Column technology and packing materials
GPC columns utilize specialized packing materials with controlled pore sizes to separate molecules based on their hydrodynamic volume. Various materials such as cross-linked polystyrene, silica gels, and organic polymers are used as stationary phases. Column design factors including length, diameter, particle size, and pore distribution significantly impact resolution, analysis time, and separation efficiency for different molecular weight ranges.Expand Specific Solutions03 Calibration and standardization methods
Accurate molecular weight determination in GPC requires proper calibration using reference standards of known molecular weights. Calibration methods include conventional calibration with narrow molecular weight standards, universal calibration based on hydrodynamic volume, and multi-detector approaches. These techniques ensure reliable and reproducible molecular weight distribution analysis across different samples and instrument configurations.Expand Specific Solutions04 Detection technologies and multi-detector systems
Modern GPC systems incorporate various detection technologies including refractive index, UV-visible, light scattering, and viscometric detectors. Multi-detector configurations allow simultaneous measurement of different molecular parameters, providing comprehensive characterization of polymers. Advanced detection systems improve sensitivity, accuracy, and the ability to analyze complex polymer structures and compositions.Expand Specific Solutions05 Specialized applications and modifications
GPC systems and columns are adapted for specialized applications including high-temperature GPC for polyolefin analysis, aqueous GPC for water-soluble polymers, and micro-GPC for small sample volumes. Modifications to traditional GPC include coupling with mass spectrometry, FTIR, or NMR for enhanced structural characterization. These specialized systems address specific analytical challenges in polymer science, pharmaceuticals, and materials research.Expand Specific Solutions
Leading Companies in GPC Systems Manufacturing
The gel permeation chromatography (GPC) systems market is currently in a mature growth phase, characterized by established technologies and steady innovation. The global market size is estimated at approximately $1.2 billion, with projected annual growth of 5-7% driven by increasing applications in pharmaceutical, polymer, and biotechnology research. The competitive landscape is dominated by specialized analytical instrument manufacturers, with Waters Technology Corp. leading the market through its extensive column portfolio and advanced detection systems. Agilent Technologies, Bio-Rad Laboratories, and Shimadzu Corp. maintain significant market shares with comprehensive product offerings. Cytiva Sweden AB (formerly GE Healthcare Life Sciences) has strengthened its position through innovations in biomolecule separation. Emerging players like Biotage AB and KNAUER are gaining traction through specialized column technologies and application-specific solutions, while pharmaceutical companies like Amgen utilize proprietary GPC systems for research applications.
Waters Technology Corp.
Technical Solution: Waters Technology Corp. has pioneered advanced gel permeation chromatography (GPC) systems with their ACQUITY Advanced Polymer Chromatography (APC) technology. This system utilizes sub-2-μm particle columns that provide significantly improved resolution and sensitivity compared to traditional GPC columns[1]. Their patented technology incorporates rigid, highly cross-linked ethylene-bridged hybrid particles that withstand high pressures while maintaining column efficiency. Waters has developed specialized column chemistries optimized for different polymer types, including their XT columns for high-temperature applications up to 220°C[2]. Their systems feature holistic integration between hardware and software, with the ACQUITY platform offering up to 10 times faster analysis and using up to 95% less solvent than traditional GPC systems[3]. Waters' patent portfolio includes innovations in column packing methods, detector technologies, and calibration techniques that enhance the accuracy of molecular weight determinations.
Strengths: Superior resolution and speed compared to conventional GPC systems; comprehensive integration of hardware and software; reduced solvent consumption; versatility across polymer types. Weaknesses: Higher initial investment costs; proprietary consumables may limit flexibility; requires specialized training for optimal operation; some applications still require traditional GPC approaches.
Cytiva Sweden AB
Technical Solution: Cytiva (formerly part of GE Healthcare Life Sciences) has developed an extensive patent portfolio around their Superdex and Superose GPC/SEC columns, which utilize proprietary composite matrix technology combining dextran and highly cross-linked agarose[1]. This unique composition provides exceptional resolution for biomolecule separation while maintaining mechanical stability. Their ÄKTA chromatography systems incorporate patented flow path designs that minimize band broadening, critical for accurate molecular weight determination[2]. Cytiva's patents cover specialized column packing methods that ensure uniform bed density and consistent performance across manufacturing batches. Their Increase SEC columns feature proprietary particle technology that enables higher flow rates without compromising resolution, allowing for faster analysis times in industrial applications[3]. Recent patent filings show innovations in automated column screening and method development tools that optimize separation conditions based on sample characteristics. Cytiva has also patented specialized calibration standards and methods specifically designed for protein and peptide molecular weight determination. Their technology includes innovations in column hardware design, particularly their patented column connectors that minimize dead volume and maintain separation efficiency.
Strengths: Exceptional performance with biological macromolecules; highly reproducible manufacturing processes; comprehensive range of pore sizes optimized for different molecular weight ranges; excellent chemical stability in aqueous buffers. Weaknesses: Limited offerings for organic solvent applications; higher cost compared to some competitors; specialized systems may require significant method development for non-standard applications; columns generally not compatible with ultra-high pressure systems.
Key Patent Analysis for GPC Column Technologies
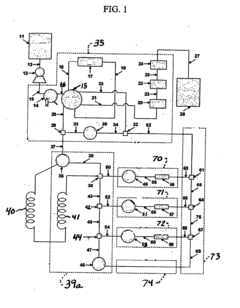
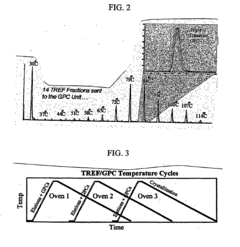
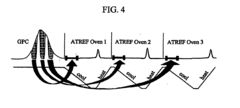
Apparatus and methods for polymer characterization
PatentInactiveEP1853905B1
Innovation
- A method and apparatus that enables increased TREF/GPC or GPC/TREF characterizations by using in-line TREF columns with controlled temperature cycles and gel permeation chromatography, allowing for simultaneous analysis of multiple polymer samples and enhanced data collection.
System and method for determining radius of gyration, molecular weight, and intrinsic viscosity of a polymeric distribution using gel permeation chromatography and light scattering detection
PatentInactiveUS20050240385A1
Innovation
- The method employs a GPC chromatograph system with multi-angle laser light-scattering, refractive index, and viscometer detectors, using novel algorithms to compute the radius of gyration for each slice, which involves fitting parameterized models simultaneously to all data collected at each slice, reducing the impact of detector noise and providing more stable estimates.
Intellectual Property Strategy in Chromatography
The intellectual property landscape surrounding gel permeation chromatography (GPC) systems and columns reveals strategic patterns that companies must navigate to maintain competitive advantage. Patent analysis shows that major chromatography companies like Waters Corporation, Agilent Technologies, and Tosoh Bioscience hold significant patent portfolios covering fundamental GPC technologies, column designs, and detection methods.
Strategic patent filing trends indicate geographical concentration in the United States, Europe, Japan, and increasingly China, reflecting the global market importance of these regions. Companies typically employ a multi-layered IP approach, protecting core technologies through composition-of-matter patents while building defensive portfolios around manufacturing processes, applications, and system integration.
Cross-licensing agreements have become increasingly common in the chromatography sector, particularly for standardized column technologies and interfaces. This collaborative approach enables companies to access complementary technologies while reducing litigation risks in a highly specialized field where innovation often builds incrementally on existing platforms.
Recent patent activity shows growing focus on specialized column materials for biomolecule separation, high-throughput systems, and miniaturized GPC technologies. The emergence of patents combining GPC with advanced detection methods like multi-angle light scattering (MALS) and mass spectrometry indicates a shift toward integrated analytical solutions.
Freedom-to-operate considerations are particularly complex in GPC, as many fundamental technologies are covered by patents dating back decades, some of which are expiring or have expired. This creates opportunities for new market entrants while established players pivot toward patenting novel applications and refinements.
Patent litigation in the chromatography space has historically focused on column chemistry and manufacturing processes rather than system design, suggesting these areas represent the highest commercial value. Companies developing novel GPC technologies should conduct thorough IP landscape analyses to identify white space opportunities, particularly in emerging application areas like nanomaterials characterization and biopharmaceutical analysis.
For sustainable competitive advantage, companies should balance aggressive patenting of truly novel innovations with strategic licensing of platform technologies, while maintaining trade secret protection for manufacturing know-how that is difficult to reverse engineer from the final product.
Strategic patent filing trends indicate geographical concentration in the United States, Europe, Japan, and increasingly China, reflecting the global market importance of these regions. Companies typically employ a multi-layered IP approach, protecting core technologies through composition-of-matter patents while building defensive portfolios around manufacturing processes, applications, and system integration.
Cross-licensing agreements have become increasingly common in the chromatography sector, particularly for standardized column technologies and interfaces. This collaborative approach enables companies to access complementary technologies while reducing litigation risks in a highly specialized field where innovation often builds incrementally on existing platforms.
Recent patent activity shows growing focus on specialized column materials for biomolecule separation, high-throughput systems, and miniaturized GPC technologies. The emergence of patents combining GPC with advanced detection methods like multi-angle light scattering (MALS) and mass spectrometry indicates a shift toward integrated analytical solutions.
Freedom-to-operate considerations are particularly complex in GPC, as many fundamental technologies are covered by patents dating back decades, some of which are expiring or have expired. This creates opportunities for new market entrants while established players pivot toward patenting novel applications and refinements.
Patent litigation in the chromatography space has historically focused on column chemistry and manufacturing processes rather than system design, suggesting these areas represent the highest commercial value. Companies developing novel GPC technologies should conduct thorough IP landscape analyses to identify white space opportunities, particularly in emerging application areas like nanomaterials characterization and biopharmaceutical analysis.
For sustainable competitive advantage, companies should balance aggressive patenting of truly novel innovations with strategic licensing of platform technologies, while maintaining trade secret protection for manufacturing know-how that is difficult to reverse engineer from the final product.
Regulatory Standards for GPC Equipment
Gel Permeation Chromatography (GPC) systems and columns are subject to stringent regulatory standards across various jurisdictions to ensure accuracy, reliability, and reproducibility of analytical results. These standards are particularly critical as GPC is widely used in pharmaceutical development, polymer characterization, and quality control processes where precision is paramount.
The International Organization for Standardization (ISO) has established several standards specifically addressing GPC equipment, including ISO 13885 series which outlines requirements for GPC instrumentation calibration and validation. These standards define acceptable performance parameters such as resolution, precision, and detection limits that manufacturers must meet to ensure compliance.
In the pharmaceutical industry, GPC equipment must adhere to guidelines set forth by regulatory bodies such as the FDA in the United States and the EMA in Europe. The FDA's 21 CFR Part 11 regulations govern electronic records and signatures, directly impacting the data management systems integrated with modern GPC equipment. Similarly, Good Manufacturing Practice (GMP) guidelines contain specific provisions for analytical instrumentation validation.
ASTM International has developed standard test methods for GPC analysis, including ASTM D6474 and ASTM D5296, which specify equipment requirements and operational parameters. These standards ensure that GPC systems can accurately determine molecular weight distributions and other critical polymer characteristics.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain monographs that reference GPC methods for specific applications, indirectly establishing requirements for equipment capabilities. USP <621> on Chromatography provides general guidelines applicable to GPC systems, including system suitability requirements.
Japanese Industrial Standards (JIS) also include specifications for GPC equipment, particularly JIS K 7252, which addresses the determination of molecular mass and molecular mass distribution of polymers. These standards influence equipment design and performance specifications for manufacturers targeting the Japanese market.
Regulatory standards also address environmental and safety aspects of GPC equipment. RoHS (Restriction of Hazardous Substances) and WEEE (Waste Electrical and Electronic Equipment) directives in Europe impact the materials and components that can be used in GPC systems, as well as end-of-life management requirements.
Calibration and traceability standards, such as those from the National Institute of Standards and Technology (NIST), provide reference materials and protocols for ensuring GPC equipment accuracy. These standards are essential for establishing traceability in analytical measurements and are often referenced in regulatory compliance documentation.
The International Organization for Standardization (ISO) has established several standards specifically addressing GPC equipment, including ISO 13885 series which outlines requirements for GPC instrumentation calibration and validation. These standards define acceptable performance parameters such as resolution, precision, and detection limits that manufacturers must meet to ensure compliance.
In the pharmaceutical industry, GPC equipment must adhere to guidelines set forth by regulatory bodies such as the FDA in the United States and the EMA in Europe. The FDA's 21 CFR Part 11 regulations govern electronic records and signatures, directly impacting the data management systems integrated with modern GPC equipment. Similarly, Good Manufacturing Practice (GMP) guidelines contain specific provisions for analytical instrumentation validation.
ASTM International has developed standard test methods for GPC analysis, including ASTM D6474 and ASTM D5296, which specify equipment requirements and operational parameters. These standards ensure that GPC systems can accurately determine molecular weight distributions and other critical polymer characteristics.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain monographs that reference GPC methods for specific applications, indirectly establishing requirements for equipment capabilities. USP <621> on Chromatography provides general guidelines applicable to GPC systems, including system suitability requirements.
Japanese Industrial Standards (JIS) also include specifications for GPC equipment, particularly JIS K 7252, which addresses the determination of molecular mass and molecular mass distribution of polymers. These standards influence equipment design and performance specifications for manufacturers targeting the Japanese market.
Regulatory standards also address environmental and safety aspects of GPC equipment. RoHS (Restriction of Hazardous Substances) and WEEE (Waste Electrical and Electronic Equipment) directives in Europe impact the materials and components that can be used in GPC systems, as well as end-of-life management requirements.
Calibration and traceability standards, such as those from the National Institute of Standards and Technology (NIST), provide reference materials and protocols for ensuring GPC equipment accuracy. These standards are essential for establishing traceability in analytical measurements and are often referenced in regulatory compliance documentation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!