Atomic-Scale Characterization Techniques For SACs
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SACs Characterization Background and Objectives
Single-atom catalysts (SACs) represent a frontier in heterogeneous catalysis research, emerging as a revolutionary class of materials where individual metal atoms are dispersed on solid supports. The concept of SACs was first formally introduced in 2011, though earlier studies had observed similar phenomena without specifically identifying them as such. These catalysts bridge the gap between homogeneous and heterogeneous catalysis, combining the high selectivity and activity of molecular catalysts with the stability and recyclability of solid catalysts.
The development of SACs has been driven by the need for more efficient utilization of precious metals in catalytic processes. Traditional nanoparticle catalysts often waste significant portions of metal atoms buried within particle cores, whereas SACs achieve 100% atom efficiency with every metal atom potentially serving as an active site. This maximizes catalytic performance while minimizing material costs, particularly important for precious metals like platinum, palladium, and rhodium.
Characterization techniques for SACs have evolved dramatically over the past decade, enabling researchers to definitively identify and study these elusive single-atom structures. Early research relied heavily on aberration-corrected transmission electron microscopy (AC-TEM) and extended X-ray absorption fine structure (EXAFS) spectroscopy. However, these techniques alone were insufficient to fully understand the complex nature of SACs, particularly regarding their electronic structure and dynamic behavior under reaction conditions.
The primary objective of SAC characterization is to unambiguously confirm the atomic dispersion of metal atoms, distinguish them from small clusters or nanoparticles, and understand their coordination environment and electronic properties. This requires pushing analytical techniques to their ultimate limits of spatial resolution and sensitivity, often necessitating complementary approaches to build a comprehensive understanding.
Recent technological advances have expanded the characterization toolkit to include scanning transmission electron microscopy coupled with electron energy loss spectroscopy (STEM-EELS), in-situ/operando X-ray absorption spectroscopy, and advanced computational modeling. These techniques aim to reveal not just the static structure of SACs but also their dynamic behavior during catalytic reactions, providing insights into reaction mechanisms at the atomic level.
The ultimate goal of SAC characterization research is to establish clear structure-performance relationships that can guide rational catalyst design. By understanding how the identity of the metal atom, the nature of the support, and the specific coordination environment influence catalytic performance, researchers aim to develop next-generation catalysts with unprecedented activity, selectivity, and stability for applications ranging from environmental remediation to energy conversion and fine chemical synthesis.
The development of SACs has been driven by the need for more efficient utilization of precious metals in catalytic processes. Traditional nanoparticle catalysts often waste significant portions of metal atoms buried within particle cores, whereas SACs achieve 100% atom efficiency with every metal atom potentially serving as an active site. This maximizes catalytic performance while minimizing material costs, particularly important for precious metals like platinum, palladium, and rhodium.
Characterization techniques for SACs have evolved dramatically over the past decade, enabling researchers to definitively identify and study these elusive single-atom structures. Early research relied heavily on aberration-corrected transmission electron microscopy (AC-TEM) and extended X-ray absorption fine structure (EXAFS) spectroscopy. However, these techniques alone were insufficient to fully understand the complex nature of SACs, particularly regarding their electronic structure and dynamic behavior under reaction conditions.
The primary objective of SAC characterization is to unambiguously confirm the atomic dispersion of metal atoms, distinguish them from small clusters or nanoparticles, and understand their coordination environment and electronic properties. This requires pushing analytical techniques to their ultimate limits of spatial resolution and sensitivity, often necessitating complementary approaches to build a comprehensive understanding.
Recent technological advances have expanded the characterization toolkit to include scanning transmission electron microscopy coupled with electron energy loss spectroscopy (STEM-EELS), in-situ/operando X-ray absorption spectroscopy, and advanced computational modeling. These techniques aim to reveal not just the static structure of SACs but also their dynamic behavior during catalytic reactions, providing insights into reaction mechanisms at the atomic level.
The ultimate goal of SAC characterization research is to establish clear structure-performance relationships that can guide rational catalyst design. By understanding how the identity of the metal atom, the nature of the support, and the specific coordination environment influence catalytic performance, researchers aim to develop next-generation catalysts with unprecedented activity, selectivity, and stability for applications ranging from environmental remediation to energy conversion and fine chemical synthesis.
Market Applications and Demand Analysis for SACs
The market for Single-Atom Catalysts (SACs) has witnessed significant growth in recent years, driven by their exceptional catalytic performance and atom-efficiency. The global catalyst market, valued at approximately 33.9 billion USD in 2022, is projected to expand at a compound annual growth rate of 4.5% through 2030, with SACs representing an increasingly important segment within this space.
Industrial sectors including petrochemicals, fine chemicals, and environmental remediation constitute the primary demand sources for SACs. In petrochemical processing, SACs offer superior selectivity and activity for hydrogenation, oxidation, and coupling reactions, enabling more efficient production pathways with reduced energy requirements. The fine chemicals and pharmaceutical industries value SACs for their ability to catalyze complex transformations with unprecedented selectivity, potentially revolutionizing synthetic routes to high-value compounds.
Environmental applications represent perhaps the most rapidly expanding market segment for SACs. Their exceptional performance in catalytic converters, water purification systems, and emissions control technologies addresses growing regulatory pressures and sustainability initiatives worldwide. Particularly noteworthy is their application in hydrogen production and fuel cell technologies, where platinum-group metal SACs demonstrate remarkable activity for hydrogen evolution reactions at significantly reduced noble metal loadings.
The renewable energy sector presents substantial growth opportunities for SACs, especially in electrocatalysis for green hydrogen production and CO2 reduction. Market analysis indicates that companies investing in SAC technology for these applications could capture significant market share in the emerging clean energy economy, with projected market values reaching billions by 2030.
Regional market assessment reveals Asia-Pacific as the dominant region for SAC demand, driven by China's aggressive investments in catalysis research and environmental technologies. North America and Europe follow closely, with demand primarily stemming from specialty chemical production and automotive emissions control applications.
Key market challenges include scaling production methods while maintaining atomic dispersion, ensuring long-term stability under industrial conditions, and developing cost-effective characterization protocols. The high cost of advanced characterization techniques required for SAC development and quality control represents a significant market barrier, particularly for smaller enterprises and research institutions.
Market forecasts suggest that as characterization technologies become more accessible and standardized, the SAC market will experience accelerated growth across multiple industrial sectors, with particularly strong demand in applications requiring precious metal catalysts where the atom-efficiency of SACs offers compelling economic advantages.
Industrial sectors including petrochemicals, fine chemicals, and environmental remediation constitute the primary demand sources for SACs. In petrochemical processing, SACs offer superior selectivity and activity for hydrogenation, oxidation, and coupling reactions, enabling more efficient production pathways with reduced energy requirements. The fine chemicals and pharmaceutical industries value SACs for their ability to catalyze complex transformations with unprecedented selectivity, potentially revolutionizing synthetic routes to high-value compounds.
Environmental applications represent perhaps the most rapidly expanding market segment for SACs. Their exceptional performance in catalytic converters, water purification systems, and emissions control technologies addresses growing regulatory pressures and sustainability initiatives worldwide. Particularly noteworthy is their application in hydrogen production and fuel cell technologies, where platinum-group metal SACs demonstrate remarkable activity for hydrogen evolution reactions at significantly reduced noble metal loadings.
The renewable energy sector presents substantial growth opportunities for SACs, especially in electrocatalysis for green hydrogen production and CO2 reduction. Market analysis indicates that companies investing in SAC technology for these applications could capture significant market share in the emerging clean energy economy, with projected market values reaching billions by 2030.
Regional market assessment reveals Asia-Pacific as the dominant region for SAC demand, driven by China's aggressive investments in catalysis research and environmental technologies. North America and Europe follow closely, with demand primarily stemming from specialty chemical production and automotive emissions control applications.
Key market challenges include scaling production methods while maintaining atomic dispersion, ensuring long-term stability under industrial conditions, and developing cost-effective characterization protocols. The high cost of advanced characterization techniques required for SAC development and quality control represents a significant market barrier, particularly for smaller enterprises and research institutions.
Market forecasts suggest that as characterization technologies become more accessible and standardized, the SAC market will experience accelerated growth across multiple industrial sectors, with particularly strong demand in applications requiring precious metal catalysts where the atom-efficiency of SACs offers compelling economic advantages.
Current Challenges in Atomic-Scale Characterization
Despite significant advancements in single-atom catalyst (SAC) characterization techniques, researchers continue to face substantial challenges when attempting to precisely identify and analyze individual atoms dispersed on support materials. The primary difficulty stems from the inherent limitations of current imaging technologies when dealing with single atoms, which represent the smallest possible unit of catalytic material.
Aberration-corrected transmission electron microscopy (AC-TEM) and scanning transmission electron microscopy (STEM), while powerful, struggle with beam-induced damage to SACs during observation. The high-energy electron beams necessary for atomic resolution often cause metal atoms to migrate or aggregate, altering the very structures being studied. This creates a fundamental paradox where the act of observation changes the subject being observed.
Detection sensitivity presents another significant hurdle. The signal-to-noise ratio when characterizing single atoms is extremely low, making it difficult to distinguish actual atomic signals from background noise. This challenge is particularly pronounced when attempting to identify lighter elements or when analyzing SACs under realistic operating conditions rather than in high-vacuum environments.
In-situ and operando characterization techniques face additional complications. Creating instrumentation capable of maintaining atomic resolution while simultaneously exposing catalysts to reaction conditions (elevated temperatures, pressures, and reactive gases) remains technically demanding. The resulting data often contains artifacts that complicate interpretation.
The three-dimensional structural determination of SACs presents unique challenges as well. While techniques like atom probe tomography offer promising approaches, they remain limited in application scope and often require destructive sample preparation that may alter the original atomic configurations.
Spectroscopic techniques such as X-ray absorption spectroscopy (XAS) provide valuable electronic and coordination information but suffer from averaging effects across the entire sample. This makes it difficult to distinguish between truly isolated single atoms and small clusters when heterogeneity exists within the sample.
Perhaps most critically, correlating atomic structure with catalytic performance remains elusive. Current characterization methods often cannot capture the dynamic structural changes occurring during catalytic cycles, leaving significant gaps in understanding structure-function relationships at the atomic level. Bridging these characterization gaps will require innovative approaches combining multiple complementary techniques and developing new methodologies specifically designed for the unique challenges posed by single-atom catalysts.
Aberration-corrected transmission electron microscopy (AC-TEM) and scanning transmission electron microscopy (STEM), while powerful, struggle with beam-induced damage to SACs during observation. The high-energy electron beams necessary for atomic resolution often cause metal atoms to migrate or aggregate, altering the very structures being studied. This creates a fundamental paradox where the act of observation changes the subject being observed.
Detection sensitivity presents another significant hurdle. The signal-to-noise ratio when characterizing single atoms is extremely low, making it difficult to distinguish actual atomic signals from background noise. This challenge is particularly pronounced when attempting to identify lighter elements or when analyzing SACs under realistic operating conditions rather than in high-vacuum environments.
In-situ and operando characterization techniques face additional complications. Creating instrumentation capable of maintaining atomic resolution while simultaneously exposing catalysts to reaction conditions (elevated temperatures, pressures, and reactive gases) remains technically demanding. The resulting data often contains artifacts that complicate interpretation.
The three-dimensional structural determination of SACs presents unique challenges as well. While techniques like atom probe tomography offer promising approaches, they remain limited in application scope and often require destructive sample preparation that may alter the original atomic configurations.
Spectroscopic techniques such as X-ray absorption spectroscopy (XAS) provide valuable electronic and coordination information but suffer from averaging effects across the entire sample. This makes it difficult to distinguish between truly isolated single atoms and small clusters when heterogeneity exists within the sample.
Perhaps most critically, correlating atomic structure with catalytic performance remains elusive. Current characterization methods often cannot capture the dynamic structural changes occurring during catalytic cycles, leaving significant gaps in understanding structure-function relationships at the atomic level. Bridging these characterization gaps will require innovative approaches combining multiple complementary techniques and developing new methodologies specifically designed for the unique challenges posed by single-atom catalysts.
State-of-the-Art Characterization Methodologies
01 Electron Microscopy Techniques for SACs Characterization
Advanced electron microscopy techniques are essential for visualizing and characterizing single-atom catalysts at the atomic scale. These include aberration-corrected transmission electron microscopy (AC-TEM), scanning transmission electron microscopy (STEM), and high-angle annular dark-field (HAADF) imaging. These techniques allow direct observation of individual metal atoms dispersed on support materials, providing crucial information about atomic dispersion, coordination environment, and structural features of SACs.- Electron Microscopy Techniques for SACs Characterization: Advanced electron microscopy techniques are essential for visualizing and characterizing single-atom catalysts at the atomic scale. These include aberration-corrected transmission electron microscopy (AC-TEM), scanning transmission electron microscopy (STEM), and high-angle annular dark-field STEM (HAADF-STEM). These techniques enable direct imaging of individual metal atoms dispersed on support materials, providing crucial information about atomic dispersion, coordination environment, and structural features of SACs.
- X-ray Absorption Spectroscopy for Electronic Structure Analysis: X-ray absorption spectroscopy (XAS) techniques, including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), are powerful tools for probing the electronic structure and local coordination environment of single atoms in catalysts. These techniques provide information about oxidation states, coordination numbers, and bond distances between the metal atoms and surrounding elements, which is crucial for understanding the catalytic mechanisms of SACs.
- Scanning Probe Microscopy for Surface Analysis: Scanning probe microscopy techniques, such as scanning tunneling microscopy (STM) and atomic force microscopy (AFM), offer atomic-scale characterization of SACs by mapping surface topography and electronic states. These techniques allow for direct visualization of single atoms on support surfaces and can provide information about the local electronic environment, which is crucial for understanding catalytic activity and selectivity at the atomic level.
- Synchrotron-based Spectroscopic Methods: Synchrotron-based spectroscopic methods provide high-resolution analysis of SACs by utilizing intense and tunable X-ray beams. These include ambient-pressure X-ray photoelectron spectroscopy (AP-XPS), X-ray emission spectroscopy (XES), and resonant inelastic X-ray scattering (RIXS). These techniques enable in-situ and operando studies of SACs under reaction conditions, revealing dynamic changes in electronic structure and coordination environment during catalytic processes.
- Computational and Multi-technique Approaches: Computational methods combined with experimental techniques provide comprehensive characterization of SACs. Density functional theory (DFT) calculations, molecular dynamics simulations, and machine learning approaches complement experimental data by predicting structural stability, electronic properties, and reaction mechanisms. Multi-technique approaches that integrate various characterization methods offer a more complete understanding of SAC structures and properties, enabling rational design of more efficient catalysts.
02 X-ray Absorption Spectroscopy for Electronic Structure Analysis
X-ray absorption spectroscopy techniques, including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), are powerful tools for probing the electronic structure and local coordination environment of single atoms in catalysts. These techniques provide information about oxidation states, coordination numbers, and bond distances, which are critical for understanding the catalytic properties and mechanisms of single-atom catalysts.Expand Specific Solutions03 Scanning Probe Microscopy for Surface Analysis
Scanning probe microscopy techniques, such as scanning tunneling microscopy (STM) and atomic force microscopy (AFM), enable detailed surface analysis of single-atom catalysts. These techniques provide atomic-resolution imaging of catalyst surfaces, allowing researchers to study the topography, electronic properties, and adsorption sites of single atoms on support materials. This information is valuable for understanding catalyst-substrate interactions and reaction mechanisms.Expand Specific Solutions04 Synchrotron-based Characterization Methods
Synchrotron-based characterization methods offer high-sensitivity analysis of single-atom catalysts. These include synchrotron radiation X-ray photoelectron spectroscopy (SR-XPS), X-ray fluorescence (XRF), and resonant inelastic X-ray scattering (RIXS). These techniques provide detailed information about the electronic structure, chemical state, and local environment of single atoms, even at extremely low concentrations, which is essential for understanding their catalytic performance.Expand Specific Solutions05 Computational and In-situ Characterization Approaches
Computational modeling combined with in-situ characterization approaches provides dynamic insights into single-atom catalyst behavior under reaction conditions. Density functional theory (DFT) calculations complement experimental techniques by predicting stable configurations and reaction pathways. In-situ techniques such as environmental TEM, operando spectroscopy, and time-resolved X-ray methods allow observation of structural changes and catalytic processes in real-time, bridging the gap between static characterization and actual working conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players in SACs
The field of Atomic-Scale Characterization Techniques for Single-Atom Catalysts (SACs) is currently in a growth phase, with an estimated market size of $300-500 million and projected annual growth of 15-20%. The competitive landscape features academic institutions leading fundamental research (Johns Hopkins University, KAIST, KIST Corp., and Institute for Basic Science) alongside industrial players developing commercial applications. SK Innovation and Akzo Nobel Chemicals are advancing industrial-scale implementation, while specialized companies like Beijing Photosynthetic Hydrogen Energy Technology are focusing on niche applications. The technology is approaching maturity in laboratory settings but remains in early commercialization stages, with key challenges in scaling production and standardizing characterization protocols across different catalyst systems.
Institute For Basic Science
Technical Solution: Institute For Basic Science (IBS) has developed advanced atomic-scale characterization techniques for single-atom catalysts (SACs) focusing on in-situ X-ray absorption spectroscopy (XAS) combined with aberration-corrected scanning transmission electron microscopy (AC-STEM). Their approach enables real-time observation of catalytic reactions at the atomic level, allowing researchers to track structural changes and electronic properties of single metal atoms during catalysis[1]. IBS has pioneered the use of synchrotron-based extended X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) techniques to determine the coordination environment and oxidation states of isolated metal atoms. Additionally, they've developed environmental TEM methods that maintain reactive gas environments while imaging single atoms, providing unprecedented insights into catalyst behavior under realistic operating conditions[3]. Their characterization platform integrates computational modeling with experimental data to establish structure-performance relationships for SACs.
Strengths: Superior atomic-resolution imaging capabilities allowing direct visualization of single atoms and their coordination environments. Integration of multiple complementary techniques provides comprehensive structural and electronic information. Weaknesses: Requires access to advanced synchrotron facilities limiting widespread application. Some techniques require ultra-high vacuum conditions that may not represent actual catalytic environments.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed a multi-modal characterization approach for single-atom catalysts combining advanced electron microscopy with synchrotron-based spectroscopic techniques. Their platform centers on aberration-corrected environmental transmission electron microscopy (AC-ETEM) that enables atomic-resolution imaging of catalysts under reaction conditions up to 1000°C and 20 mbar gas pressure[2]. This is complemented by in-situ X-ray absorption spectroscopy (XAS) that provides element-specific information about the electronic structure and coordination environment of single atoms. The university has pioneered the development of machine learning algorithms to analyze spectroscopic data, enabling automated identification of active sites and reaction mechanisms[4]. Their characterization suite also includes in-situ Raman spectroscopy and temperature-programmed techniques that correlate structural changes with catalytic performance. Johns Hopkins researchers have successfully applied these techniques to characterize various SACs including Pt, Pd, and Au atoms on oxide and carbon supports.
Strengths: Exceptional capability to correlate atomic structure with catalytic performance through multi-modal characterization. Advanced data analysis methods including AI for processing complex spectroscopic datasets. Weaknesses: High technical complexity requiring specialized expertise and equipment. Limited throughput for screening large catalyst libraries due to time-intensive analysis procedures.
Key Breakthroughs in Atomic Resolution Imaging
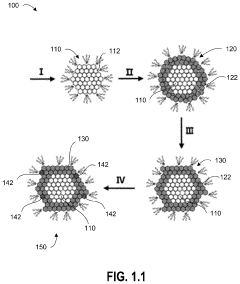
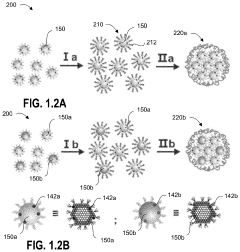
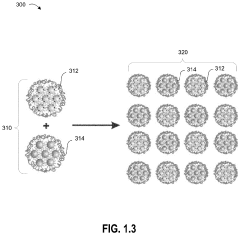
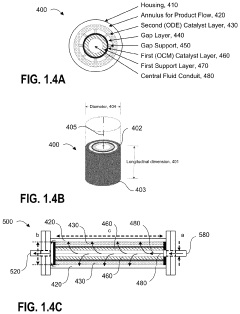
Single-atom-based catalyst systems
PatentActiveUS11273433B2
Innovation
- A single-atom-based catalyst system with controlled hierarchical structures is developed, comprising core-shell nanoparticles and superlattices, optimized through lattice-strain engineering and 3D printing, to enhance the selectivity and activity of single-atom catalytic sites for the direct conversion of methane to ethylene.
Environmental Impact and Sustainability of SACs
The environmental impact of Single-Atom Catalysts (SACs) represents a critical dimension in their development and application trajectory. SACs offer significant sustainability advantages over traditional catalysts due to their atomic-level efficiency. By maximizing atom utilization to nearly 100%, SACs dramatically reduce the consumption of precious metals like platinum, palladium, and rhodium, which face increasing scarcity and extraction challenges.
This atom-efficient design translates directly into reduced environmental footprints across the catalyst lifecycle. Mining operations for precious metals typically generate substantial ecological damage through habitat destruction, water pollution, and energy-intensive extraction processes. The minimal metal requirements of SACs proportionally decrease these environmental burdens, representing a substantial step toward more sustainable catalytic technologies.
In operational contexts, SACs demonstrate enhanced performance metrics that further contribute to their environmental benefits. Their superior catalytic activity often enables reactions to proceed under milder conditions—lower temperatures and pressures—resulting in significant energy savings across industrial applications. This energy efficiency translates to reduced carbon emissions when scaled to industrial production levels.
The application of advanced characterization techniques has revealed that many SACs exhibit exceptional selectivity, minimizing unwanted by-products and reducing waste streams. This selectivity is particularly valuable in environmental remediation applications, where SACs have shown promise in water purification, air pollution control, and the degradation of persistent organic pollutants with minimal secondary contamination.
Lifecycle assessments of SAC technologies indicate potential challenges in recycling and recovery processes. While the atomically dispersed nature of active sites enhances catalytic performance, it may complicate end-of-life recovery compared to traditional nanoparticle catalysts. Research into sustainable recovery methods for spent SACs represents an emerging priority to ensure closed-loop material cycles.
The long-term stability of SACs under industrial conditions remains an area requiring further investigation from a sustainability perspective. Atomic migration and aggregation can reduce catalyst longevity, potentially offsetting initial material efficiency gains. Advanced characterization techniques are instrumental in understanding degradation mechanisms and developing stabilization strategies that extend operational lifetimes without compromising environmental benefits.
As climate change concerns intensify, the role of SACs in green chemistry applications continues to expand. Their implementation in renewable energy systems, carbon capture technologies, and sustainable chemical manufacturing positions them as enabling technologies for broader environmental transitions across multiple industries.
This atom-efficient design translates directly into reduced environmental footprints across the catalyst lifecycle. Mining operations for precious metals typically generate substantial ecological damage through habitat destruction, water pollution, and energy-intensive extraction processes. The minimal metal requirements of SACs proportionally decrease these environmental burdens, representing a substantial step toward more sustainable catalytic technologies.
In operational contexts, SACs demonstrate enhanced performance metrics that further contribute to their environmental benefits. Their superior catalytic activity often enables reactions to proceed under milder conditions—lower temperatures and pressures—resulting in significant energy savings across industrial applications. This energy efficiency translates to reduced carbon emissions when scaled to industrial production levels.
The application of advanced characterization techniques has revealed that many SACs exhibit exceptional selectivity, minimizing unwanted by-products and reducing waste streams. This selectivity is particularly valuable in environmental remediation applications, where SACs have shown promise in water purification, air pollution control, and the degradation of persistent organic pollutants with minimal secondary contamination.
Lifecycle assessments of SAC technologies indicate potential challenges in recycling and recovery processes. While the atomically dispersed nature of active sites enhances catalytic performance, it may complicate end-of-life recovery compared to traditional nanoparticle catalysts. Research into sustainable recovery methods for spent SACs represents an emerging priority to ensure closed-loop material cycles.
The long-term stability of SACs under industrial conditions remains an area requiring further investigation from a sustainability perspective. Atomic migration and aggregation can reduce catalyst longevity, potentially offsetting initial material efficiency gains. Advanced characterization techniques are instrumental in understanding degradation mechanisms and developing stabilization strategies that extend operational lifetimes without compromising environmental benefits.
As climate change concerns intensify, the role of SACs in green chemistry applications continues to expand. Their implementation in renewable energy systems, carbon capture technologies, and sustainable chemical manufacturing positions them as enabling technologies for broader environmental transitions across multiple industries.
Standardization and Reproducibility Challenges
The standardization and reproducibility of atomic-scale characterization techniques for Single-Atom Catalysts (SACs) represent significant challenges in advancing this field. Despite the remarkable progress in developing sophisticated characterization methods, the scientific community continues to struggle with establishing universal protocols that ensure consistent and comparable results across different research groups and facilities.
One of the primary challenges lies in sample preparation methodologies, which can significantly influence characterization outcomes. The ultra-sensitive nature of SACs means that minor variations in preparation conditions—such as exposure time to ambient conditions, substrate cleaning procedures, or precursor purity—can lead to substantially different results. This variability makes it difficult to establish whether observed differences between studies reflect genuine material properties or merely procedural inconsistencies.
Instrument calibration presents another critical challenge. Advanced characterization techniques like aberration-corrected STEM, XAFS, and in-situ spectroscopies require precise calibration to deliver accurate atomic-scale information. However, calibration standards for SAC characterization remain largely non-standardized, with different research groups employing varied reference materials and calibration methodologies, further complicating cross-study comparisons.
Data processing and interpretation frameworks also lack standardization. The extraction of meaningful information from raw characterization data often involves complex algorithms and modeling approaches that vary between research groups. Without standardized data processing protocols, the same raw data can yield different conclusions depending on the analytical methods employed, undermining reproducibility in the field.
Environmental control during characterization represents a particularly challenging aspect of SAC analysis. Single atoms are exceptionally sensitive to environmental conditions, with factors such as temperature fluctuations, vacuum quality, and electron beam effects potentially altering the catalyst structure during observation. These variables must be meticulously controlled and reported to ensure reproducible characterization.
The scientific community has begun addressing these challenges through collaborative initiatives aimed at establishing best practices and standardized protocols. International round-robin testing programs, where identical samples are characterized across multiple facilities, have proven valuable in identifying sources of variability. Additionally, efforts to develop certified reference materials specifically designed for SAC characterization are underway, though still in early stages.
Addressing these standardization and reproducibility challenges will require concerted effort from academic institutions, industry partners, and standards organizations. The development of comprehensive reporting guidelines that detail all relevant experimental parameters would significantly enhance reproducibility and facilitate meaningful comparisons between studies, ultimately accelerating progress in SAC development and implementation.
One of the primary challenges lies in sample preparation methodologies, which can significantly influence characterization outcomes. The ultra-sensitive nature of SACs means that minor variations in preparation conditions—such as exposure time to ambient conditions, substrate cleaning procedures, or precursor purity—can lead to substantially different results. This variability makes it difficult to establish whether observed differences between studies reflect genuine material properties or merely procedural inconsistencies.
Instrument calibration presents another critical challenge. Advanced characterization techniques like aberration-corrected STEM, XAFS, and in-situ spectroscopies require precise calibration to deliver accurate atomic-scale information. However, calibration standards for SAC characterization remain largely non-standardized, with different research groups employing varied reference materials and calibration methodologies, further complicating cross-study comparisons.
Data processing and interpretation frameworks also lack standardization. The extraction of meaningful information from raw characterization data often involves complex algorithms and modeling approaches that vary between research groups. Without standardized data processing protocols, the same raw data can yield different conclusions depending on the analytical methods employed, undermining reproducibility in the field.
Environmental control during characterization represents a particularly challenging aspect of SAC analysis. Single atoms are exceptionally sensitive to environmental conditions, with factors such as temperature fluctuations, vacuum quality, and electron beam effects potentially altering the catalyst structure during observation. These variables must be meticulously controlled and reported to ensure reproducible characterization.
The scientific community has begun addressing these challenges through collaborative initiatives aimed at establishing best practices and standardized protocols. International round-robin testing programs, where identical samples are characterized across multiple facilities, have proven valuable in identifying sources of variability. Additionally, efforts to develop certified reference materials specifically designed for SAC characterization are underway, though still in early stages.
Addressing these standardization and reproducibility challenges will require concerted effort from academic institutions, industry partners, and standards organizations. The development of comprehensive reporting guidelines that detail all relevant experimental parameters would significantly enhance reproducibility and facilitate meaningful comparisons between studies, ultimately accelerating progress in SAC development and implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!